Abstract
Objective
The aim of the study was to assess for the presence of vulvar lichen planus (LP) in association with human papillomavirus (HPV)–independent squamous cell carcinoma (SCC).
Materials and Methods
We performed a clinicohistopathologic review of consecutive vulvectomies and wide local excisions for HPV-independent vulvar or vaginal SCC from 2007 to 2017. Data collected included site of SCC, adjacent precursor lesions and dermatoses, dermatologic treatment, and outcome.
Results
There were 43 cases of primary HPV-independent vulvar SCC treated by excision, but no vaginal cancers. Eighteen women (42%) had a preoperative diagnosis of lichen sclerosus (LS); none had a diagnosis of LP. Topical corticosteroids were prescribed in 19 (44%) of 43, with 4 women placed on maintenance therapy. Tumors arose from the labia minora, labia majora, and periclitoris, but not from vestibule or perianus. On histopathological review, LS was present in 41 (95%) of 43 specimens, 1 had a nonspecific lichenoid reaction, and 1 had lichen simplex; both of the latter had subsequent biopsies showing LS. Lichen planus was not seen in association with SCC. Differentiated vulvar intraepithelial neoplasia (dVIN) was present in 38 (88%) of 43 specimens, whereas 1 had acanthosis with altered differentiation and 4 (9%) had no precursor lesion. Differentiated vulvar intraepithelial neoplasia had standard, basaloid, and hypertrophic morphology, superficially resembling erosive LP in 9 (24%) of 38 and hypertrophic LP in 6 (16%) of 38.
Conclusions
Lichen planus was not seen in association with HPV-independent vulvar SCC, whereas LS was underrecognized and inadequately treated in this group. Pathologists should be aware that dVIN may superficially resemble erosive or hypertrophic LP.
Key Words: lichen planus, lichen sclerosus, differentiated vulvar intraepithelial neoplasia, HPV-independent, vulvar squamous cell carcinoma
There are 2 types of vulvar squamous cell carcinoma (SCC): approximately 30% of cases are human papillomavirus (HPV)–dependent and the other 70% are usually associated with lichen sclerosus (LS).1 Multiple studies suggest that vulvar SCC occurs in 5% of women with LS, and a recent prospective cohort study suggested that effective treatment mitigates this risk.2 Vulvovaginal lichen planus (LP) has also been described in association with vulvar neoplasia in several case reports and series, and SCC is occasionally noted in women with erosive LP during long-term follow-up.3–6 Limitations of this literature include lack of histopathologic confirmation of the LP diagnosis, inadequate follow-up, and failure to identify if neoplasia could be related to HPV or other carcinogens.3,4
The largest study to date reported 38 cases of LP-associated SCC and differentiated vulvar intraepithelial neoplasia (dVIN), occurring at a single European center.6 However, this series did not detail the histopathologic diagnostic criteria applied for the diagnosis of LP nor how LP was distinguished from LS and did not account for the possibility of comorbid LP and LS.7 A recent publication describes the difficulty in distinguishing the basaloid variant of dVIN from regenerative erosive LP, raising the question of neoplastic precursor lesions being mistaken for LP.8
The aims of this study are to assess consecutive excisions of primary HPV-independent vulvar and vaginal cancers for peritumoral LP, LS, and precursor lesions and to place these findings in the context of clinical diagnosis and management of SCC-associated vulvar dermatoses.
MATERIALS AND METHODS
The Pathology New South Wales Hunter New England database was searched for vulvectomies and excisions of vulvar or vaginal cancer submitted between 2007 and 2017 inclusive. Cases of recurrent cancer were excluded. Human papillomavirus–independent status was confirmed by routine histopathology and, when in doubt, through nonblock staining of p16 on adjacent VIN. Any case with previous or concurrent high-grade squamous intraepithelial lesion and/or positive p16 was excluded. The Hunter New England Research Ethics and Governance Unit approved this retrospective histopathologic case series (HREC 15/11/18/5.02), and signed written consent was obtained for use of clinical photographs.
Histopathologic review was performed of slides stained with hematoxylin and eosin (H&E). The tumor morphology was assessed as keratinizing or verrucous SCC. Verrucous SCC is a well-differentiated nonmetastasizing neoplasia with minimal suprabasilar nuclear atypia; spread occurs through an expansile blunt interface.9 The location of the tumor was determined through assessment of deep anatomic structures, site-specific stromal appendages, and the following perilesional epithelial types: squamous mucosa, mucocutaneous junction, hairless skin, and hair bearing skin. The peritumoral epithelium was then inspected for precursor lesions and adjacent dermatologic diseases.
Lichen sclerosus (LS) required basal layer degeneration seen as vacuolar change, apoptotic bodies, and/or squamatization, in combination with sclerosis or thick fibrosis of the papillary dermis and a closely applied band-like lymphocytic infiltrate.10 Squamatization is defined as a change in morphology of normal basilar keratinocytes to horizontally disposed cells with a mature squamous appearance.11 Classic LP required hyperkeratosis, hypergranulosis, sawtooth or spiky acanthosis, evidence of basal layer damage, a band-like lymphocytic infiltrate, and absence of dermal homogenization.12 Hypertrophic LP required the changes of classic LP with superimposed lichenification seen as irregular elongated rete ridges and papillary dermal fibrosis.10,12 Erosive LP required epithelial thinning, often with erosion, a closely applied lymphocytic infiltrate, and either the degenerative or regenerative epithelial patterns.7,8 The regenerative pattern showed altered maturation, increased nucleus-to-cytoplasm ratio, and mitoses. Cases of a lichenoid pattern that lacked diagnostic features of LS or LP were labeled nonspecific lichenoid reaction.
Features of dVIN included a thickened hyperkeratotic or parakeratotic stratum corneum, acanthosis with elongated and branching rete ridges, premature maturation, enlarged squamous cells with large vesicular nuclei above the basal layer, and basal layer atypia characterized by the following 4 findings: increased mitoses, nuclear enlargement, pleomorphism, and hyperchromasia.13 We also looked for the basaloid variant of dVIN in which the epidermis is replaced by a homogenous population of abnormal keratinocytes.8,14 Basaloid dVIN was distinguished from regenerative erosive LP by the presence of marked nuclear pleomorphism, supported by an aberrant positive or negative p53; high-grade squamous intraepithelial lesion was excluded by a negative p16.8 Vulvar acanthosis with altered differentiation (VAAD) is a descriptive term for an unusual epithelial appearance with marked verruciform hyperplasia, plaque-like parakeratosis, hypogranulosis, a layer of pale-staining squamous cells, premature maturation, and absence of basal atypia.9 Vulvar acanthosis with altered differentiation was first described as a possible precursor lesion for verrucous SCC; alternatively, it may be associated with keratinizing SCC or may be a reactive phenomenon that resolves with treatment of the underlying dermatologic condition.
Clinical data collected included age, rurality, body mass index, diabetes mellitus status, tumor location and number, surgical stage, and outcome. Referrals and provider notes were reviewed for documentation of a clinical diagnosis of LS or LP occurring before cancer surgery. Additional data collected included previous and subsequent vulvar biopsies, treatment prescribed, and presence of a dermatologic diagnosis written on the pathology request form and in the histopathology report. Descriptive statistics were performed; clinical and histopathologic characteristics were compared with Fisher exact test.
RESULTS
There were 43 excisions for primary HPV-independent vulvar SCC; no vaginal cases were encountered. The mean age was 76 years, with 4 women 50 years or younger (Table 1). The diagnosis of LS was recorded in clinical notes before cancer surgery in 18 (42%) of 43, and there were no preoperative diagnoses of LP. There were no significant differences between women with and without a diagnosis of LS with regard to age, body mass index, diabetes status, rurality, notation of suspected or confirmed LS on the histopathology request form, surgical stage, and cancer outcome. A preoperative diagnosis was associated with postoperative documentation of LS (13/18 [72%] vs 7/25 [28%], p = .006) but no significant difference in treatment. Topical corticosteroids prescribed were high potency in 11 (58%) of 19, medium in 6 (32%), and unspecified in 2 (11%). One woman was referred postoperatively to a vulvar specialist for LS management.
TABLE 1.
Clinical Characteristics of Women With HPV-Independent Vulvar Cancer, Stratified by Preoperative Diagnosis of LS
Tumor locations included labia minora, labia majora, and periclitoral; cancers did not originate at the vestibule or perianus. On review of excision specimens, LS was present in 41 (95%) of 43, 1 had a nonspecific lichenoid reaction, and 1 had lichen simplex chronicus; both of the latter had subsequent biopsies showing LS (Table 2). There were no significant differences in tumor characteristics, precursor lesions, or histopathologic appearance of LS, when stratified by preoperative LS diagnosis. One woman who underwent excision of a right labial SCC with adjacent LS and dVIN had a concurrent punch biopsy of a noncontiguous perianal lesion that showed classic LP.
TABLE 2.
Histopathologic Characteristics of HPV-Independent Vulvar Cancer, Stratified by Preoperative Diagnosis of LS
There were 3 morphologies of dVIN identified: standard, basaloid, and hypertrophic, and more than 1 type could be seen in the same specimen (see Figures 1–3). Of 38 cases with dVIN, 14 (37%) had a negative p16 to substantiate the HPV-independent diagnosis; this included all specimens with basaloid dVIN and 5 with standard morphology. Subepithelial sclerosis, the cardinal histopathologic feature of LS, accompanied dVIN in 72% of cases. Verrucous carcinoma and hypertrophic dVIN appeared as a spectrum of disease, rather than 2 distinct entities. Vulvar acanthosis with altered differentiation was more often seen in combination with dVIN than in isolation and was observed in cases both with and without verrucous SCC (see Figure 4). In addition to VAAD as described by Nascimento et al.,9 2 cases demonstrated other morphologies of “acanthosis with altered differentiation” seen as marked hyperkeratosis and hypergranulosis disproportionate to the acanthosis or as parakeratosis without the underlying pale band of squamous cells (see Figure 5). Those cases showed mild basal nuclear enlargement and occasional mitoses but lacked nuclear pleomorphism and hyperchromasia, so they did not fulfill criteria for basal atypia as seen in dVIN.
FIGURE 1.

A, A 50-year-old with a 20-year history of LS and 2 opposing tumors of keratinizing SCC and dVIN in a field of LS. B, Standard dVIN morphology is accompanied by dermal sclerosis (H&E ×200).
FIGURE 3.
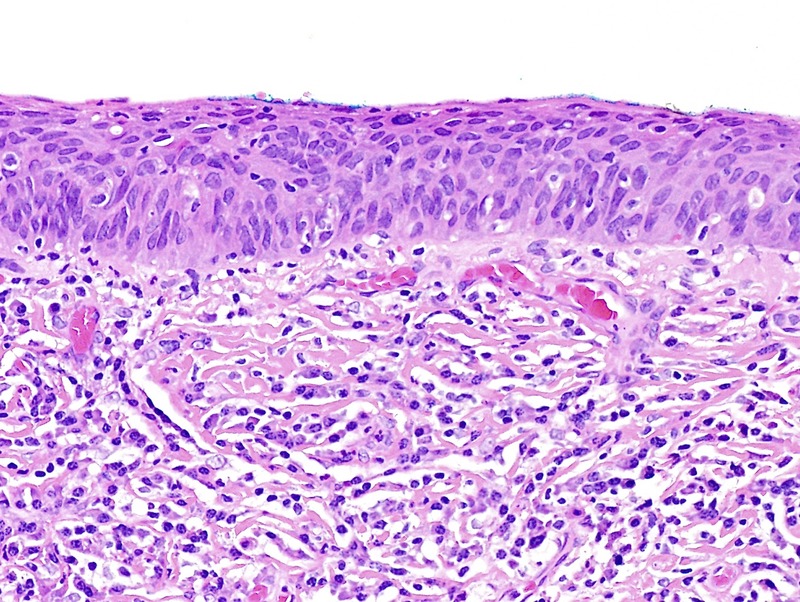
Basaloid dVIN seen as thinned epithelium, full-thickness atypia, stromal fibrosis, and a dense lymphoplasmacytic infiltrate; p16 was negative (H&E ×200).
FIGURE 4.
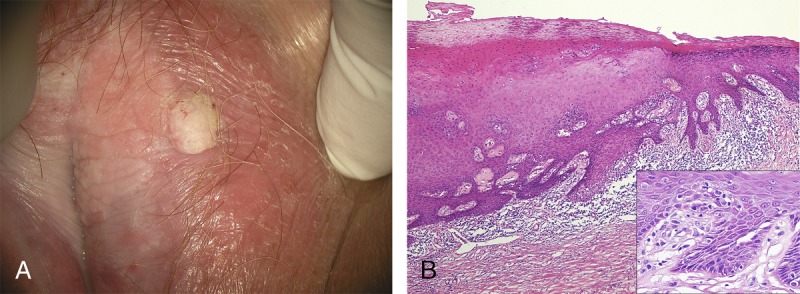
A, A 74-year-old with verrucous SCC, dVIN, and VAAD on background of pallor, lichenification, and architectural change characteristic of LS. B, VAAD adjacent to dVIN is demonstrated by thickened epithelium, parakeratosis, and an underlying pale band, with a sharp transition to the spiky rete ridges and basilar atypia (inset) of standard dVIN. Throughout there is a dense lymphocytic infiltrate and stromal sclerosis (H&E ×40, inset H&E ×400).
FIGURE 5.

An area of acanthotic morphology without basal layer atypia showed a variable appearance of the stratum corneum and granular cell layer (H&E ×100).
FIGURE 2.
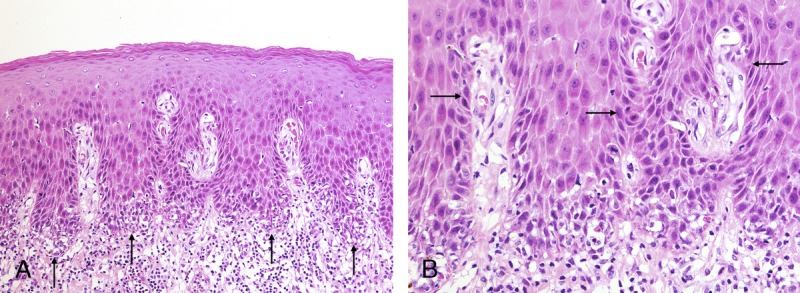
A, This example of hypertrophic dVIN displays basal layer degeneration at the tips of rete ridges (arrows) and dense lymphocytic infiltrate (H&E ×100). B, Basilar atypia away from the tips of rete ridges (arrows) differentiates dVIN from hypertrophic LP (H&E ×200).
The diagnosis of LS was written in 33 (77%) of pathology reports, but 14 (42%) of these cases never had a clinical diagnosis recorded. During postoperative follow-up, 8 additional women received diagnoses of LS by punch biopsy, 6 (75%) of whom were treated. Mean follow-up was 3 years (range = 1–8 years). Among 11 (26%) women who died of disease, the mean interval from diagnosis was 3.3 years. Eight (42%) of 19 women prescribed topical corticosteroids had no evidence of recurrent cancer during follow-up, compared with 7 (29%) of 24 of untreated women; this was not statistically significant.
DISCUSSION
For an 11-year period, 95% of excisions for HPV-independent vulvar SCC showed histopathologic evidence of LS. The other 5% had subsequent biopsies showing LS. There was no evidence of hypertrophic, classic, or erosive LP in the SCC specimens. These results suggest that vulvar SCC associated with LP is rare.
There are several reasons to doubt an association between vulvovaginal LP and SCC. Erosive LP is usually seen on nonkeratinized epithelium of vestibule and vagina, but primary HPV-independent vaginal cancer has not been reported and vestibular tumors are uncommon, although establishing an origin site medial to the mucocutaneous junction is difficult if there is dermatosis-associated architectural change or the cancer is locally advanced.5 The proposed mechanism for LS-associated neoplasia is the scar-cancer hypothesis, in which the combination of altered epithelial-stromal interface and chronic epithelial damage and repair leads to accumulation of carcinogenic mutations.15,16 Classically, scar cancers arise from burns and chronic ulcers; in the case of LS, the scar is the band of abnormal stromal collagen and the damaged epithelium results from T-cell–mediated attack on basilar keratinocytes.15 Lichen planus lacks significant scarring, and chronic inflammation alone is a weak potential driver for carcinogenesis. Finally, controversy continues regarding the malignant potential of oral LP, with that literature likewise afflicted by problems with clinical and histopathologic diagnoses, documentation of comorbidities, and inadequate follow-up. The mouth is a carcinogen-rich environment in which multiple pathways result in a common end point of atypical epithelium; toxic exposures may cause antigenic alterations in mucosal basal cells, triggering a secondary lichenoid reaction.4,17 In an effort to reduce the possibility of misattribution to LP, consensus criteria were constructed on diagnosis of oral lichenoid lesions.17 No similar criteria exist for vulvovaginal disease.
Pathologic assessment of peritumoral dermatoses and precursor lesions is challenging and presents many opportunities for misdiagnosis. Previous studies have documented that dVIN may be misdiagnosed as LS and that p53 overexpression can occur both in benign and neoplastic conditions.9,13,18,19 This study highlights several other pitfalls in assessment of these specimens. The basaloid variant of dVIN occurred in 21% of cancers and sometimes was the only precursor lesion present; clues to distinguish it from regenerative erosive LP include parakeratosis, minimal epithelial thickening, abnormal mitotic figures, and more nuclear hyperchromasia. The hypertrophic variant of dVIN was seen in 14% and is a mimic for hypertrophic LP; the presence of atypical nuclei away from the inflamed dermoepidermal junction is the major distinguishing characteristic. Another feature that may cause diagnostic confusion is pseudoepitheliomatous hyperplasia, a benign squamoproliferative condition that occurs within LP, LS, and nodular prurigo, and histopathologically resembles SCC.20 Mistaking pseudoepitheliomatous hyperplasia for SCC leads to unnecessary surgical intervention and attribution of the purported cancer to the adjacent dermatosis.21 Finally, cases of LS with edematous, fibrotic, or localized collagen change may be misinterpreted as LP or a nonspecific lichenoid reaction.7,22
Distinguishing between LP and LS also presents difficulties to clinicians. The adage that LS is a disease of keratinized skin is incorrect as LS can extend into the vestibule and vagina and rarely may present as an isolated lesion in squamous mucosa.11 Hypertrophic LP and lichenified LS both demonstrate a combination of erythema and pallor, often accompanied by architectural change, erosions, excoriations, and superinfection. The diagnosis of comorbid LP and LS requires a high index of suspicion and is confirmed with 2 well-placed biopsies; thus, this phenomena is likely underreported.7 However, this study suggests that LS may be overlooked even when the clinical appearance is typical and peritumoral LS is noted on the pathology report. This is especially problematic because adjacent LS is associated with a three-fold risk of local recurrence of vulvar SCC and a five-fold risk of second field tumors. In contrast, margin status is not related to HPV-independent SCC recurrence risk.23
Despite recent evidence to suggest that a tailored long-term regimen of topical corticosteroids reduces the risk of primary vulvar cancer, only 4 women in this high-risk group were ever prescribed maintenance therapy.2 Heterogeneity in international clinical guidelines likely contributes to a laissez-faire approach to long-term surveillance and treatment of LS. European guidelines state that “maintenance treatment with either topical steroids or calcineurin inhibitors is recommended as it seems to prevent severe relapses” and that frequency of steroid application is determined by symptoms.24 British guidelines restrict maintenance treatment to women with persistent symptoms or appearance of active disease, with follow-up annually by a general practitioner for stable disease and specialist opinion for “atypical or poorly controlled” disease or previous neoplasia.25 North American guidelines state that the evidence for maintenance therapy is conflicting and do not discuss referral to vulvar specialists.26 Australian guidelines have recently been updated to advise chronic regular use of a topical corticosteroid potent enough to maintain remission and that management should be in consultation with an expert.27 None of these documents specifies the role of gynecologic oncologists in management of vulvar dermatoses; moreover, there is no mention of LS treatment in national oncologic guidelines for management of vulvar cancer.28,29 Most gynecologic oncologists have limited training and experience in long-term care of dermatoses, so involvement of a vulvar specialist in the follow-up of women with dVIN and HPV-independent SCC could offer advantages both in quality of life and reduced recurrence rates, with research urgently needed to assess this hypothesis.30
This study adds to the literature on the relationship between keratinizing SCC, verrucous SCC, and VAAD. The acronym “VAAD” was devised as a descriptive term for an unusual acanthotic lesion that lacks the basal atypia of dVIN and is remarkable for plaque-like parakeratosis with conspicuous underlying pallor; it was originally described as a precursor to verrucous SCC but has since been identified with keratinizing SCC.9,31 We observed variants of “acanthosis with altered differentiation” in cancer excision specimens that do not fulfill all criteria of the original VAAD description and agree that the nomenclature and definition should be revised to accommodate the various manifestations of this maturational abnormality.31 The potential for reversibility of these lesions with aggressive treatment of the underlying dermatosis remains unclear. We found no clear histopathologic distinction between hypertrophic dVIN and verrucous SCC, and 4 (9%) of 43 cases showed coexistent verrucous and keratinizing SCC. Previous histopathologic studies of both vulvar and oral verrucous SCC specimens demonstrate foci of atypia or invasion consistent with conventional SCC in 20% to 35%; this is a high rate of comorbidity if these were synchronous-unrelated primaries.32–34 From a clinical perspective, women with verrucous SCC have a substantial risk of concomitant or subsequent keratinizing SCC and should be managed similarly to women with dVIN.
Inherent to the retrospective design, limitations of this study include incomplete data, practice differences between clinicians, and the potential for selection bias. This study did not capture advanced vulvar or vaginal cancers confirmed by an external biopsy and referred directly for chemoradiation. A particular shortcoming of clinical notes was that details about LS symptoms and signs were lacking, as was documentation of the frequency of steroid use when prescribed “as needed.” Women from outer regional areas often returned to their local doctor for ongoing care after several postoperative visits with gynecologic oncology; notes from these consultations were unavailable, which may have underestimated the number of women with diagnosis and treatment of LS during prolonged postsurgical follow-up. Immunohistochemistry for p16 was not universally obtained in cases of standard and hypertrophic dVIN; the potential for misdiagnosis was mitigated by the clinical context and expert pathology review. Universal clinical photography would have permitted a more nuanced description of tumor origin and location and severity of the adjacent dermatosis.
In summary, LP was not encountered in excision specimens for vulvar SCC occurring for more than a decade. Lichen sclerosus was histopathologically demonstrated in association with all HPV-independent SCC cases, either adjacent to the tumor or at a subsequent vulvar biopsy. Thus, several criteria should be satisfied before attributing vulvar cancer to LP: LS should be thoroughly considered and excluded, there should be clinicopathologic concordance in the diagnosis of LP, precursor lesions should be distinguished from LP, and the lesion should be a keratinizing or verrucous SCC arising in a field of LP.

Footnotes
The authors have declared they have no conflicts of interest.
T.D. is supported by the Australian Government Research Training Program Scholarship.
This study was approved by Hunter New England Research Ethics and Governance unit (HREC 15/11/18/5.02).
REFERENCES
- 1.Chiesa-Vottero A, Dvoretsky PM, Hart WR. Histopathologic study of thin vulvar squamous cell carcinomas and associated cutaneous lesions: a correlative study of 48 tumors in 44 patients with an analysis of adjacent VIN types and lichen sclerosus. Am J Surg Path 2006;30:310–8. [DOI] [PubMed] [Google Scholar]
- 2.Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol 2015;151:1061–7. [DOI] [PubMed] [Google Scholar]
- 3.Simpson RC, Murphy R. Is vulval erosive lichen planus a premalignant condition? Arch Dermatol 2012;148:1314–6. [DOI] [PubMed] [Google Scholar]
- 4.Ramos-e-Silva M, Jacques CD, Carneiro SC. Premalignant nature of oral and vulva lichen planus: facts and controversies. Clin Dermatol 2010;28:563–7. [DOI] [PubMed] [Google Scholar]
- 5.Derrick EK, Ridley CM, Kobza-Black A, et al. A clinical study of 23 cases of female anogenital carcinoma. Br J Dermatol 2000;143:1217–23. [DOI] [PubMed] [Google Scholar]
- 6.Regauer S, Reich O, Eberz B. Vulvar cancer in women with vulvar lichen planus: a clinicopathological study. J Am Acad Dermatol 2014;71:698–707. [DOI] [PubMed] [Google Scholar]
- 7.Day T, Moore S, Bohl TJ, et al. Cormorbid lichen planus and lichen sclerosus. J Low Genit Tract Dis 2017;21:204–8. [DOI] [PubMed] [Google Scholar]
- 8.Day T, Bowden N, Jaaback K, et al. Distinguishing erosive lichen planus from differentiated vulvar intraepithelial neoplasia. J Low Genit Tract Dis 2016;20:174–9. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento AF, Granter SR, Cviko A, et al. Vulvar acanthosis with altered differentiation: a precursor to verrucous carcinoma? Am J Surg Pathol 2004;28:638–43. [DOI] [PubMed] [Google Scholar]
- 10.Patterson JW, Hosler GA. Tissue reaction patterns, Section 2, and The dermis, Section 4. In: Patterson JW, ed. Weedon's Skin Pathology. 4th ed vol. 37–80 London: Churchill Livingstone Elsevier; 2016:36–54, 237–244. [Google Scholar]
- 11.Day T, Burston K, Dennerstein G, et al. Vestibulovaginal sclerosis versus lichen sclerosus. Int J Gynecol Pathol. 2017. Doi: 10.1097/PGP.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Lazar A. Lichenoid and interface dermatitis, Chapter 7. In: Calonje E, Brenn T, Lazar A, et al., eds. McKee's Pathology of the Skin With Clinical Correlations. 4th ed London: Elsevier; 2012:219–58. [Google Scholar]
- 13.Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol 2000;24:429–41. [DOI] [PubMed] [Google Scholar]
- 14.Ordi J, Alejo M, Fusté V, et al. HPV-negative vulvar intraepithelial neoplasia (VIN) with basaloid histologic pattern: an unrecognized variant of simplex (differentiated) VIN. Am J Surg Pathol 2009;33:1659–65. [DOI] [PubMed] [Google Scholar]
- 15.Scurry J, Whitehead J, Healey M. Histology of lichen sclerosus varies according to site and proximity to carcinoma. Am J Dermatopathol 2001;23:413–8. [DOI] [PubMed] [Google Scholar]
- 16.Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol 1998;29:932–48. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg E. Oral lichen planus: a benign lesion. J Oral Maxillofac Surg 2000;58:1278–85. [DOI] [PubMed] [Google Scholar]
- 18.Van de Nieuwenhof HP, Bulten J, Hollema H, et al. Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol 2011;24:297–305. [DOI] [PubMed] [Google Scholar]
- 19.Pinto AP, Miron A, Yassin Y, et al. Differentiated vulvar intraepithelial neoplasia contains Tp53 mutations and is genetically linked to vulvar squamous cell carincoma. Mod Pathol 2010;23:404–12. [DOI] [PubMed] [Google Scholar]
- 20.Lee ES, Allen D, Scurry J. Pseudoepitheliomatous hyperplasia in lichen sclerosus of the vulva. Int J Gynecol Pathol 2003;22:57–62. [DOI] [PubMed] [Google Scholar]
- 21.Ashton KA, Scurry J, Rutherford J, et al. Nodular prurigo of the vulva. Pathology 2010;44:565–7. [DOI] [PubMed] [Google Scholar]
- 22.Weyers W. Hypertrophic lichen sclerosus sine sclerosis: clues to histopathologic disgnosis when presenting as psoriasiform lichenoid dermatitis. J Cutan Pathol 2015;42:118–29. [DOI] [PubMed] [Google Scholar]
- 23.Yap JK, Fox R, Leonard S, et al. Adjacent lichen sclerosus predicts local recurrence and second field tumous in women with vulvar squamous cell carincoma. Gynecol Oncol 2016;142:420–6. [DOI] [PubMed] [Google Scholar]
- 24.Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol 2015;29:e1–43. [DOI] [PubMed] [Google Scholar]
- 25.Neill SM, Lewis FM, Tatnall FM, et al. British Association of Dermatologists' guideline for the management of lichen sclerosus. Br J Dermatol 2010;163:672–82. [DOI] [PubMed] [Google Scholar]
- 26.Boardman L, Kennedy C. Diagnosis and management of vulvar skin conditions. Obstet Gynecol 2008;111:1243–53. [DOI] [PubMed] [Google Scholar]
- 27.Anogenital Skin Conditions: lichen sclerosus. Victoria, Australia: Therapeutic Guidelines Ltd 2017. Available at: http://www.tg.com.au. Accessed December 1, 2017.
- 28.Koh WJ, Greer BE, Abu-Rustum NR, et al. Vulvar cancer, version 1.2017, clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:92–120. [DOI] [PubMed] [Google Scholar]
- 29.British Gynaecological Cancer Society and Royal College of Obstetricians and Gynaecologists. Guidelines for the diagnosis and management of vulvar carcinoma, May 2014. Available at: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/vulval-carcinoma-guidelines-for-the-diagnosis-and-management-of/. Accessed December 1, 2017.
- 30.Jones RW, Scurry J, Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol 2008;198:e1–3. [DOI] [PubMed] [Google Scholar]
- 31.Watkins JC, Howitt BE, Horowitz NS, et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod Pathol 2017;30:448–58. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Li Q, Shang X, et al. Verrucous carcinoma of the vulva: a 20 year retrospective study and literature review. J Low Genit Tract Dis 2016;20:114–8. [DOI] [PubMed] [Google Scholar]
- 33.Haidopoulos D, Diakomanolis E, Rodolakis A, et al. Coexistence of verrucous and squamous carcinoma of the vulva. Aust N Z J Obstet Gynaecol 2005;45:60–3. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu A, Tamura A, Ishikawa O. Invasive squamous cell carcinoma arising from verrucous carcinoma: recognition of verrucous carcinoma of skin as an in-situ carcinoma. Eur J Dermatol 2006;16:439–42. [PubMed] [Google Scholar]


