Abstract
The significance of semaphorin3A (sema3A) in regulating immune-mediated inflammation is widely reported. There are multiple mechanisms involved in the process of sema3A-mediated regulation. One of them is the ability of sema3A to maintain a sufficient regulation of both T-cell and B-cell activation. Because it is involved in the pathogenesis of many autoimmune, infectious, and malignant diseases, sema3A turns to be a promising therapeutic tool to be studied and applied in these diseases.
Keywords: Semaphorin 3A, immune mediated diseases, atopic diseases
Introduction
Semaphorins are a large family of secreted and membrane-bound proteins, characterized by a homologous cysteine-rich domain of approximately 500 amino acids in an N-terminal extracellular domain, called the semaphorin domain. Classes 2 and 3 are secreted proteins, whereas classes 1, 4, 5, and 6 are membrane bound (1, 2). In addition to being involved in tumor angiogenesis, metastasis, and tumor survival, semaphorins are continuously reported to be important regulators of immune-mediated responses, thus also called immune semaphorins. They are expressed on most immune cells, such as T, B, and macrophages. Semaphorins were shown to be involved in all phases of both normal and pathological immune responses (3–5). In the last decade, semaphorin3A (sema3A) was recognized as one of the most active semaphorins in the modulation of inflammatory conditions, therefore receiving special attention. The expression of sema3A and its receptors, neuropilin-1 (NP-1), neuropilin-2 (NP-2), and plexins, were found to be increased on differentiating macrophages and activated T cells, suggesting that they may play the key role in immune-mediated diseases (5, 6).
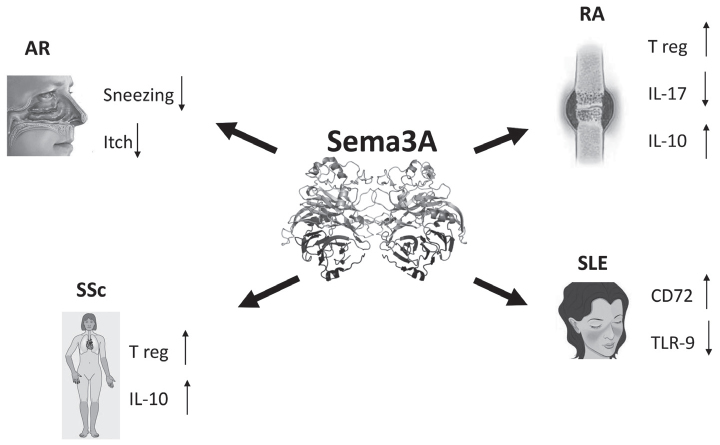
When sema3A was added to co-culture of dendritic and T cells, it significantly inhibited allogeneic T-cell proliferation. In addition, by acting directly on T cells, sema3A blocked anti-CD3/CD28-induced T-cell proliferation. In contrast, when endogenous sema3A was neutralized by blocking antibodies, one could see the reverse in T-cell proliferation, suggesting that the inhibition of T-cell proliferation is sema3A dependent. Similarly, sema3A was also found to be involved in the regulation of human thymocyte migration and proven to inhibit triggered chemotaxis by CXCL12 (7, 8). Of the many immune-regulatory functions of sema3A, one should mention its involvement in the maintenance of self-tolerance. Semaphorin3A triggers a pro-apoptotic process by sensitizing leukemic T cells to Fas (CD95)-mediated apoptosis. It provokes Fas translocation into lipid raft micro domains before binding with agonistic antibody or FasL (CD95L). Disruption of lipid rafts reduces sensitivity to Fas-mediated apoptosis in the presence of sema3A. The present study emphasizes the role of sema3A in controlling Fas-mediated apoptosis and the prevention of immune-mediated inflammation (9). Because of its above-noted characteristics, sema3A can be used in the treatment of immune-mediated diseases. Below, we discuss sema3A involvement in specific immune-mediated diseases (Figure 1).
Figure 1.
Semaphorin 3A is a unique regulatory molecule playing a role in the management of rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (Ssc), and allergic rhinitis (AR).
Semaphorin3A and rheumatoid arthritis (RA)
The levels of sema3A (a repellent of sympathetic nerve fibers) were found to be increased in the synovial tissue of patients with RA and in association with synovial damage (10). Taking into consideration the regulatory function of sema3A, researchers looked to sema3A as a good candidate in treating RA. In a study by M. Catalano, the effect of injected sema3A was examined in a mouse model of collagen-induced arthritis (CIA). The administration of plasmid DNA encoding sema3A significantly reduced the incidence, disease severity, and articular damage when compared with mice injected with empty plasmid. On the one hand, sema3A reduced anti-collagen IgG levels and decreased the release of relevant pro-inflammatory cytokines such as IL-17 and IFN-gamma. On the other, it increased the serum level of the anti-inflammatory cytokine IL-10 (11). In line with these findings, sema3A expression was found to be decreased on CD4+ T cells of RA patients, whereas NP-1 expression on these cells was increased. CD4+NP-1+T cells, known as T regulatory cells and a source of IL-10, were reported to be decreased in RA. In this case, the addition of sema3A into a co-culture with T cells acts directly on CD4+NP-1+T cells and increases IL-10 production, supporting its therapeutic beneficial effect in RA (11). In a recent study, the expression of sema3A in synovial tissues of RA patients was investigated. Disease activity and the histological features of synovial tissues were also assessed in relation to sema3A expression. Human synovial tissues were obtained from established RA patients, compared those of patients suffering from osteoarthritis (OA), and assessed for sema3A, VEGF-A, and NP-1mRNA expression, using quantitative real-time polymerase chain reaction (qPCR). The protein expression of sema3A in synovial lining cells was decreased in RA tissues compared with that in OA samples. Sema3A mRNA levels correlated with the inflammation score, namely with the extent of lymphocyte infiltration (R=0.50, p=0.004). Thus, the correlation of sema3A expression in RA synovial tissues may become a therapeutic target in RA (12).
Semaphorin3A and systemic lupus erythematosus (SLE)
Hypothesizing that sema3A may have a similar role in SLE, we assessed its serum levels in SLE patients. Sema3A serum levels in SLE patients were found to be significantly lower than in RA patients (55.04±16.30 ng/mL vs. 65.54±14.82 ng/mL, p=0.018) and lower yet than in normal individuals (55.04±16.30 ng/mL vs. 74.41±17.60 ng/mL, p<0.0001). Serum sema3A levels in SLE patients were low in negative correlation with SLE disease activity, renal damage, and the presence of relevant autoantibodies (R=−0.89, p<0.0001) (13). Being the source of autoantibodies and pro-inflammatory cytokines, the regulatory status of B cells was evaluated. The expression of sema3A on B regulatory (Breg) cells, namely CD19+CD25high and NP-1+ was analyzed. As expected sema3A expression on these cells was significantly lower in SLE patients when compared to B cells from normal individuals, suggesting this to be in part responsible for B-cell auto-reactivity in SLE. Our other finding was the down-regulation of NP-1 expression on B cells from SLE patients, suggesting that both sema3A and NP-1 are essential in the process of regulation autoimmunity in SLE (13). Auto-reactive B cells in SLE are characterized by the over-expression of TLR-9 and increased production of IL-6 and IFN-gamma. This increased expression was found to be in positive association with SLE disease severity and with anti-dsDNA antibody production. Considering sema3A to be an important regulator in autoimmune diseases, we analyzed the possibility of lowering TLR-9 expression on B cells by co-culturing them with sema3A. We found that the addition of sema3A to activated B cells of SLE patients decreased the expression of TLR-9, strengthening the idea of using sema3A as a therapeutic agent for SLE (13). The expression of CD72 (a regulatory molecule) on B cells is critical in the process of regulating/suppressing B-cell over-activity. The ligation of CD72 on B cells was found to down-regulate B-cell receptor-related signaling, thereby maintaining self-tolerance (14). With this in mind, we conducted a study, seeking to evaluate the expression of CD72 on activated B cells from individuals with SLE and healthy individuals. We founded that CD72 expression was significantly lower in patients with SLE when compared to that in healthy controls. This lower expression of CD72 in patients with SLE correlated inversely with SLE disease activity and was associated with lupus nephritis, the presence of anti-dsDNA antibodies, and with low levels of complement. Our important finding was that co-culturing of purified B cells with recombinant sema3A resulted in significant up-regulation of CD72 on B cells from normal controls and patients with SLE, although lower than in normal controls. The improvement of CD72 expression following the addition of soluble sema3A may lead to its established usage as a beneficial therapy in many autoimmune diseases (14).
Semaphorin3A and other rheumatic diseases
The possible involvement of Treg and Breg cells in the pathogenesis of familial Mediterranean fever (FMF) was previously suggested. Being a marker of both Treg and Breg cells, soluble and membrane-bound sema3A was assessed in 17 patients with FMF during attacks and in remission. Eight patients with smoldering disease and 12 healthy controls were included. The serum level of sema3A was recorded as much lower in FMF patients during attacks or remission compared to serum levels in healthy controls (242±9.8 ng/mL vs. 232±22.7 ng/mL vs. 323±160 ng/mL respectively, p<0.05). In addition, the expression of sema3A on Treg (57.2%±8.3 during attack vs. 77.2%±10.3 in remission vs. 88.7%±3.6% normal controls, p<0.01, respectively) and Breg cells (69.5%±9 during attack vs. 83.4%±5.8 in remission vs. 82.6%±6.4% normal controls, p<0.05, respectively) was significantly lower in patients with FMF during attacks, but was normalized in remission. It is therefore possible that healthy Treg and Breg cells, namely the proper expression of sema3A are required to end FMF attacks (15). In another recent study, the involvement of sema3A in systemic sclerosis (SSc) was also reported. Here, 27 patients with SSc, 42 patients with SLE, and 28 healthy controls were studied. Serum levels of sema3A and the expression of sema3A on Treg cells were assessed in all studied individuals. Serum levels of sema3A were found to be significantly lower in patients with SSc compared to healthy individuals (14.38±5.7 vs. 27.14±8.4 ng/mL, p<0.0001), but similar to that in SLE (15.7±4.3 ng/mL). The expression of sema3A on Treg cells was also significantly lower in patients with SSc when compared to normal controls (61.7±15.7% vs. 88.7±3.7% (p<0.0001). The lower serum level of sema3A was inversely correlated with disease duration (r=−0.4, p=0.036) and correlated with low C4 levels (r=0.66, p=0.026). Thus, the finding of reduced sema3A expression on Treg cells in patients with SSc may reflect their impaired regulatory function, thereby contributing to our understanding of the immune pathogenesis of this disease. This finding suggests that increasing the sema3A level in the serum and/or on Treg cells may serve a future strategy for follow-up and treatment (16, 17).
Semaphorin3A and inflammatory bowel disease (IBD)
The possible involvement of semaphorins in peripheral immune responses and bowel tissue inflammation of patients suffering from Crohn’s disease (CD) and ulcerative colitis (UC) was also evaluated. Serum levels of sema3A and sema4A, their expression on Treg cells, and their tissue expression in bowel biopsies were assessed in CD, UC, acute diverticulitis (a disease control), and in healthy individuals as normal controls. The percentage (%) of sema3A expressing Treg cells with active CD (64.5%±14.49%) and active UC (49.8%±16.45%) was significantly lower when compared to that of healthy controls (88.7%±3.6%) p<0.001 and 0.0001, respectively. This finding was observed to be in negative correlation with CD activity. Serum levels of sema4A were significantly lower in patients with CD and UC compared to that of both control groups. In addition, sema4A was highly expressed in lymphocytes of lamina propria of CD and UC patients, but was absent in patients in acute diverticulitis or in normal individuals. An altered percentage of sema3A expressing Treg cells in patients with inflammatory bowel disease (IBD) is suggested to be partially responsible for their failure in suppressing T-cell-induced inflammation in IBD, and therefore sema3A could become a therapeutic candidate for improving immune-mediated bowel inflammation. Sema4A expression was found to be increased in bowel biopsies of CD and UC, but not in normal bowel tissues. Accordingly, it was suggested that it acts as a regulatory factor in preventing local tissue inflammation in IBD (18).
Semaphorin3A in atopic diseases
The involvement of regulatory cells such as Treg cells in allergic rhinitis (AR) and asthma is well recorded, making sema3A a regulatory molecule and a good candidate to be assessed in atopic diseases (19). Sema3A, previously shown to restrict innervation of sensory neurons, was presumed to play a role in the pathogenesis of AR. Namely it was hypothesized that an alteration in the expression of sema3A in the nasal mucosa might contribute to the hyper-innervation of nasal neurons that we see in nasal epithelium. Indeed, the expression of sema3A in the nasal epithelium of ovalbumin-sensitized AR in a mouse model was found to be significantly decreased. This was observed to be in association with sneezing and nasal rubbing in this murine model, along with an increased nerve fiber density in the lamina propria of the turbinate. Furthermore, when recombinant sema3A was administered intra-nasally to these mice, sneezing and nasal rubbing symptoms were alleviated. This was an additional study showing evidence that sema3A may provide a novel therapy for AR and other atopic disorders such as conjunctivitis and bronchial asthma (21, 22). Semaphorins are well reported to be regulators of airway smooth muscle (ASM) hyperplasia. With this in mind, the sema3A receptor NP-1 was shown to be well expressed on ASM. When exogenous sema3A was added to these cells, it inhibited their growth factor-induced proliferation. This inhibitory effect was associated with decreased tyrosine phosphorylation of PDGFR, down-regulation of Raci activation, STAT3 and GSK-3B phosphorylation. These findings suggest a functional contribution of sema3A-NP-1 axis in airway remodeling and that sema3A could become a useful therapy in treating hyper-reactive airway disorders (21, 22).
Semaphorin3A in atopic dermatitis (AD)
Epidermal hyper innervation in AD is activated by various external stimuli causing enhanced itching. This condition is regulated in part by the nerve repulsion factor sema3A. Aiming to gauge the contribution of sema3A in regulating this skin disorder, the application of sema3A ointment in the NC/Nga mouse model of AD was evaluated. Transepidermal water loss (TEWL) was measured before and after each treatment. The degree of dermatitis and scratching behavior were also scored. Topical application of sema3A, betamethasone, and tacrolimus ointments improved scratching behavior and the dermatitis score in treated mice compared with control mice. A significant improvement of TEWL was recorded only in sema3A ointment-treated mice. The number of inflammatory cells, such as CD4 effector T cells and eosinophils were reduced in the epidermis of sema3A treated mice compared with controls (23). In another study, sema3A treatment has been shown to inhibit nerve growth factor-induced sprouting of sensory nerves, and the normalization of hyper innervation in AD. Here also, sema3A resulted in suppression of itching and the amelioration of intractable pruritus in AD (24).
The above results are summarized in Table 1.
Table 1.
The expression of sema3A in serum and regulatory cells in immune mediated diseases
| Disease | Sema3A in Serum | Sema3A in Treg Cells | Sema3A in Breg Cells |
|---|---|---|---|
| Rheumatoid arthritis | Reduced | Reduced | ND |
| Systemic lupus erythematosus | Reduced | Equal to normal controls | Reduced |
| Familial Mediterranean fever | Reduced during attacks | Reduced during attacks | Reduced during attacks |
| Systemic sclerosis | Reduced | Reduced | Reduced |
| Inflammatory bowel disease | Equal to normal controls | Reduced during attacks | ND |
| Atopic dermatitis | ND | ND | ND |
| Atopic diseases | ND | ND | ND |
Conclusion
Semaphorin 3A is well known for its suppressive\regulatory effect on many inflammatory processes. Its low level of expression on both T and B regulatory cells in autoimmune diseases, such as SLE and RA, contributes to the failure of self-tolerance in these diseases. Thus, sema3A should be considered a promising therapeutic tool that could be applied in a wide spectrum of immune-mediated diseases.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.Z., T.E.; Design - V.Z., T.E.; Supervision - V.Z., T.E.; Resources - V.Z., T.E.; Materials - V.Z., T.E.; Data Collection and/or Processing - V.Z., T.E.; Analysis and/or Interpretation - V.Z., T.E.; Literature Search - V.Z., T.E.; Writing Manuscript - V.Z., T.E.; Critical Review - V.Z., T.E.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 3.Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14:669–78. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Tamagnone L, Comoglio PM. Signaling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000;10:377–83. doi: 10.1016/S0962-8924(00)01816-X. [DOI] [PubMed] [Google Scholar]
- 5.Mendes-da-Cruz DA, Lepelletier Y, Brignier AC, Smaniotto S, Renand A, Milpied P, et al. Neuropilins, semaphorins, and their role in thymocyte development. Ann N Y Acad Sci. 2009;1153:20–28. doi: 10.1111/j.1749-6632.2008.03980.x. [DOI] [PubMed] [Google Scholar]
- 6.Vadasz Z, Toubi E. Semaphorins: Their Dual Role in Regulating Immune-Mediated Diseases. CRAI. 2014;47:17–25. doi: 10.1007/s12016-013-8360-4. [DOI] [PubMed] [Google Scholar]
- 7.Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, et al. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc Natl Acad Sci USA. 2007;104:5545–50. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia F, Lepelletier Y, Smaniotto S, Hadj-Slimane R, Dardenne M, Hermine O, et al. Inhibitory effect of semaphorin-3A, a known axon guidance molecule, in the human thymocyte migration induced by CXCL12. J Leukoc Biol. 2012;91:7–13. doi: 10.1189/jlb.0111031. [DOI] [PubMed] [Google Scholar]
- 9.Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, et al. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008;111:2290–9. doi: 10.1182/blood-2007-06-096529. [DOI] [PubMed] [Google Scholar]
- 10.Miller LE, Weidler C, Falk W, Angele P, Schaumburger J, Schölmerich J, et al. Increased prevalence of semaphorin 3C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1156–63. doi: 10.1002/art.20110. [DOI] [PubMed] [Google Scholar]
- 11.Catalano A. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J Immunol. 2010;185:6373–83. doi: 10.4049/jimmunol.0903527. [DOI] [PubMed] [Google Scholar]
- 12.Takagawa S, Nakamura F, Kumagai K, Nagashima Y, Goshima Y, Saito T. Decreased semaphorin3A expression correlates with disease activity and histological features of rheumatoid arthritis. BMC Musculoskelet Disord. 2013;14:40. doi: 10.1186/1471-2474-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vadasz Z, Haj T, Halasz K, Rosner I, Slobodin G, Attias D, et al. Semaphorin 3A is a marker for disease activity and a potential immunoregulator in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:146. doi: 10.1186/ar3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadasz Z, Haj T, Balbir A, Peri R, Rosner I, Slobodin G, et al. A regulatory role for CD72 expression on B cells in systemic lupus erythematosus. Semin Arthritis Rheum. 2014;43:767–71. doi: 10.1016/j.semarthrit.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Rimar D, Rosner I, Slobodin G, Rozenbaum M, Halasz K, Jiries N, et al. Semaphorin3A, a potential immune regulator in familial Mediterranean fever. Clin Exp Rheumatol. 2016;34:52–5. [PubMed] [Google Scholar]
- 16.Rimar D, Nov Y, Rosner I, Slobodin G, Rozenbaum M, Halasz K, et al. Semaphorin3A: an immunoregulator in systemic sclerosis. Rheumatol Int. 2015;35:1625–30. doi: 10.1007/s00296-015-3269-2. [DOI] [PubMed] [Google Scholar]
- 17.Vadasz Z, Rimar D. New potential biomarkers for disease activity and fibrosis in systemic sclerosis. IMAJ. 2014;16:629–30. [PubMed] [Google Scholar]
- 18.Vadasz Z, Raines T, Nakhleh A, Haj T, Bejar J, Halasz K, et al. The involvement of immune semaphorins in the pathogenesis of inflammatory bowel disease. PLoS One. 2015;10:e0125860. doi: 10.1371/journal.pone.0125860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadasz Z, Haj T, Toubi E. The role of B regulatory cells and semaphorin3A in atopic diseases. Int Arch Allergy Immunol. 2014;163:245–51. doi: 10.1159/000360477. [DOI] [PubMed] [Google Scholar]
- 20.Movassagh H, Tatari N, Shan L, Koussih L, Alsubait D, Khattabi M, et al. Human airway smooth muscle cell proliferation from asthmatics is negatively regulated by semaphorin3A. Oncotarget. 2016;7:80238–51. doi: 10.18632/oncotarget.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawaki H, Nakamura F, Aihara M, Nagashima Y, Komori-Yamaguchi J, Yamashita N, et al. Intranasal administration of semaphorin3A alleviates sneezing and nasal rubbing in a murine model of allergic rhinitis. J Pharmacol Sci. 2011;117:34–44. doi: 10.1254/jphs.11005FP. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka J, Tanaka H, Mizuki N, Nomura E, Ito N, Nomura N, et al. Semaphorin 3A controls allergic and inflammatory responses in experimental allergic conjunctivitis. Int J Ophthalmol. 2015;8:1–10. doi: 10.3980/j.issn.2222-3959.2015.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negi O, Tominaga M, Tengara S, Kamo A, Taneda K, Suga Y, et al. Topically applied semaphorin3A ointment inhibits scratching behavior and improves skin inflammation in NC/Nga mice with atopic dermatitis. J Dermatol Sci. 2012;66:37–43. doi: 10.1016/j.jdermsci.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Tominaga M, Takamori K. Itch and nerve fibers with special reference to atopic dermatitis: therapeutic implications. J Dermatol. 2014;41:205–12. doi: 10.1111/1346-8138.12317. [DOI] [PubMed] [Google Scholar]