Supplemental Digital Content is available in the text.
Keywords: adenosine, angiography, coronary angiography, coronary artery disease, hyperemia
Abstract
Background—
Quantitative flow ratio (QFR) is a novel diagnostic modality for functional testing of coronary artery stenosis without the use of pressure wires and induction of hyperemia. QFR is based on computation of standard invasive coronary angiographic imaging. The purpose of WIFI II (Wire-Free Functional Imaging II) was to evaluate the feasibility and diagnostic performance of QFR in unselected consecutive patients.
Methods and Results—
WIFI II was a predefined substudy to the Dan-NICAD study (Danish Study of Non-Invasive Diagnostic Testing in Coronary Artery Disease), referring 362 consecutive patients with suspected coronary artery disease on coronary computed tomographic angiography for diagnostic invasive coronary angiography. Fractional flow reserve (FFR) was measured in all segments with 30% to 90% diameter stenosis. Blinded observers calculated QFR (Medis Medical Imaging bv, The Netherlands) for comparison with FFR. FFR was measured in 292 lesions from 191 patients. Ten (5%) and 9 patients (5%) were excluded because of FFR and angiographic core laboratory criteria, respectively. QFR was successfully computed in 240 out of 255 lesions (94%) with a mean diameter stenosis of 50±12%. Mean difference between FFR and QFR was 0.01±0.08. QFR correctly classified 83% of the lesions using FFR with cutoff at 0.80 as reference standard. The area under the receiver operating characteristic curve was 0.86 (95% confidence interval, 0.81–0.91) with a sensitivity, specificity, negative predictive value, and positive predictive value of 77%, 86%, 75%, and 87%, respectively. A QFR–FFR hybrid approach based on the present results enables wire-free and adenosine-free procedures in 68% of cases.
Conclusions—
Functional lesion evaluation by QFR assessment showed good agreement and diagnostic accuracy compared with FFR. Studies comparing clinical outcome after QFR- and FFR-based diagnostic strategies are required.
Clinical Trial Registration—
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02264717.
Functional evaluation is the established diagnostic standard for the assessment of intermediate coronary artery stenosis in stable coronary artery disease.1–3 Present guidelines recommend fractional flow reserve (FFR) to assess obstruction-mediated ischemia, and numerous studies have documented favorable clinical outcome of FFR-guided coronary interventions.4–6 Many centers have not fully adopted an FFR-guided treatment strategy.7,8 Underlying causes include the need for drug-induced hyperemia causing patient discomfort, a prolonged procedure time, and reimbursement systems not favoring FFR-based diagnostic strategies. To overcome these limitations, several attempts to derive FFR with methods based on 3-dimensional imaging modalities have been undertaken.9–12 Quantitative flow ratio (QFR; Medis medical imaging system bv, The Netherlands) is a computation of FFR based on (1) a 3-dimensional reconstruction of the stenotic vessel rendered from 2 angiographic projections, and (2) contrast flow frame count. The optimal approach for QFR computation was evaluated in the FAVOR pilot study (Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve from Diagnostic Coronary Angiography) where computation by frame count–based estimation of contrast flow velocity showed favorable results.13 Hence, this method was applied in the WIFI II study (Wire-Free Functional Imaging II) and is denoted as QFR. In WIFI II, we aimed to evaluate the feasibility and diagnostic performance of QFR in consecutive prospectively enrolled patients.
See Editorial by Albaghdadi and Jaffer
Methods
Study Design
WIFI II was a predefined prospective, observational, investigator-initiated Dan-NICAD substudy with paired assessment of QFR and pressure wire–based FFR. Patients with suspected coronary artery disease and indication for coronary computed tomographic angiography (CTA) evaluation were enrolled at 2 referring centers (Department of Cardiology, Hospitalsenheden Midt, Silkeborg, Denmark; and Department of Cardiology, Hospitalsenheden Vest, Herning, Denmark) between September 11, 2014, and March 31, 2016.14 Patients with stenosis identified by coronary CTA were referred for invasive coronary angiography (ICA) and formed the study population of WIFI II. Lesions with diameter stenosis (DS) of 30% to 90% in vessels with a reference diameter >2.0 mm by visual estimate entered the analyzed population. Angiographic and procedural exclusion criteria were (1) <2 projections with visible stenosis; (2) stenosis in the ostium of the right coronary artery or the left main coronary artery; (3) no administration of intracoronary nitrates; and (4) pressure wire position not documented angiographically. All patients provided informed written consent. The Danish Data Protection Agency and The Central Denmark Region Committees on Health Research Ethics approved the study. At least 2 authors (J.W. and N.R.H.) had access to all data presented. All authors are responsible for the data integrity. The study material will not be made available to other researchers for competitive reasons.
Invasive Coronary Angiography
ICA was scheduled within 4 weeks after completing a coronary CTA. The procedure was performed with 6F catheters through a radial or femoral access and administration of 250 μg of intracoronary nitroglycerine. All lesions with 30% to 90% DS by visual assessment were evaluated by 2 projections rotated around the axis of the vessel with minimum 25° separation aiming for minimal foreshortening and no vessel overlap. Acquisitions were acquired manually at 15 frames per second and with forceful contrast injections.
FFR Assessment
FFR was measured according to the prespecified protocol. Volcano FFR (San Diego, CA) and St. Jude medical FFR (Saint Paul, MN) systems were used. Hyperemia was induced by administration of 140 μg min−1 kg−1 adenosine in a femoral or brachial vein. Fluctuating hemodynamics was evaluated as decreased hyperemia, and the infusion speed was increased accordingly. Limited drift with a ratio of distal pressure/proximal pressure (Pd/Pa) at the guiding catheter tip ranging from 0.96 to 1.04 was accepted. For FFR values between 0.76 and 0.84, only minor drift values between 0.98 and 1.02 were accepted.
FFR Core Laboratory Analysis
Blinded observers analyzed the waveforms for all traces at the central core laboratory (Interventional Coronary Imaging Core Laboratory; Aarhus University Hospital, Denmark). The FFR readings were assessed for achievement of hyperemia, no significant loss of Pa and Pd, no severe dampening, and acceptable drift. Each analysis provided Pd and Pa values at baseline and intravascular FFR values. Resting Pd/Pa was noted if at least 8 s of stable value was identified in the trace before induction of hyperemia. A core laboratory reading of FFR ≤0.80 was used as diagnostic cutoff value.
QFR Computation
QFR was computed using the QAngio XA 3-dimensional (3D) 1.1 software package (Medis Medical Imaging System bv, The Netherlands) by an observer blinded to the FFR readings. The methodology was described previously.13 In brief, a 3D model of the vessel of interest was constructed based on automated contouring of the vessel in 2 angiographic projections. Subsequently, estimated contrast flow velocity (eCFV) was determined using frame count analysis by indicating the frames where contrast entered and exited the segmented part of the vessel. The eCFV obtained during resting conditions was automatically converted into a virtual hyperemic flow velocity using a quadratic function. Finally, the application computed the pressure drop along the segmented vessel enabling QFR reading at any point along the vessel. QFR ≤0.80 was used as diagnostic cutoff value.
Flow Velocity Estimation
The frame count method used is modified from conventional trombolysis in myocardial infarction frame counting by only counting frames of contrast transit in the reconstructed vessel or subsegments.13 The flow characteristics were described for all cases with eCFV at baseline conditions.
Two-Dimensional Quantitative Coronary Angiography
All angiograms were assessed by standard 2D quantitative coronary angiography analysis (QAngioXA 7.3; Medis Medical Imaging System bv, The Netherland) by a separate core laboratory (ClinFact, Leiden, The Netherlands). The observers only received diagnostic angiographic runs and were blinded to any potential treatment and to FFR and QFR results.
Repeatability Analysis
Repeated analysis included the last 40 lesions analyzed by the same observer 6 months after completing the last QFR computation. Repeated analysis was performed using the same angiographic frames.
FFR–QFR Hybrid Strategy
A hybrid diagnostic approach using QFR as the main diagnostic method and only performing FFR measurements in a grey zone around the cut point was constructed. QFR limits were determined by area under the receiver operating curve curve analysis for which sensitivity and specificity were both >90% compared with FFR. The QFR–FFR hybrid method was modeled assuming that lesions with QFR values between the 90% limits follow the FFR binary cutoff. The fraction of potential adenosine-free and wire-free procedures were calculated (Figure I in the Data Supplement).
Power Calculation
On the basis of data from the FAVOR pilot study,10 we estimated the sensitivity and specificity of QFR to be 0.74 and 0.91, respectively. Combined with a presumed hemodynamically significant (FFR≤0.80) stenosis prevalence of 30%, 220 lesions were required to obtain sufficient power with 10% precision on each side of the confidence interval (CI). To account for FFR- and QFR-related exclusions, 250 lesions with FFR measurement were needed.
Statistical Analysis
Baseline results are presented as mean and SD or range as appropriate. Feasibility of QFR was determined as the fraction of successful wire FFR measurements where the corresponding QFR was computed. The correlation between QFR and FFR was assessed with Spearman correlation coefficient. As FFR is a vessel-specific index, measurements from multiple lesions assessed with FFR in the same patient were assumed to be independent. A Bland–Altman plot and Wilcoxon signed-rank test were used to visualize and compare QFR and FFR. An extension of the Wilcoxon rank-sum test was used to detect trends across strata of stenosis severities. Mann–Whitney U test, unpaired t test, Fisher exact test (when 2 groups), and χ2 test (when ≥2 groups) were used to test for numeric and binary differences in baseline characteristics when grouped by FFR–QFR concordance/discordance. Multiple linear regression was performed to identify predictors of increased absolute difference between FFR and QFR. Variables with clinical importance (age, sex, body mass index, hypertension, vessel, %DS, and FFR) and a P value <0.20 were included in the model. Because of non-normal distribution of the absolute QFR–FFR difference, data were transformed by (log(abs_dif+0.01)). Diagnostic performance was assessed with a nonparametric analysis of area under the receiver operating characteristic curve, and separate QFR cut points were derived for which sensitivity and specificity were each >90%. On the basis of the Fractional Flow Reserve to Determine the Approriateness of Angioplasty in Moderate Coronary Stenosis (DEFER) trial results, the performance analysis was repeated excluding FFR values between 0.77 and 0.83, where the FFR classification certainty is around 80% for repeated measurements.15 P values <0.05 were considered significant. Analysis was performed using STATA version 13 (StataCorp, College Stadion, TX).
Results
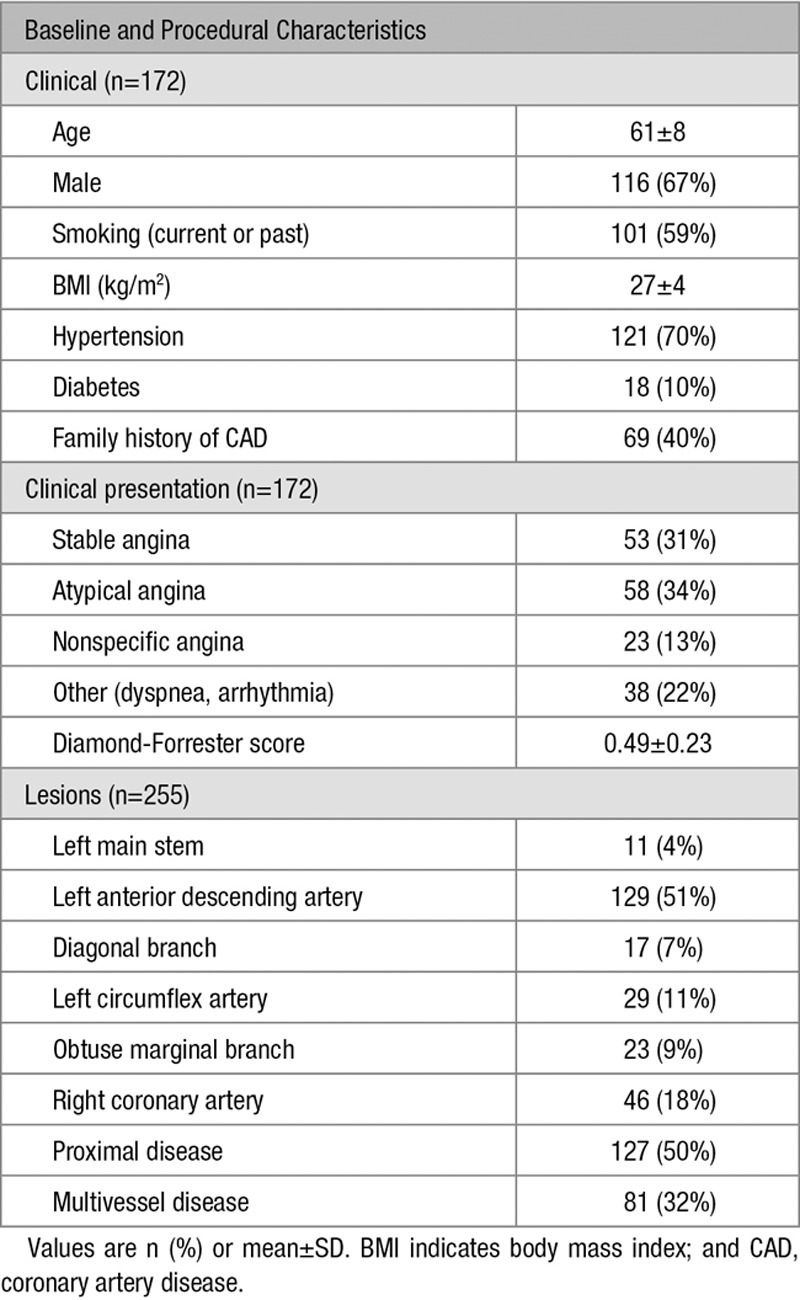
In the Dan-NICAD study, 362 patients were referred for ICA, and FFR was measured in 292 lesions from 191 patients. Ten patients (5%) were excluded because of insufficient FFR documentation and 9 patients (5%) because of predefined angiographic and procedural exclusion criteria. Exclusions were because of ostial left main coronary artery stenosis (n=1), missing angiographic projections (n=2), no nitroglycerine administration (n=2), ostial right coronary artery disease (=1), angiographic storage issue (n=1), and no documentation of pressure wire position (n=2; Figure 1; Figure II in the Data Supplement), leaving 172 patients with 255 lesions in the group entering analysis. Multivessel disease was observed in 32% of the patients. Investigated lesions had a mean DS of 50±12% (2D quantitative coronary angiography). Baseline characteristics are listed in Table 1 and Table I in the Data Supplement.
Figure 1.
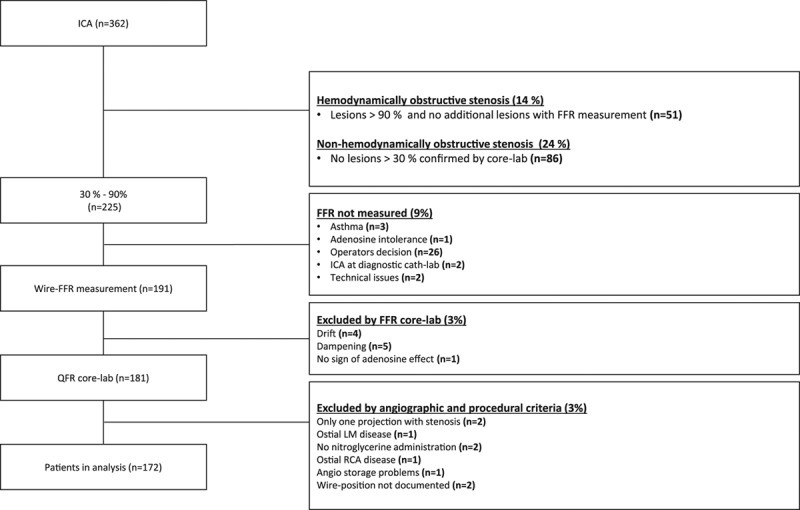
Study enrollment flowchart. Numbers n are study patients. ICA indicates invasive coronary angiography; FFR, fractional flow reserve; LM, left main stem; QFR, quantitative flow ratio; and RCA, right coronary artery.
Table 1.
Baseline Characteristics
Pressure and Flow Characteristics
Lesions had a mean FFR of 0.82±0.11 and median FFR of 0.85 (Inter Quartile Range, 0.77–0.90) (Figure 2), with a mean Pa and Pd of 87±17 and 72±18 mm Hg, respectively. Positive FFR (≤0.80) was identified in 36% (n=86) of the patients. Mean eCFV was 0.19±0.07 m/s. Resting Pd/Pa was analyzed in 223 (92%) traces with an average Pd/Pa of 0.94±0.05.
Figure 2.
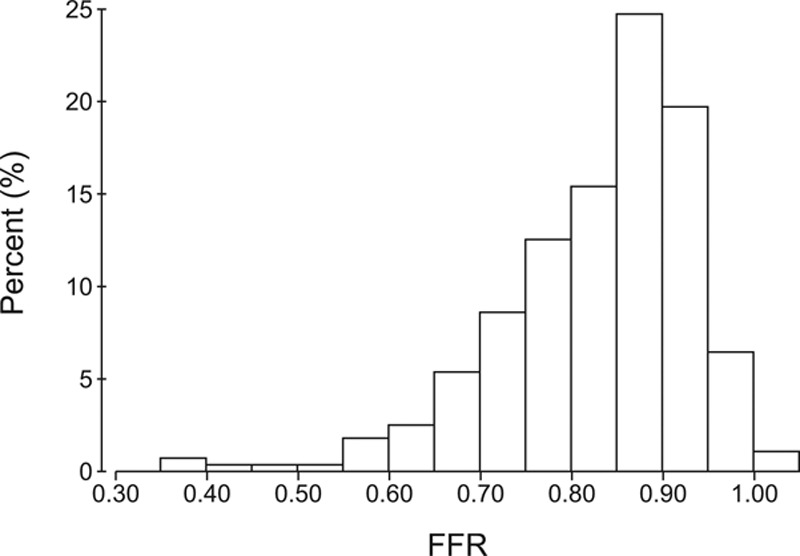
Lesion distribution. Distribution of measurements according to fractional flow reserve (FFR). Median FFR was 0.85 (range 0.39–1.04). Twenty-one percent of the lesions were in the FFR 0.77 to 0.83 interval.
QFR Computation Feasibility
QFR computation was successful in 240 of 255 (95%) lesions. Unsuccessful QFR computations were because of overlap at the lesion segment of interest (n=6), excessive foreshortening in stenotic segments (n=7), insufficient contrast flow quality (n=1), and inability to contour a tight stenosis because of poor contrast filling (n=1).
Correlation and Agreement
Median QFR was 0.84 (IQR, 0.77–0.89). QFR computation showed a correlation of r=0.70 (P<0.0001; Figure 3) and precision with a mean difference of 0.01±0.08 (P=0.08) with FFR. Bland–Altman plot and scatter plots are presented in Figure 3. Stratified by FFR values, the agreement was −0.14±0.23 for FFR values <0.55; −0.03±0.11 for FFR 0.55 to 0.65; −0.01±0.10 for FFR 0.65 to 0.75; −0.02±0.05 for FFR 0.75 to 0.85; and 0.03±0.07 for FFR >0.85. Increasing degree of difference between QFR and FFR (Ptrend<0.0001) was observed for increasing stenosis severity based on 3D quantitative coronary angiography. The mean difference between mean QFR for repeated QFR computation was 0.00±0.06 (P=0.65).
Figure 3.
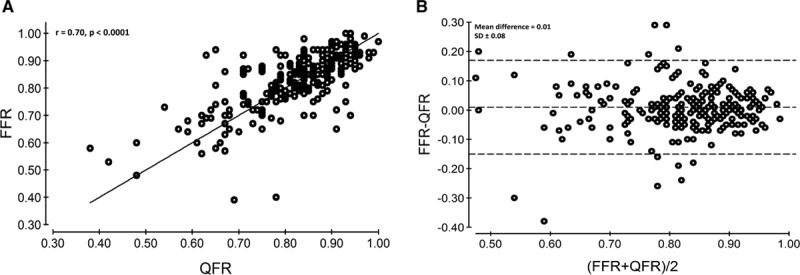
Correlation and agreement. Good correlation and agreement of quantitative flow ratio (QFR) and fractional flow reserve (FFR) was observed (r=0.70; mean difference=0.01; A). Bland–Altman plot; dashed line illustrates the mean difference±2 SD (B).
Diagnostic Performance
Comparison of QFR with FFR as reference standard resulted in an AUC for QFR of 0.86 (95% CI, 0.81–0.91). Accuracy was 83% for QFR computation with 66 true positives, 132 true negatives, 20 false positives, and 22 false negatives. Overall sensitivity, specificity, positive predictive value, and negative predictive value were 77% (95% CI, 66–85), 86% (95% CI, 79–91), 75% (95% CI, 65–84), and 87% (95% CI, 80–92), respectively (Table 2). QFR computation performed significantly better than DS derived from 2D quantitative coronary angiography (AUC 0.86 versus 0.61; P<0.0001) with FFR as gold standard (Figure 4). Mean difference between QFR and FFR was 0.01±0.08 for the left anterior descending artery, 0.01±0.05 for diagonal branches, 0.02±0.10 for the right coronary artery, 0.03±0.08 for the left circumflex artery, 0.02±0.09 for obtuse marginal branches, and 0.02±0.04 for the left coronary main artery. We found no per vessel difference for mean difference between QFR and FFR (P=0.10). A total of 123 (52%) patients had >1 lesion with paired FFR and QFR assessment. There was no difference in mean difference±SD for FFR–QFR when grouped by multiple lesions versus single lesion (multiple lesions 0.01±0.09 versus single lesion 0.01±0.07; P=0.38). There was no difference in clinical characteristics stratified by FFR/QFR correspondence (Table II in the Data Supplement). Low FFR was a predictor of increased absolute difference between QFR and FFR after adjusting for body mass index, %DS, and vessel location (Table III in the Data Supplement).
Table 2.
Diagnostic Performance
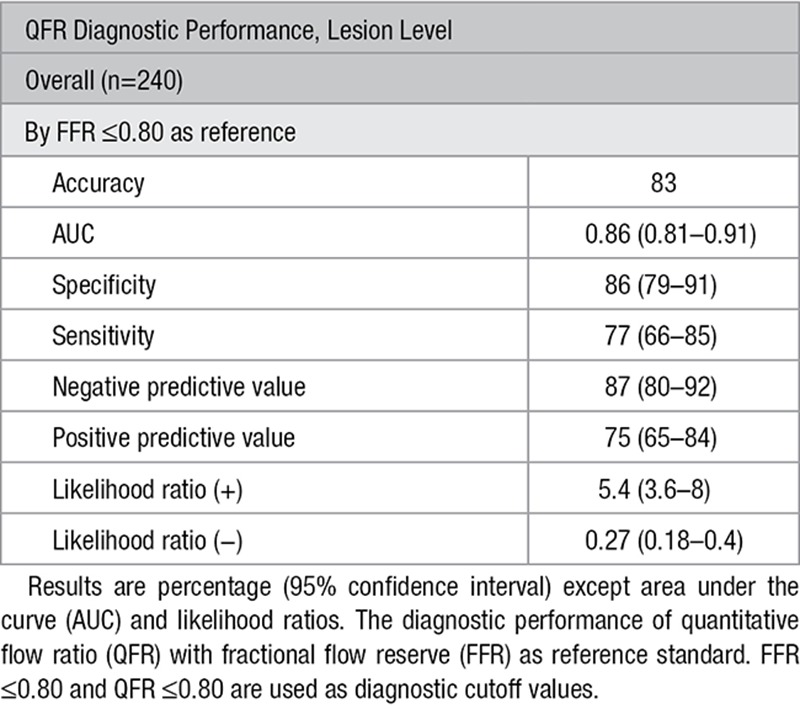
Figure 4.
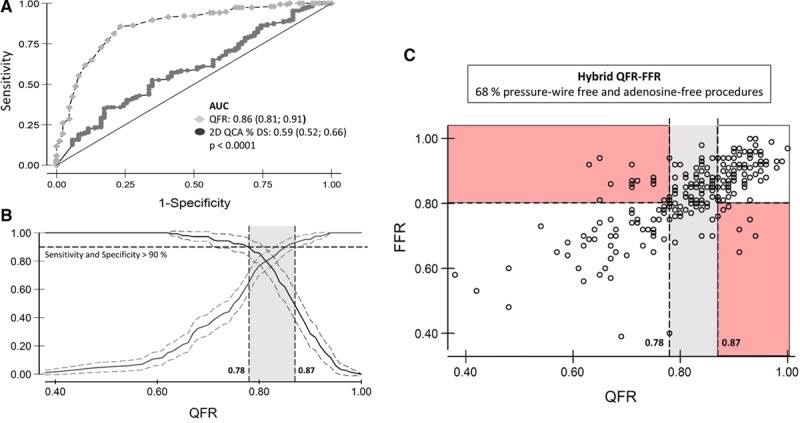
Diagnostic performance and clinical application of quantitative flow ratio (QFR). Receiver operating characteristic analysis comparing QFR to 2-dimensional (2D) quantitative coronary angiography (QCA) with fractional flow reserve (FFR) as reference standard (A) and identification of QFR cut points to yield a sensitivity and specificity >90% compared with FFR (B) for use in a hybrid QFR–FFR model (C). AUC indicates area under the receiver operating characteristic curve.
Diagnostic Cutoff Values
QFR accuracy improved when excluding cases with FFR values in the range of 0.77 to 0.83 (83%–87%; P=0.002) around the diagnostic cut point. By AUC analysis, the QFR limits to yield specificity and sensitivity >90% were 0.78 and 0.87, respectively. A QFR–FFR hybrid approach with the 0.78 and 0.87 limits was modeled and indicated that QFR based on the present results enables wire-free and adenosine-free procedures in 68% of cases (Figure 4). The limits to yield >95% accuracy were 0.71 and 0.90 resulting in wire-free and adenosine-free procedure in 42% of cases.
Discussion
WIFI II is the first adequately powered study to assess the feasibility and diagnostic performance of functional lesion evaluation by QFR. We showed that QFR was feasible and provided good diagnostic performance compared with conventional wire–based FFR assessment in prospectively enrolled patients with intermediate risk.
Comparison to the Existing Literature
Several approaches for approximation of FFR by computation of invasive- or noninvasive-derived angiographic images have been introduced16 (Table IV in the Data Supplement). Coronary CTA-based FFR computation has demonstrated promising results by improving diagnostic accuracy of coronary CTA.17–21 Published CTA-based FFR methods are based on computational fluid dynamics and assumptions on physiological models for coronary blood flow. QFR differs from these approaches by avoiding the need for computational fluid dynamics that requires extensive computing. QFR is derived from fluid dynamic equations incorporating vessel geometry, assumptions on flow through a stenosis, and vessel tapering in relation to side branch exits as described in detail by Tu et al.13 Hence, the inherited limitations of fixed boundary conditions are minimized, and computation is performed without noticeable delay using a Windows-based computer improving in-procedure feasibility of QFR. We found an agreement of QFR to FFR comparable and slightly better than most studies comparing CTA-based FFR and FFR (Table IV in the Data Supplement); only the NXT trial (Diagnostic Performance of Noninvasive Fractional Flow Reserve Derived From Coronary Computed Tomography Angiography) by Nørgaard et al showed a better agreement with FFR. However, likewise for the WIFI II results, the SD of 0.074 was higher for more severe lesions in the NXT trial (the NXT Bland–Altman plot illustrates substantial scatter for lesions with FFR <0.80). Thus, because only 17% had FFR ≤0.80 in NXT (36% in WIFI II), the SDs are not comparable. QFR- and CTA-based FFR computation strategies are potentially feasible at different steps in the diagnostic process and may therefore complement each other rather than compete. The results from WIFI II are in line with the FAVOR pilot study that investigated the optimal approach for QFR computation.13 We observed a lower AUC and a higher SD which may be caused by the stricter inclusion of consecutive patients in WIFI II and an intention on excluding as few as possible based on impaired angiographic quality. Further, all QFR computations in the FAVOR pilot study were performed by the highly trained inventor of QFR (S.T.) which might attribute further to the observed difference. Several alternative approaches for ICA-derived FFR computation exist. Morris et al11 documented a good agreement between their virtual-FFR method and FFR. However, the lesion distribution and study design was different (20% with FFR≤0.80, 24 hours computation time, and inclusion of lesions both pre- and post-stenting, Table III in the Data Supplement). The overall agreement of virtual-FFR with FFR was better than for QFR reported in this study, but importantly, the SD of virtual-FFR with FFR as reference for prestent measurements was 0.10 and thus similar to 0.08 for QFR in this report.11 The virtual functional assessment index proposed by Papafaklis et al9 is not equivalent to FFR but showed a good diagnostic performance in a retrospective study with FFR as reference standard. The virtual functional assessment index computation time was reported to an average of 15 minutes with 7 minutes contributed to computational fluid dynamics simulations. Because QFR does not require the computational fluid dynamics step, QFR is potentially faster increasing the clinical feasibility.9 A direct comparison between time to FFR and QFR during diagnostic angiography is assessed in FAVOR II E-J (Clinical Trial Registration—URL: https://www.clinicaltrials.gov. Unique identifier: NCT02959814).
Comparison to FFR
The gold standard FFR is extensively validated with a single cutoff value of 0.80 or 0.75. More prognostic information is derived from FFR as the numeric value of FFR has a continuous relationship with outcomes, meaning that lower values gain more benefit from revascularization, whereas values around the FFR cut point of 0.80 approach the percutaneous coronary intervention-related event rate.22 Diagnostic accuracy of QFR improved significantly if only compared with FFR values outside an FFR interval of 0.77 to 0.83 (83% improved to 87%; P=0.002). Reclassification of FFR diagnosis for repeated measurements of values around the cut point indicates a sealing for the theoretical achievable diagnostic precision for any modality compared with FFR.15 This phenomenon related to all diagnostic tests with a dichotomous cut off emphasizes the limitation of dichotomous interpretation of FFR. In WIFI II, almost one fourth of the stenoses were within the range of 0.77 to 0.83.
QFR–FFR Hybrid Approach
As for all virtual indices that were compared with FFR (Table IV in the Data Supplement), the reported agreement and diagnostic performance of QFR with FFR as reference showed variation that might limit the individual patient decisions. This was likewise observed for the initial instantaneous wave–free ratio FFR comparison studies. However, instantaneous wave–free ratio was recently shown to provide noninferior clinical outcome compared with FFR.23,24 Awaiting evidence that a QFR-based strategy yields clinical outcome comparable to FFR, a hybrid QFR–FFR diagnostic approach could be a first step in clinical integration. On the basis of the assumption applied in the (instantaneous wave–free ratio)-FFR hybrid strategies,25,26 standard pressure wire–based FFR assessment would be required in 32% of the cases in a QFR–FFR hybrid strategy (Figure 4). The potential two-third reduction in need for pressure wires and medical-induced hyperemia may improve overall patient comfort and lead to substantial cost reductions. The modeled hybrid approach is hypothesis generating and should be tested in an independent population.
Combined Pressure and Flow
The QFR algorithm is sensitive to the underlying patient-specific eCFV.13 Estimation of flow velocity based on modified contrast frame counting is not validated against invasively measured coronary flow velocity, and contrast injection may cause changes in hemodynamics itself.27 However, our eCFV values at baseline are numerically consistent with recent data from the Iberian-Dutch-English (IDEAL) collaborators.28 QFR was tuned to match FFR that presently is the best-validated lesion-specific index, but the definition of a true clinical gold standard for identification of lesion-specific ischemic potential is continuously developing and refined. Previous studies documented a possible discriminative power of simultaneous pressure and flow measurements that has to be further elucidated by addressing the importance of discordance between hemodynamic parameters for patient outcomes.29,30 Hence, QFR could develop as routine diagnostic tool pending further validation to reflect the stenosis-specific pressure gradient while being able to complement with flow characteristics.
Clinical Feasibility of QFR
The promising results of this study warrant the validation by in-procedure QFR assessment. The early version of the QFR application used in the trial required user interaction to delineate the lumen contours and for contrast flow estimation. Further, the effect of standardized contrast injection (ie, standard dose or automated injection) has not yet been clarified. User interaction could cause variation in analysis and particularly in patients with diffuse disease combined with suboptimal angiographic image quality. This is reflected in the reported SD for repeated analysis (0.06) which is larger than the imprecision for repeated FFR measurement of 0.02.31 Interobserver agreement was not assessed in this study. Before QFR solutions are implemented in clinical practice, analysis training and elaborate, evidence-based standard operating procedures are important to ensure uniform, consistent, and repeatable QFR analysis.
Limitations
QFR analysis was performed after receiving the application based on the final QFR algorithm; thus, in-procedure feasibility was not evaluated in WIFI II. As experience with QFR at study start was limited, the importance of optimal angiographic views and optimal image quality was not fully appreciated during the early phase of the study. In-procedure QFR may improve results as the direct feedback to the percutaneous coronary intervention operator after early identification of insufficient angiographic quality could improve overall performance of QFR. Dedicated bifurcation QFR analysis software was not available during the QFR analysis in WIFI II, and results derived in lesions involving a large side branch might therefore improve with future iterations of QFR. A common analysis protocol was applied to all lesion subsets, but specific lesions types or localizations requiring an adjusted algorithm or different analysis approach cannot be ruled out with the present level of evidence. As patients were referred to ICA based on coronary CTA, a portion of patients presented with nonobstructive coronary artery disease without lesions of >30% DS and were not eligible for the study procedure. Further, the results presented should be interpreted by appreciating the lesion distribution with a dominant proportion of nonischemic lesions which might have impacted the specificity and negative predictive value results (Figure 2). However, the setup of WIFI II reflects normal clinical practice when examining intermediate-risk patients.
Conclusions
Functional lesion evaluation by QFR measurement is feasible and shows good agreement and diagnostic accuracy compared with FFR in patients with intermediate stenosis. QFR may emerge as a safe and cost-reducing diagnostic modality potentially improving the utilization of physiological guided decision making. The promising results call for a randomized comparison of clinical outcome after QFR- and FFR-based diagnostic strategies in a multicenter setup.
Sources of Funding
The study was funded by Acarix, Medis Medical Imaging bv, the participating hospitals, and the National Key Research and Development Program of China (Grant No. 2016YFC0100500) and the National Natural Science Foundation of China (Grant No. 31500797).
Disclosures
J. Westra received research support from Medis Medical Imaging System bv. Dr Tu received research support from Medis Medical Imaging System bv and Pulse Medical Imaging. Dr Maeng has received institutional grants from Volcano. Dr Christiansen received Institutional research grants from Boston Scientific, St. Jude Medical, and Volcano. Dr Holm has received institutional research grants from Biosensors, Abbott, Cordis, Medtronic, Biotronik, Reva Medical, Elixir, Medis Medical Imaging, and Boston Scientific, and speaker fees from Boston Scientific, St. Jude Medical, and Terumo. Dr Reiber is the CEO of Medis Medical Imaging bv that provided the software for this study. The other authors report no conflicts.
Supplementary Material
Footnotes
The Data Supplement is available at http://circimaging.ahajournals.org/lookup/suppl/doi:10.1161/CIRCIMAGING.117.007107/-/DC1.
CLINICAL PERSPECTIVE
We showed that quantitative flow ratio (QFR) had superior diagnostic precision compared with quantitative coronary angiography. A QFR-based strategy could therefore be an alternative in centers or healthcare systems where pressure wire–based diagnostics are not possible. QFR solutions may be combined with or integrated into most digital angiographic systems, making physiology-based diagnostic strategies available to a wider population. In centers using fractional flow reserve routinely, a hybrid strategy with QFR may reduce the need for costly pressure wire interrogation and the discomfort of medial-induced hyperemia. Clinical implementation of QFR requires verification of multicenter in-procedure feasibility and detailed evaluation of precision in complex lesion subsets. Recommendation of QFR as an equal alternative to fractional flow reserve requires proven noninferiority of a QFR-based diagnostic strategy compared with fractional flow reserve–based strategies in adequately powered randomized trials.
References
- 1.Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, Heyndrickx GR, Bartunek J, Vanderheyden M, Barbato E, Wijns W, De Bruyne B. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35:2831–2838. doi: 10.1093/eurheartj/ehu094. doi: 10.1093/eurheartj/ehu094. [DOI] [PubMed] [Google Scholar]
- 2.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 3.White CW, Wright CB, Doty DB, Hiratza LF, Eastham CL, Harrison DG, Marcus ML. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? N Engl J Med. 1984;310:819–824. doi: 10.1056/NEJM198403293101304. doi: 10.1056/NEJM198403293101304. [DOI] [PubMed] [Google Scholar]
- 4.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 5.De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nüesch E, Jüni P FAME 2 Trial Investigators. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. doi: 10.1056/NEJMoa1408758. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 6.van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrøm T, Klauss V, MacCarthy PA, Manoharan G, Oldroyd KG, Ver Lee PN, Van’t Veer M, Fearon WF, De Bruyne B, Pijls NH FAME Study Investigators. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet. 2015;386:1853–1860. doi: 10.1016/S0140-6736(15)00057-4. doi: 10.1016/S0140-6736(15)00057-4. [DOI] [PubMed] [Google Scholar]
- 7.Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, Pyxaras S, Rusinaru D, Di Gioia G, Pellicano M, Barbato E, Van Mieghem C, Heyndrickx GR, De Bruyne B, Wijns W. Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv. 2014;7:751–759. doi: 10.1161/CIRCINTERVENTIONS.114.001608. doi: 10.1161/CIRCINTERVENTIONS.114.001608. [DOI] [PubMed] [Google Scholar]
- 8.Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60:2337–2339. doi: 10.1016/j.jacc.2012.08.990. doi: 10.1016/j.jacc.2012.08.990. [DOI] [PubMed] [Google Scholar]
- 9.Papafaklis MI, Muramatsu T, Ishibashi Y, Lakkas LS, Nakatani S, Bourantas CV, Ligthart J, Onuma Y, Echavarria-Pinto M, Tsirka G, Kotsia A, Nikas DN, Mogabgab O, van Geuns RJ, Naka KK, Fotiadis DI, Brilakis ES, Garcia-Garcia HM, Escaned J, Zijlstra F, Michalis LK, Serruys PW. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire - fractional flow reserve. EuroIntervention. 2014;10:574–583. doi: 10.4244/EIJY14M07_01. doi: 10.4244/EIJY14M07_01. [DOI] [PubMed] [Google Scholar]
- 10.Tu S, Barbato E, Köszegi Z, Yang J, Sun Z, Holm NR, Tar B, Li Y, Rusinaru D, Wijns W, Reiber JH. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv. 2014;7:768–777. doi: 10.1016/j.jcin.2014.03.004. doi: 10.1016/j.jcin.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Morris PD, Ryan D, Morton AC, Lycett R, Lawford PV, Hose DR, Gunn JP. Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions: results from the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) study. JACC Cardiovasc Interv. 2013;6:149–157. doi: 10.1016/j.jcin.2012.08.024. doi: 10.1016/j.jcin.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014;63:1145–1155. doi: 10.1016/j.jacc.2013.11.043. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, Nef H, Tebaldi M, Murasato Y, Lansky A, Barbato E, van der Heijden LC, Reiber JH, Holm NR, Wijns W FAVOR Pilot Trial Study Group. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the International Multicenter FAVOR Pilot Study. JACC Cardiovasc Interv. 2016;9:2024–2035. doi: 10.1016/j.jcin.2016.07.013. [Google Scholar]
- 14.Nissen L, Winther S, Isaksen C, Ejlersen JA, Brix L, Urbonaviciene G, Frost L, Madsen LH, Knudsen LL, Schmidt SE, Holm NR, Maeng M, Nyegaard M, Bøtker HE, Bøttcher M. Danish study of Non-Invasive testing in Coronary Artery Disease (Dan-NICAD): study protocol for a randomised controlled trial. Trials. 2016;17:262. doi: 10.1186/s13063-016-1388-z. doi: 10.1186/s13063-016-1388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petraco R, Sen S, Nijjer S, Echavarria-Pinto M, Escaned J, Francis DP, Davies JE. Fractional flow reserve-guided revascularization: practical implications of a diagnostic gray zone and measurement variability on clinical decisions. JACC Cardiovasc Interv. 2013;6:222–225. doi: 10.1016/j.jcin.2012.10.014. doi: 10.1016/j.jcin.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Chu M, Dai N, Yang J, Westra J, Tu S. A systematic review of imaging anatomy in predicting functional significance of coronary stenoses determined by fractional flow reserve. Int J Cardiovasc Imaging. 2017;33:975–990. doi: 10.1007/s10554-017-1085-3. doi: 10.1007/s10554-017-1085-3. [DOI] [PubMed] [Google Scholar]
- 17.Nørgaard BL, Hjort J, Gaur S, Hansson N, Bøtker HE, Leipsic J, Mathiassen ON, Grove EL, Pedersen K, Christiansen EH, Kaltoft A, Gormsen LC, Mæng M, Terkelsen CJ, Kristensen SD, Krusell LR, Jensen JM. Clinical use of coronary CTA-derived FFR for decision-making in stable CAD. JACC Cardiovasc Imaging. 2017;10:541–550. doi: 10.1016/j.jcmg.2015.11.025. doi: 10.1016/j.jcmg.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Renker M, Schoepf UJ, Wang R, Meinel FG, Rier JD, Bayer RR, II, Möllmann H, Hamm CW, Steinberg DH, Baumann S. Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol. 2014;114:1303–1308. doi: 10.1016/j.amjcard.2014.07.064. doi: 10.1016/j.amjcard.2014.07.064. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH, Doh JH, Koo BK, Min JK, Erglis A, Yang HM, Park KW, Lee HY, Kang HJ, Kim YJ, Lee SY, Kim HS. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv. 2014;7:72–78. doi: 10.1016/j.jcin.2013.05.024. doi: 10.1016/j.jcin.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A, Lin FY, Dunning AM, Budoff MJ, Malpeso J, Leipsic J, Min JK. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging. 2013;6:881–889. doi: 10.1161/CIRCIMAGING.113.000297. doi: 10.1161/CIRCIMAGING.113.000297. [DOI] [PubMed] [Google Scholar]
- 21.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–1997. doi: 10.1016/j.jacc.2011.06.066. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 22.Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, Domínguez-Franco AJ, Dupouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jiménez-Navarro MF, Katritsis DG, Kocaman SA, Koo BK, López-Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodés-Cabau J, Sedlis SP, Takeishi Y, Tonino PA, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne B, Gould KL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–1654. doi: 10.1016/j.jacc.2014.07.973. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- 23.Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Öhagen P, Olsson H, Omerovic E, Calais F, Lindroos P, Maeng M, Tödt T, Venetsanos D, James SK, Kåregren A, Nilsson M, Carlsson J, Hauer D, Jensen J, Karlsson AC, Panayi G, Erlinge D, Fröbert O iFR-SWEDEHEART Investigators. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813–1823. doi: 10.1056/NEJMoa1616540. doi: 10.1056/NEJMoa1616540. [DOI] [PubMed] [Google Scholar]
- 24.Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle E, Krackhardt F, Bojara W, Going O, Härle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Canas Silva P, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria-Barrero E, Meuwissen M, Piek JJ, van Royen N, Sezer M, Di Mario C, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824–1834. doi: 10.1056/NEJMoa1700445. doi: 10.1056/NEJMoa1700445. [DOI] [PubMed] [Google Scholar]
- 25.Petraco R, Park JJ, Sen S, Nijjer SS, Malik IS, Echavarría-Pinto M, Asrress KN, Nam CW, Macías E, Foale RA, Sethi A, Mikhail GW, Kaprielian R, Baker CS, Lefroy D, Bellamy M, Al-Bustami M, Khan MA, Gonzalo N, Hughes AD, Francis DP, Mayet J, Di Mario C, Redwood S, Escaned J, Koo BK, Davies JE. Hybrid iFR-FFR decision-making strategy: implications for enhancing universal adoption of physiology-guided coronary revascularisation. EuroIntervention. 2013;8:1157–1165. doi: 10.4244/EIJV8I10A179. doi: 10.4244/EIJV8I10A179. [DOI] [PubMed] [Google Scholar]
- 26.Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruyne B, Davies JE, Escaned J, Fearon WF, Gould KL, Johnson NP, Kirtane AJ, Koo BK, Marques KM, Nijjer S, Oldroyd KG, Petraco R, Piek JJ, Pijls NH, Redwood S, Siebes M, Spaan JAE, van ‘t Veer M, Mintz GS, Stone GW. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014;63:1253–1261. doi: 10.1016/j.jacc.2013.09.060. doi: 10.1016/j.jacc.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 27.Leone AM, Martin-Reyes R, Baptista SB, Amabile N, Raposo L, Franco Pelaez JA, Trani C, Cialdella P, Basile E, Zimbardo G, Burzotta F, Porto I, Aurigemma C, Rebuzzi AG, Faustino M, Niccoli G, Abreu PF, Slama MS, Spagnoli V, Telleria Arrieta M, Amat Santos IJ, de la Torre Hernandez JM, Lopez Palop R, Crea F. The Multi-center Evaluation of the Accuracy of the Contrast MEdium INduced Pd/Pa RaTiO in Predicting FFR (MEMENTO-FFR) Study. EuroIntervention. 2016;12:708–715. doi: 10.4244/EIJV12I6A115. doi: 10.4244/EIJV12I6A115. [DOI] [PubMed] [Google Scholar]
- 28.Nijjer SS, de Waard GA, Sen S, van de Hoef TP, Petraco R, Echavarría-Pinto M, van Lavieren MA, Meuwissen M, Danad I, Knaapen P, Escaned J, Piek JJ, Davies JE, van Royen N. Coronary pressure and flow relationships in humans: phasic analysis of normal and pathological vessels and the implications for stenosis assessment: a report from the Iberian-Dutch-English (IDEAL) collaborators. Eur Heart J. 2016;37:2069–2080. doi: 10.1093/eurheartj/ehv626. doi: 10.1093/eurheartj/ehv626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–11. doi: 10.1161/CIRCINTERVENTIONS.113.001049. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 30.Petraco R, van de Hoef TP, Nijjer S, Sen S, van Lavieren MA, Foale RA, Meuwissen M, Broyd C, Echavarria-Pinto M, Foin N, Malik IS, Mikhail GW, Hughes AD, Francis DP, Mayet J, Di Mario C, Escaned J, Piek JJ, Davies JE. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7:492–502. doi: 10.1161/CIRCINTERVENTIONS.113.000926. doi: 10.1161/CIRCINTERVENTIONS.113.000926. [DOI] [PubMed] [Google Scholar]
- 31.Johnson NP, Johnson DT, Kirkeeide RL, Berry C, De Bruyne B, Fearon WF, Oldroyd KG, Pijls NHJ, Gould KL. Repeatability of fractional flow reserve despite variations in systemic and coronary hemodynamics. JACC Cardiovasc Interv. 2015;8:1018–1027. doi: 10.1016/j.jcin.2015.01.039. doi: 10.1016/j.jcin.2015.01.039. [DOI] [PubMed] [Google Scholar]


