Abstract
Purpose of review
Functional neuroimaging with PET and SPECT is a commonly used tool in presurgical evaluation. The following article reviews the literature of PET and SPECT in presurgical assessment of epilepsies published in the last year.
Recent findings
FDG-PET adds concomitant information in temporal and extratemporal lobe epilepsy in adults and children. The pattern of hypometabolism in FDG-PET is a good additional predictor or seizure outcome in TLE with mesial temporal sclerosis or negative MRI. There is growing evidence that diagnostic value of FDG-PET increases with postprocessing. Although several methods were applied in the reviewed literature, all of them seem to outperform the visual analysis. Imaging of the epileptic focus with ictal SPECT is depending on short injection latencies. It is particularly useful in patients with nonlesional MRI and mostly of extratemporal localization. Areas of hyperperfusion remote of SOZ are reflecting the epileptic network. Combining more concordant investigations including PET and SPECT in MRI-negative evaluation adds to better presurgical stratification and therefore, better postsurgical outcome. FET-PET shows increased uptake in status epilepticus.
Summary
PET and SPECT are important investigations to localize the epileptic focus in temporal lobe and nonlesional extratemporal epilepsies. Postprocessing for both modalities is important to increase diagnostic value.
Keywords: epilepsy, functional neuroimaging, computer assisted image processing, positron-emission tomography, single photon emission computed tomography
INTRODUCTION
Localizing epileptogenic foci is of interest in presurgical evaluation of drug refractory epilepsy. Functional neuroimaging with nuclear medicine tracers using positron emission tomography (PET) or single photon emission computed tomography (SPECT) are well established techniques and are recommended in presurgical evaluation by the neuroimaging subcommission of the International League Against Epilepsy (ILAE) [1].
PET is primarily used to image the glucose metabolism by using 18F-fluorodeoxyglucose (FDG) tracer. Particularly in temporal lobe epilepsy (TLE), ipsilateral metabolic activity for glucose might be reduced [2,3]. However, the area of temporal hypometabolism might extend beyond the seizure onset zone (SOZ) indicating rather a lateralizing than localizing value [4]. Unilateral hypometabolism in FDG-PET is predictive of good outcome particularly in TLE with nonlesional MRI or equivocal ictal EEG recording [5]. Bilateral hypometabolism may predict less favorable surgical outcome [6]. Localizing the epileptogenic zone in neocortical epilepsies with FDG-PET has also been reported [7,8]. Postprocessing of FDG-PET was shown to increase sensitivity detecting the epileptogenic area [8,9].
Functional imaging of regional cerebral blood flow (rCBF) is shown by SPECT using either 99mTc-hexamethyl-propylene-amine-oxime (HMPAO) or 99mTc-ethylene-cysteine-diethylester (ECD) [10]. Detection of rCBF is seen as secondary marker of neuronal activity. Sensitivity and specificity for the epileptogenic focus is low with interictal SPECT but significantly increased with ictal application and comparison with interictal perfusion patterns [11–13]. Subtraction interictal from ictal SPECT and coregistration to MRI (SISCOM) further increased sensitivity and specificity [14]. Ictal SPECT requires video– Electroencephalography (EEG) monitoring for early injection within a seizure. Hence, it is applied in cases with discordant or negative MRI results mostly extratemporal epilepsies and has shown to be helpful in planning of intracranial electrode placement or identification of SOZ [15–17]. Complex partial seizures and early injection time have been prognostic markers for a highly localizing SPECT [16,18,19]. Different patterns of hyperperfusion have been described in correlation to temporal seizure semiology and propagation as well as injection latency in patients with focal cortical dysplasia (FCD) [20,21].
The aim of this review is to give an overview of the publications on PET and SPECT in focus localization of epilepsy published between November 2016 and November 2017.
Box 1.
no caption available
PET IN TEMPORAL LOBE EPILEPSY WITH MESIAL TEMPORAL SCLEROSIS
Mesial temporal sclerosis is the most common histopathological finding in epilepsy surgery [22]. There are four studies focusing on mTLE with MTS only. Two studies showed that restricted rather than widespread hypometabolism in FDG-PET is correlated with Engel class Ia outcome [23▪▪,24]. The larger study assessed outcome of 97 patients with histologically proven mesial temporal sclerosis (MTS) and long postsurgical follow-up (mean >6 years) in relation to postprocessed FDG-PET and voxel-based morphometry (VBM) of MRI [23▪▪]. Eighty-five percentage of patients were classified with outcome Engel I (45% Engel IA). IA outcome showed ipsilateral anterior mesial temporal hypometabolism only. Non-IA outcome showed different pattern for left and right MTS whereas right MTS non-IA outcome was associated with ipsilateral mesial frontal and perisylvian hypometabolism, correlated left MTS non-IA outcome with contralateral frontoinsular hypometabolism and posterior white matter hypermetabolism. Furthermore, outcome Engel I and II without IA showed similar temporal patterns as IA outcome but with more extratemporal and in left sided MTS contralateral involvement. Failures (outcomes III and IV) showed less temporal hypometabolism sparing the hippocampus and more extratemporal hypometabolism bilaterally. These findings were concordant with electroclinical features but independent of atrophy in VBM. A smaller study of 18 patients with histologically proven MTS and 2-year surgical follow-up showed similar results [24]. Predictors for non-IA outcome were lateralizing rather than localizing EEG, lateralizing rather than localizing hypometabolism in FDG-PET and history of status epilepticus.
The extend of ipsilateral hypometabolism in TLE with MTS is correlated to early onset of disease in hippocampal area and longer disease duration in amygdala area indicating premature insult affecting more hippocampal areas whereas disease duration seems to have a progressive effect on decreased metabolic activity of amygdala [25].
PET IN TEMPORAL LOBE EPILEPSY OTHER THAN MESIAL TEMPORAL SCLEROSIS-RELATED
Localizing hypometabolism of FDG-PET in lesional TLE without MTS was shown in approximately 40% of patients only in a small sub-cohort [26]. High-density electric source imaging (hd-ESI) showed a higher localizing value of 72% in another sub-cohort. As not all patients had all investigations performed, direct comparison of modalities remains limited.
LOCALIZING VALUE OF PET IN MRI-NEGATIVE EPILEPSIES
A large single centre study on 142 MRI-negative neocortical epilepsies compared two clinical protocols within this cohort. The first protocol considered surgical resection with one concordant investigation (interictal EEG, ictal EEG, FDG-PET or ictal SPECT) in line with the seizure semiology [27▪▪]. The second protocol applied from a specific date required concordant results of two or more investigations, that is, interictal/ictal EEG and functional neuroimaging were localizing to the same epileptogenic lobe. Seizure-free outcome improved significantly (47.2 versus 75.5%). Concordance between two or more investigations and localizing PET were predictive for seizure-free outcome in a multivariate analysis. The outcome in the second group is remarkable for MRI-negative neocortical epilepsy surgery. PET was visually reported and postprocessed with SPM. A large cohort of 109 patients with MRI-negative neocortical epilepsy showed correlation to concordant functional neuroimaging with the highest odds ratio of 0.3 for FDG-PET (other results are discussed in SPECT section) [28▪▪]. In a smaller study of 36 patients with MRI-negative epilepsy, hypometabolism in FDG-PET was correlated to favorable outcome in temporal as well as extratemporal lobe epilepsy [29]. Interestingly, favorable (Engel I and II) outcome was achieved in histopathology of FCD type I in 10 pediatric patients of which 5 had no abnormality in PET but were all seizure free. This is an unusual good outcome for this histopathology. Similar results were shown in a study of 26 patients (10 temporal) with nonlesional 3T MRI, in which all underwent invasive presurgical evaluation. Concordance of FDG-PET hypometabolism with ECoG SOZ in TLE correlated with seizure-free outcome (Engel IA) whereas TLE patients without PET lateralization had outcome Engel IIA–IV [30]. No predictive value was found for visually assessed PET localization in extratemporal lobe epilepsy. A comprehensive review of outcome in MRI-negative temporal lobe epilepsy with concordant FGD-PET hypometabolism indicates outcome rates similar to TLE with MTS [31▪▪].
PET IN OTHER PRESURGICAL EVALUATION
Fronto-orbital SOZ is a rare localization. A recent Canadian multicenter study described 16 patients with this entity and reviewed the literature [32]. In their patient cohort, only one patient had localizing PET hypometabolism, another four more extensive areas of decreased metabolic activity (nine in total). This is in line with other investigations, for example, interictal scalp EEG, which often extends over the ipsilateral fronto-temporal area.
Patients who failed to become seizure-free after epilepsy surgery were reevaluated with FDG-PET with co-registration to MRI, MRI and EEG monitoring. Out of 16 patients, all showed concordant results for extension of the previous surgery with 4 showing evidence of tumor recurrence [33]. One patient with acute seizures had 18F-Dopa-PET, MRI and routine EEG concordant to site of previous surgery and was operated without further video-EEG monitoring.
A large pediatric cohort with 166 patients showed highest localizing value for FDG-PET [34]. Interestingly, most patients had only interictal video–EEG monitoring. Concordance of MRI, PET and EEG abnormality showed the best predictive value for favorable postsurgical outcome.
An interesting study compared histopathological classification of FCDs to patterns of MRI+/PET+, MRI-/PET+ and MRI-/PET- but could not show any correlations [35▪]. As in other studies, FDG-PET hypometabolism correlated with younger age of onset.
LOCALIZATION OF 18F-FLUORODEOXYGLUCOSE-PET WITH DIFFERENT METHODS OF REVIEWING/POSTPROCESSING
Several studies focused on the difference of PET image postprocessing or reviewing. Co-registration of PET or SPECT or both to MRI showed reduction in need for invasive monitoring in a cohort of 166 pediatric epilepsy patients and significantly higher rates of seizure-free outcome after 1 and 2 years postsurgery [36▪▪]. Co-registration of PET to gray matter-segmented MRI increased concordance to ictal EEG localization compared with visual nonsegmented PET analysis [37▪]. Semiquantitative analysis of FDG-PET in 39 patients with possible FCD on MRI compared with visual analysis showed comparable pick-up rate in TLE but much higher pick-up rate in frontal lobe epilepsy [38▪]. Whereas visual analysis in FLE failed to detect any PET abnormality in 50% of patients and was only concordant with EEG SOZ in 22%, semiquantitative analysis showed hypometabolism concordant to EEG SOZ in 72% (Fig. 1). Interestingly, four patients with FLE who initially had negative MRI result were re-reviewed positively after semiquantitative PET showed an area with hypometabolism. Semiquantitative analysis was done with an integrated software on the PET scanner, which added only a few minutes to the PET reviewing time.
FIGURE 1.
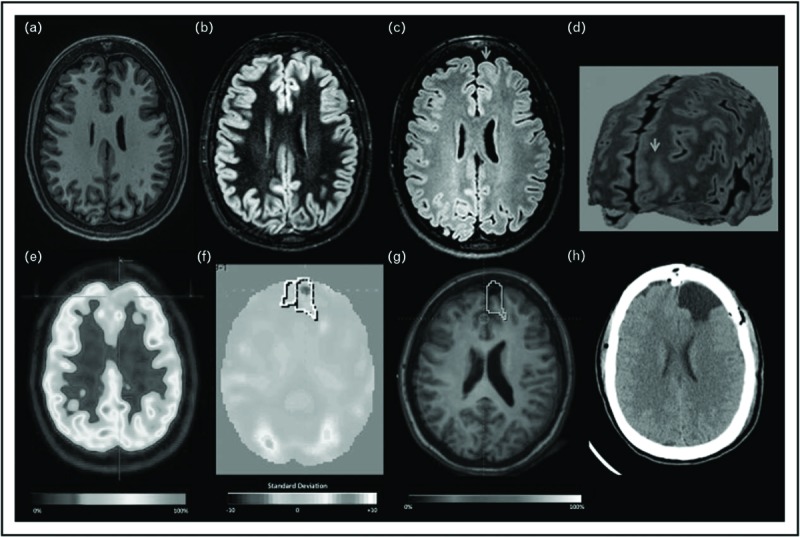
Focal cortical dysplasia II-B left frontal lobe. (a–d) T1, double inversion recovery, FLAIR axial MRI, and curvilinear reconstruction, showing abnormal cortical thickening, focal increased signal, white and gray matter junction blurred (arrow). (e) Visual PET initially was considered normal. (f) Quantitative-PET showed −3.6 SD in the left frontal lobe. (g) Coregistration MRI and PET. (h) Axial CT after surgery resection (for color coding please refer to online version). Reproduced with permission from from Coelho et al.[38▪].
A combined surface analysis of MRI and FDG-PET by a machine-learning approach was developed to detect FCDs in 28 patients with histologically proven FCDs [39▪▪]. The classifier outperformed both, conventional postprocessing and visual analysis (93 versus 82 versus 68%). Different patterns of lesion distribution allowed to differentiate between FCD I–IIa and IIb. Surgical outcome was as expected, better in the FCD IIb group (91% positive outcome) compared with the other FCDs (71%).
COMPARISON OF PET/COMPUTED TOMOGRAPHY AND PET/MAGNETIC RESONANCE
Two studies focused on FDG-PET images acquired on PET/MR. Attenuation correction and results on PET/MR are not inferior to PET/CT in a prospective comparative study in 35 patients with focal epilepsy [40]. Another prospective study reported 54 patients undergoing presurgical assessment with so far 9 patients operated [41]. PET/MR showed concordant abnormality in both modalities in five patients, in one modality in three and no abnormality in one patient.
CAUSE OF 18F-FLUORODEOXYGLUCOSE-PET HYPOMETABOLISM
The reason for the more widespread hypometabolism remains unclear. No correlation of hippocampal metabolic activity was found in comparison with temporal hypometabolism whereas hypometabolism in focal cortical dysplasias showed a correlation to reduced mitochondrial complex IV function [42,43]. A new study showed now in four TLE patients that propagation of interictal epileptic activity recorded by magnetoencephalography (MEG) calculated by a statistical voxel by voxel analysis corresponds to the area of reduced hypometabolism in FDG-PET [44▪▪]. These findings are supported by a study investigating ictal high frequency oscillations (HFOs) and hypometabolism showing a correlation in temporal lobe epilepsy [45]. Interestingly, there was no correlation for extratemporal lobe epilepsies and for lower frequency bands. In contrast is another recent study comparing FDG-PET hypometabolism and hypermetabolism to intracranial ECoG SOZ and interictal discharges [46▪▪]. Hypometabolic areas showed a good correlation to SOZ on the lobar level but were up to 3 cm distant from SOZ in detailed analysis with a sensitivity of up to 0.39 and a specificity of 0.9.
A study on remote areas with reduced metabolic activity in PET in 28 patients with hypothalamic hamartoma correlated with the impairment in cognitive function indicating remote network nodes involved [47]. It is not reported if these areas are also impaired by interictal epileptic activity.
OTHER PET TRACERS AND LOCALIZATION
One very interesting study on 18F-fluoroethyl- L-tyrosine amino acid (FET)-PET in patients with brain tumors and status epilepticus revealed increased FET uptake with status activity (Fig. 2) [48▪▪]. Histopathological studies showed no sign of tumor progression, therefore, increased cerebral amino acid transport was most likely related to status. This is an interesting finding and deserves further studies identifying if amino acid transport activity is increased in SOZ or related to seizure activity as well.
FIGURE 2.
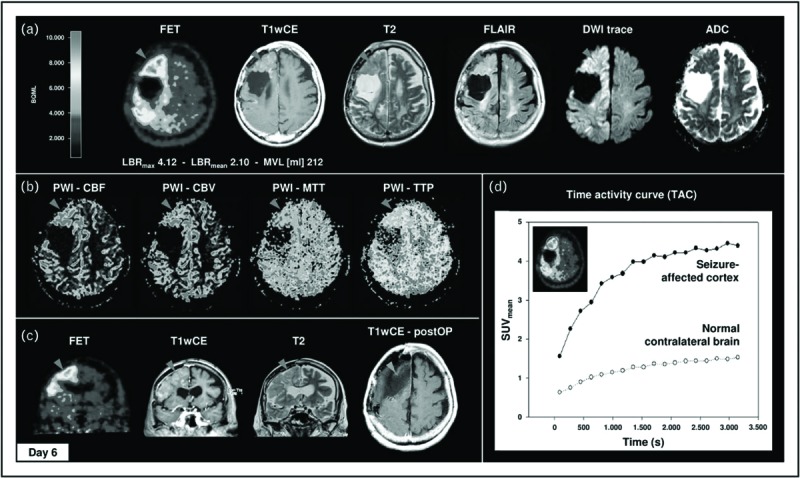
Widespread 18F-FET uptake, vasogenic and cytotoxic edema, contrast enhancement, and hyperperfusion with strict gyral pattern during nonconvulsive SE. Case 3 demonstrates a 66-year-old woman with clinically stable right frontal oligodendroglioma WHO II without residual tumor. In 2014, the patient presented with repeated CPS followed by treatment-resistant nonconvulsive SE. 18F-FET PET revealed distinct elevated cortical 18F-FET uptake of right hemisphere with frontal and parietal accentuation (LBRmax 4.42; LBRmean 2.45), corresponding to cortical contrast enhancement in T1wCE, marked gyral vasogenic (T2/FLAIR, cortical swelling), and cytotoxic (DWI/ADC) edema (a) and cortical hyperperfusion in DSC-PWI (b). (c) Clinical deterioration in combination with MRI and 18F-FET PET imaging was interpreted as tumor recurrence. Therefore, patient underwent subtotal frontal lobe resection without any histologic evidence of tumor progression. (d) Additional 18F-FET kinetic analysis of right frontal lesion and normal contralateral brain demonstrated SUVmean time–activity course curve pattern with continuously increasing 18F-FET uptake without washout. CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time; TTP, time to peak (for color coding please refer to online version). Reproduced with permission from Hutterer et al.[48▪▪].
ICTAL SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY AND INJECTION LATENCY
In a cohort of 95 pediatric epilepsy patients, 60 had localizing SISCOM with 85% indicating focal hyperperfusion concordant to lobe of EEG SOZ [49]. Short injection time (mean 18 s) was seen with concordant results whereas longer injection latency was apparent in discordant (mean 27 s) and nonlocalizing (mean 35 s) SISCOMs being statistically significant differences. No significant differences were seen for MRI findings, epilepsy cause or seizure duration. Concordance of SISCOM, SOZ and resected area was correlated with 6-month postoperative seizure freedom. The authors conclude that injection delay should be shorter then 25 s. A more systematical approach to injection time and SISCOM threshold to avoid detection related to seizure propagation showed a recommendation of injection latency below 35 s [50].
LOCALIZING VALUE OF SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY IN MRI-NEGATIVE EPILEPSY
A large cohort of 109 MRI-negative patients showed Engel IA outcome after 1 year of 54.1% and almost 60% seizure-free at last follow-up [28▪▪]. Localizing patterns of neuroimaging showed odd ratio of 0.37 for ictal SPECT. Concordant results in presurgical assessment, presence of aura and complete resection of SOZ including interictal frequent spikes in ECoG were further positive predictors for favorable surgical outcome. In another study on MRI-negative epilepsy patients, intracranial electrodes were implanted guided by VBM of MRI (11 patients) or ictal SPECT results (3 patients) [51]. Thirteen of 14 patients underwent resective surgery and 57% were seizure-free postsurgically. Less successful were the SPECT results reported in a study investigating high-density scalp EEG in FLE [52]. Although 12/14 patients had localizing hd-EEG results, only 3/12 had localizing SISCOM.
STRONG CONNECTIVITY BETWEEN HYPERPERFUSED AREAS
SISCOM hyperperfused and hypoperfused areas were correlated to corticocortical-evoked responses from cortical electrostimulation in sEEG in 31 patients with epilepsy [53▪]. Hyperperfused areas in SISCOM remote from SOZ showed strong correlation to evoked potentials indicating network connectivity.
NEW METHODS
A new approach of joint ictal/interictal reconstruction method was developed and tested on phantom studies as well as on 35 patients [54]. The new method showed better performance than standard subtraction method. Another study investigated arterial spin labelling (ASL) MRI to identify postictal hypoperfusion as marker of SOZ [55]. Twenty patients were investigated but only two underwent surgical resection. Interestingly, comparison to FDG-PET and SISCOM showed higher rate of concordance of postictal hypoperfusion in ASL MRI to SOZ in EEG.
REVIEWS
Several reviews on PET and SPECT in presurgical evaluation were published during this period [31▪▪,56▪▪–59▪▪,60▪].
CONCLUSION
Functional Imaging with PET and SPECT adds concomitant imaging information on localizing the epileptic focus. A recent review on surgical treatment of TLE suggested to use FDG-PET only in MRI-negative patients [61]. The literature of the past year, strongly supports the use of FDG-PET in TLE, even in patients with MTS. These finding support an earlier publication on added value of PET from an economical view [62]. There is growing evidence that diagnostic value of FDG-PET is increasing with postprocessing. Several methods were applied in the reviewed literature and all of them seem to outperform the visual analysis. In SPECT, machine-learning approaches may further increase the specificity of results. Hyperperfused areas in SISCOM remote from SOZ indicate nodes of the epileptic network.
SPECT literature further supports the indication in nonlesional MRI and mostly of extra-temporal localization and short injection latencies. Combining more concordant investigations in MRI-negative evaluation adds to better presurgical stratification and therefore, postsurgical outcome. Re-reviewing of negative MRI with PET and SPECT results may help to find subtle lesions, keeping in mind that negative MRI is highly dependent on imaging technique and reviewers experience [63]. Only sparse recommendations exist for use of PET and SPECT in presurgical evaluation and application in clinical practice shows broad variation [1,64]. Future research should focus on a structured approach gathering evidence for functional imaging in presurgical evaluation and a standardized computer-assisted processing. FET-PET, indicating that increased amino acid transport is caused by status, warrants further investigation if it is as another biomarker for the SOZ.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Neuroimaging Subcommision of the International League Against Epilepsy Commission on diagnostic strategies: recommendations for functional neuroimaging of persons with epilepsy. Epilepsia 2000; 41:1350–1356. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, Kuhl DE, Phelps ME, Mazziotta JC. Interictal cerebral glucose metabolism in partial epilepsy and its relation to EEG changes. Ann Neurol 1982; 12:510–517. [DOI] [PubMed] [Google Scholar]
- 3.Stefan H, Pawlik G, Böcher-Schwarz HG, et al. Functional and morphological abnormalities in temporal lobe epilepsy: a comparison of interictal and ictal EEG, CT, MRI, SPECT and PET. J Neurol 1987; 234:377–384. [DOI] [PubMed] [Google Scholar]
- 4.Vinton AB, Carne R, Hicks RJ, et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain 2007; 130 (Pt 2):548–560. [DOI] [PubMed] [Google Scholar]
- 5.Willmann O, Wennberg R, May T, et al. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy: a meta-analysis. Seizure 2007; 16:509–520. [DOI] [PubMed] [Google Scholar]
- 6.Koutroumanidis M, Hennessy MJ, Seed PT, et al. Significance of interictal bilateral temporal hypometabolism in temporal lobe epilepsy. Neurology 2000; 54:1811–1821. [DOI] [PubMed] [Google Scholar]
- 7.Carne RP, O’Brien TJ, Kilpatrick CJ, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain 2004; 127 (Pt 10):2276–2285. [DOI] [PubMed] [Google Scholar]
- 8.Kim YK, Lee DS, Lee SK, et al. 18F-FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med 2002; 43:1167–1174. [PubMed] [Google Scholar]
- 9.Mayoral M, Marti-Fuster B, Carreño M, et al. Seizure-onset zone localization by statistical parametric mapping in visually normal 18F-FDG PET studies. Epilepsia 2016; 57:1236–1244. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien TJ, Brinkmann BH, Mullan BP, et al. Comparative study of 99mTc-ECD and 99mTc-HMPAO for peri-ictal SPECT: qualitative and quantitative analysis. J Neurol Neurosurg Psychiatry 1999; 66:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biersack HJ, Reichmann K, Winkler C, et al. 99mTc-labelled hexamethylpropyleneamine oxime photon emission scans in epilepsy. Lancet 1985; 2:1436–1437. [DOI] [PubMed] [Google Scholar]
- 12.Biersack HJ, Linke D, Brassel F, et al. Technetium-99m HM PAO brain SPECT in epileptic patients before and during unilateral hemispheric anesthesia (Wada test): report of three cases. J Nucl Med 1987; 28:1763–1767. [PubMed] [Google Scholar]
- 13.Grünwald F, Menzel C, Pavics L, et al. Ictal and interictal brain SPECT imaging in epilepsy using technetium-99m-ECD. J Nucl Med 1994; 35:1896–1901. [PubMed] [Google Scholar]
- 14.O’Brien TJ, So EL, Mullan BP, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology 1998; 50:445–454. [DOI] [PubMed] [Google Scholar]
- 15.Ahnlide J-A, Rosén I, Lindén-Mickelsson Tech P, Källén K. Does SISCOM contribute to favorable seizure outcome after epilepsy surgery? Epilepsia 2007; 48:579–588. [DOI] [PubMed] [Google Scholar]
- 16.von Oertzen TJ, Mormann F, Urbach H, et al. Prospective use of subtraction ictal SPECT coregistered to MRI (SISCOM) in presurgical evaluation of epilepsy. Epilepsia 2011; 52:2239–2248. [DOI] [PubMed] [Google Scholar]
- 17.Kudr M, Krsek P, Marusic P, et al. SISCOM and FDG-PET in patients with nonlesional extratemporal epilepsy: correlation with intracranial EEG, histology, and seizure outcome. Epileptic Disord 2013; 15:3–13. [DOI] [PubMed] [Google Scholar]
- 18.Van Paesschen W, Dupont P, Van Driel G, et al. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain 2003; 126 (Pt 5):1103–1111. [DOI] [PubMed] [Google Scholar]
- 19.Lee SK, Lee S-Y, Yun C-H, et al. Ictal SPECT in neocortical epilepsies: clinical usefulness and factors affecting the pattern of hyperperfusion. Neuroradiology 2006; 48:678–684. [DOI] [PubMed] [Google Scholar]
- 20.Shin WC, Hong SB, Tae WS, Kim SE. Ictal hyperperfusion patterns according to the progression of temporal lobe seizures. Neurology 2002; 58:373–380. [DOI] [PubMed] [Google Scholar]
- 21.Dupont P, Van Paesschen W, Palmini A, et al. Ictal perfusion patterns associated with single MRI-visible focal dysplastic lesions: implications for the noninvasive delineation of the epileptogenic zone. Epilepsia 2006; 47:1550–1557. [DOI] [PubMed] [Google Scholar]
- 22.Blumcke I, Spreafico R, Haaker G, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med 2017; 377:1648–1656. [DOI] [PubMed] [Google Scholar]
- 23▪▪.Chassoux F, Artiges E, Semah F, et al. 18F-FDG-PET patterns of surgical success and failure in mesial temporal lobe epilepsy. Neurology 2017; 88:1045–1053. [DOI] [PubMed] [Google Scholar]; This study on FDG-PET and voxel-based morphometry in 97 patients with histologically proven MTS and long postsurgical follow-up shows clearly different patterns in distribution of hypometabolism according to outcome as well as for left and right TLE with prediction of Engel IA outcome.
- 24.Farooque P, Hirsch L, Levy S, et al. Surgical outcome in adolescents with mesial temporal sclerosis: Is it different? Epilepsy Behav 2017; 69 (Suppl C):24–27. Study on 18 adolescent patients with TLE and mesial temporal sclerosis with postsurgical follow-up of at least 2 years. Risk factors for not achieving postoperative seizure control were lateralized rather than localized ictal EEG onset, lateralized rather than localized hypometabolism in FDG-PET and history of status epilepticus. [DOI] [PubMed] [Google Scholar]
- 25.Leiva-Salinas C, Quigg M, Elias WJ, et al. Earlier seizure onset and longer epilepsy duration correlate with the degree of temporal hypometabolism in patients with mesial temporal lobe sclerosis. Epilepsy Res 2017; 138 (Suppl C):105–109. Study of 18 patients with mTLE and successful epilepsy surgery with histopathologically confirmed hippocampal sclerosis assessing FGD-PET and MRI volumetry. Earlier epilepsy onset and longer disease duration (independent of age at onset) correlated with decreased metabolic activity of ipsilateral sclerotic hippocampus and amygdala, respectively. No such correlation was found to volumetric changes. Methodologically well designed study. [DOI] [PubMed] [Google Scholar]
- 26.Feng R, Hu J, Wu J, et al. Comprehensive preoperative work-up and surgical treatment of low grade tumor/benign lesion related temporal lobe epilepsy. J Clin Neurosci 2017; 39 (Suppl C):203–208. Presurgical workup and postoperative follow-up of 60 patients with lesional TLE except hippocampal sclerosis. Thirty-five patients had FDG-PET, which showed reduced metabolic activity in 39.4%. Dense array EEG (22 patients) was showing higher localizing value 72.7%. No difference in outcome between restricted and extended lesionectomy. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Moon H-J, Kim DW, Chung C-K, et al. Change of patient selection strategy and improved surgical outcome in MRI-negative neocortical epilepsy. J Epilepsy Res 2016; 6:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large single centre study on MRI-negative patients undergoing epilepsy surgery with two different protocols. Changing to two or more concordant diagnostic modalities (interict/ictal EEG, FDG-PET, SPECT) instead of one increased excellent outcome (Engel I) by 75%. Localizing PET was an independent significant variable for excellent outcome. PET was analyzed with postprocessing (SPM).
- 28▪▪.Kim DW, Lee SK, Moon H-J, et al. Surgical treatment of nonlesional neocortical epilepsy: long-term longitudinal study. JAMA Neurol 2017; 74:324–331. [DOI] [PubMed] [Google Scholar]; Description of 10-year cohort of 109 MRI-negative patients with postsurgical follow-up; 54.1% were seizure-free after 1 year. Favourable surgical outcome parameters were localizing patterns in FDG-PET and ictal SPECT amongst others.
- 29.Isler C, Ozkara C, Kucukyuruk B, et al. Seizure outcome of patients with magnetic resonance imaging–negative epilepsies: still an ongoing debate. World Neurosurg 2017; 106 (Suppl C):638–644. Study on 36 patients (33 with postsurgical follow-up) showed good correlation of FDG-PET hypometabolism and outcome in temporal as well as extratemporal epilepsy (most patients FLE). Interestingly, histopathology of FCD I showed the most favorable outcome. [DOI] [PubMed] [Google Scholar]
- 30.Kogias E, Klingler J-H, Urbach H, et al. 3 Tesla MRI-negative focal epilepsies: presurgical evaluation, postoperative outcome and predictive factors. Clin Neurol Neurosurg 2017; 163:116–120. Study of 26 patients (10 temporal), which were nonlesional on 3T MRI and had intracranial EEG recordings. FDG-PET concordant with ECoG seizure onset in TLE showed most favorable results (Engel I and II). [DOI] [PubMed] [Google Scholar]
- 31▪▪.Muhlhofer W, Tan Y-L, Mueller SG, Knowlton R. MRI-negative temporal lobe epilepsy—what do we know? Epilepsia 2017; 58:727–742. Comprehensive review of nonlesional temporal lobe epilepsy and epilepsy surgery. [DOI] [PubMed] [Google Scholar]; The review covers advanced diagnostic techniques such as electric source imaging (ESI), MRI postprocessing, PET and SPECT. Particular emphasis is on concordant EEG and FDG-PET results but nonlesional MRI, where surgical outcome is comparable to mTLE patients with hippocampal sclerosis.
- 32.Chibane IS, Boucher O, Dubeau F, et al. Orbitofrontal epilepsy: case series and review of literature. Epilepsy Behav 2017; 76 (Suppl C):32–38. This study reports 16 patients with orbitofrontal epilepsy. Half were nonlesional on MRI, 4/9 showed hypometabolism extending beyond the fronto-orbital area and ictal SPECT showed orbitofrontal hyperperfusion in 1/5 patients. Fourteen proceeded to surgery. General outcome was favourable (71% Engel I). [DOI] [PubMed] [Google Scholar]
- 33.Reed CM, Dewar S, Fried I, et al. Failed epilepsy surgery deserves a second chance. Clin Neurol Neurosurg 2017; 163 (Suppl C):110–115. Report of 17 patients who failed to become seizure-free after surgery. Re-evaluation with FDG-PET, MRI and EEG monitoring in all but one patients showed concordant results for extension of the previous surgery (four tumor recurrence). One patient had concordant repeat MRI, EEG and 18F-Dopa-PET, hence, no further EEG monitoring and 10/17 patients had good outcome (Engel I and II). [DOI] [PubMed] [Google Scholar]
- 34.Wang G-B, Long W, Li X-D, et al. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) combined with positron emission tomography-computed tomography (PET-CT) and video-electroencephalography (VEEG) have excellent diagnostic value in preoperative localization of epileptic foci in children with epilepsy. Med Sci Monit 2017; 23:1–10. Comparison of MRI, VEEG and FDG-PET in 166 pediatric patients undergoing epilepsy surgery and 1-year postoperative follow-up. FDG-PET had the highest localizing value but most VEEGs were interictal only. The diagnostic value was highest with all three methods combined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Halac G, Delil S, Zafer D, et al. Compatibility of MRI and FDG-PET findings with histopathological results in patients with focal cortical dysplasia. Seizure 2017; 45 (Suppl C):80–86. [DOI] [PubMed] [Google Scholar]; Comparison of FDG-PET results in histopathologically proven focal cortical dysplasia in 71 patients (28 FCD I, 43 FCD II; 53 MRI lesional, 21 temporal). Age of onset was younger in patients with PET indicating hypometabolism. Categorized groups of MRI+/PET+, MRI-/PET+ and MRI-/PET- did not correspond to histopathological diagnoses.
- 36▪▪.Perry MS, Bailey L, Freedman D, et al. Coregistration of multimodal imaging is associated with favourable two-year seizure outcome after paediatric epilepsy surgery. Epileptic Disord 2017; 19:40–48. [DOI] [PubMed] [Google Scholar]; In a cohort of 115 pediatric epilepsy patients, outcome of epilepsy surgery was correlated to co-registration of MRI with SPECT, FDG-PET or both. the co-registered group (59%) was less likely to undergo invasive monitoring and had significantly higher rates of seizure freedom at 1 and 2 years postoperatively.
- 37▪.Elkins KC, Moncayo VM, Kim H, Olson LD. Utility of gray-matter segmentation of ictal-Interictal perfusion SPECT and interictal 18F-FDG-PET in medically refractory epilepsy. Epilepsy Res 2017; 130 (Suppl C):93–100. [DOI] [PubMed] [Google Scholar]; This study assessed visual FDG-PET and ictal/interictal SPECT analysis of cortical structures only by gray matter segmentation of MRI and co-registration. Segmented PET showed significantly higher concordance to ictal EEG compared with conventional nonsegmented visual PET analysis, segmented or nonsegmented ictal SPECT analysis.
- 38▪.Coelho M, Cristina V, Morita ME, et al. Automated online quantification method for 18F-FDG positron emission tomography/CT improves detection of the epileptogenic zone in patients with pharmacoresistant epilepsy. Front Neurol 2017; 8:453. [DOI] [PMC free article] [PubMed] [Google Scholar]; In 39 patients with possible FCD FDG-PET was assessed visually and with semiquantitative analysis. No difference in pick-up rate of hypometabolism was seen in TLE but a much higher pick-up rate with semiquantitative analysis is reported in FLE.
- 39▪▪.Tan Y-L, Kim H, Lee S, et al. Quantitative surface analysis of combined MRI and PET enhances detection of focal cortical dysplasias. NeuroImage 2017; 166:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; New approach to analyze MRI and PET by a classifier (machine learning) to detect FCD. Classifier outperformed postprocessing and visual analysis. Different patterns of lesion distribution may allow to differentiate types of FCDs (I–IIa versus IIb).
- 40.Paldino MJ, Yang E, Jones JY, et al. Comparison of the diagnostic accuracy of PET/MRI to PET/CT-acquired FDG brain exams for seizure focus detection: a prospective study. Pediatr Radiol 2017; 47:1500–1507. Prospective comparative study of FDG-PET on PET/CT and PET/MR in 35 patients with focal epilepsy. MR attenuation correction and results of PET/MR were not inferior to PET/CT. [DOI] [PubMed] [Google Scholar]
- 41.Johnson D, Witte R, Britton J, et al. PET/MR and preoperative localization of seizure foci in patients with medication-refractory epilepsy. J Nucl Med 2017; 58 (Suppl 1):633.Abstract reporting 54 epilepsy patients with FDG-PET/MR of which 9 underwent epilepsy surgery so far. Results for PET and MRI are described as well as postsurgical outcome (78% seizure free). [Google Scholar]
- 42.Vielhaber S, Von Oertzen JH, Kudin AF, et al. Correlation of hippocampal glucose oxidation capacity and interictal FDG-PET in temporal lobe epilepsy. Epilepsia 2003; 44:193–199. [DOI] [PubMed] [Google Scholar]
- 43.Tenney JR, Rozhkov L, Horn P, et al. Cerebral glucose hypometabolism is associated with mitochondrial dysfunction in patients with intractable epilepsy and cortical dysplasia. Epilepsia 2014; 55:1415–1422. [DOI] [PubMed] [Google Scholar]
- 44▪▪.Shibata S, Matsuhashi M, Kunieda T, et al. Magnetoencephalography with temporal spread imaging to visualize propagation of epileptic activity. Clin Neurophysiol 2017; 128:734–743. [DOI] [PubMed] [Google Scholar]; Propagation of interictal epileptic activity recorded by magnetoencephalography (MEG) is calculated on a voxel by voxel statistical approach and compared with areas of hypometabolism in FDG-PET. It is shown in four patients that area of spread corresponds to the area of reduced metabolism.
- 45.Lamarche F, Job A, Deman P, et al. Correlation of FDG-PET hypometabolism and SEEG epileptogenicity mapping in patients with drug-resistant focal epilepsy. Epilepsia 2016; 57:2045–2055. A study investigating correlation of ictal HFOs with hypometabolism in FDG-PET, postprocessed with SPM. Results showed correlation only in temporal lobe epilepsy and not for lower frequency oscillations in ECoG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪▪.Jeong J-W, Asano E, Kumar Pilli V, et al. Objective 3D surface evaluation of intracranial electrophysiologic correlates of cerebral glucose metabolic abnormalities in children with focal epilepsy. Hum Brain Mapp 2017; 38:3098–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Thirty-seven paediatric epilepsy surgery patients (31 nonlesional) had FDG-PET and intracranial EEG prior to surgery. PET was quantified by SPM including a pseudonormal control group. Intracranial interictal and ictal discharge localization was compared with areas of abnormal metabolic activity. Hypometabolic areas are a good indicator of the affected lobe but have limited localizing value.
- 47.Wagner K, Schulze-Bonhage A, Urbach H, et al. Reduced glucose metabolism in neocortical network nodes remote from hypothalamic hamartomas reflects cognitive impairment. Epilepsia 2017; 58 (Suppl 2):41–49. FDG-PET study of 29 patients with hypothalamic hamartoma and correlation to cognitive function. Patients with impaired cognitive function showed remote areas of reduced glucose metabolism. [DOI] [PubMed] [Google Scholar]
- 48▪▪.Hutterer M, Ebner Y, Riemenschneider MJ, et al. Epileptic activity increases cerebral amino acid transport assessed by 18F-fluoroethyl-l-tyrosine amino acid PET: a potential brain tumor mimic. J Nucl Med 2017; 58:129–137. [DOI] [PubMed] [Google Scholar]
- 49.Stamoulis C, Verma N, Kaulas H, et al. The promise of subtraction ictal SPECT co-registered to MRI for improved seizure localization in pediatric epilepsies: affecting factors and relationship to the surgical outcome. Epilepsy Res 2017; 129 (Suppl C):59–66. Study of ictal SPECT in 95 paediatric epilepsy surgery patients with 60 SISCOM indicating focal hyperperfusion. Short injection latency was correlated with positive SISCOM result. Concordance of SISCOM with scalp EEG and resected area correlated with seizure freedom 1-year postsurgery. No correlation of SISCOM and MRI lesion was found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramchuankiat S, Jarumaneeroj P, Limotai C, et al. Impact of injection time on migration of SPECT seizure onset in temporal lobe epilepsy. Conf Proc IEEE Eng Med Biol Soc 2017; 2017:1465–1468. Study on injection time of ictal SPECT in TLE. With injection latency of more than 35 s at a threshold of +2SD seizure propagation was found in the images. [DOI] [PubMed] [Google Scholar]
- 51.Delev D, Quesada CM, Grote A, et al. A multimodal concept for invasive diagnostics and surgery based on neuronavigated voxel-based morphometric MRI postprocessing data in previously nonlesional epilepsy. J Neurosurg 2017; 16:1–9. Cohort of 14 MRI-negative patients, who were ROI-guided, implanted with intracranial electrodes. Eleven ROIs were obtained from MRI postprocessing, the remaining three from ictal SPECT. In 13 patients, 57% were seizure-free postsurgery. [DOI] [PubMed] [Google Scholar]
- 52.Feyissa AM, Britton JW, Van Gompel J, et al. High density scalp EEG in frontal lobe epilepsy. Epilepsy Res 2017; 129 (Suppl C):157–161. Validation of SOZ detection with high-density EEG, also compared with ictal SPECT. Only 3/12 patients had localizing SISCOM whereas 12/14 had localizing high-density EEG. [DOI] [PubMed] [Google Scholar]
- 53▪.Tousseyn S, Krishnan B, Wang ZI, et al. Connectivity in ictal single photon emission computed tomography perfusion: a cortico-cortical evoked potential study. Brain 2017; 140:1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study on 36 patients with ictal SPECT and intracranial electrodes with cortical electrostimulation triggering remote discharges (evoked potentials). It is nicely shown that corticocortical-evoked potentials are significantly increased mostly in the hyperperfusion areas of ictal SPECT. Findings indicate that SPECT hyperperfusion areas are part of an electrical epileptic network.
- 54.Rakvongthai Y, Fahey F, Borvorntanajanya K, et al. Joint reconstruction of Ictal/inter-ictal SPECT data for improved epileptic foci localization. Med Phys 2017; 44:1437–1444. Description of a new method of SPECT postprocessing. Simultaneous joint reconstruction of ictal/interictal SPECT scans was slightly superior to conventional coregistration and subtraction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaxiola-Valdez I, Singh S, Perera T, et al. Seizure onset zone localization using postictal hypoperfusion detected by arterial spin labelling MRI. Brain 2017; 140:2895–2911. Study on arterial spin labelling postictal MRI in 20 patients undergoing presurgical evaluation for drug refractory focal epilepsy (only to operated). Comparison with FDG-PET and SISCOM showed higher diagnostic yield of ASL for detection of seizure onset zone in EEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪▪.Theodore WH. Presurgical focus localization in epilepsy: PET and SPECT. Semin Nucl Med 2017; 47:44–53. [DOI] [PubMed] [Google Scholar]; Comprehensive review of PET and SPECT studies in presurgical evaluation of epilepsy including research applications.
- 57▪▪.Mountz JM, Patterson CM, Tamber MS. Pediatric epilepsy: neurology, functional imaging, neurosurgery. Semin Nucl Med 2017; 47:170–187. [DOI] [PubMed] [Google Scholar]; Comprehensive review on pediatric epilepsy surgery including functional imaging. Review of PET and SPECT diagnostics and results. Particular section dedicated on ictal SPECT application and image processing.
- 58▪▪.Kumar A, Chugani HT. The role of radionuclide imaging in epilepsy, Part 1: sporadic temporal and extratemporal lobe epilepsy. J Nucl Med Technol 2017; 45:14–21. [DOI] [PubMed] [Google Scholar]; Dedicated review/CME article on PET and SPECT in temporal and extratemporal lobe epilepsy. Main focus is on clinical application and detection of the epileptogenic zone. A variety of tracers used mainly in research and its findings is discussed as well. Very informative.
- 59▪▪.Kumar A, Chugani HT. The role of radionuclide imaging in epilepsy, part 2: epilepsy syndromes. J Nucl Med Technol 2017; 45:22–29. [DOI] [PubMed] [Google Scholar]; Dedicated review/CME article on PET and SPECT in epilepsy syndromes such as epileptic spams, tuberous sclerosis, Lennox–Gastaut syndrome and more, focusing on scientific and diagnostic findings including epilepsy surgery assessment.
- 60▪.Verger A, Lagarde S, Maillard L, et al. Brain molecular imaging in pharmacoresistant focal epilepsy: Current practice and perspectives. Rev Neurol (Paris) 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; Comprehensive review of SPECT and PET in presurgical assessment of epilepsy. Clinical application, value of postprocessing and research on additional tracer is highlighted.
- 61.Jones AL, Cascino GD. Evidence on use of neuroimaging for surgical treatment of temporal lobe epilepsy: a systematic review. JAMA Neurol 2016; 73:464–470. [DOI] [PubMed] [Google Scholar]
- 62.O’Brien TJ, Miles K, Ware R, et al. The cost-effective use of 18F-FDG PET in the presurgical evaluation of medically refractory focal epilepsy. J Nucl Med 2008; 49:931–937. [DOI] [PubMed] [Google Scholar]
- 63.Von Oertzen J, Urbach H, Jungbluth S, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry 2002; 73:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mouthaan BE, Rados M, Barsi P, et al. Current use of imaging and electromagnetic source localization procedures in epilepsy surgery centers across Europe. Epilepsia 2016; 57:770–776. [DOI] [PubMed] [Google Scholar]


