Supplemental Digital Content is available in the text.
Key Words: oncolytic virus, anticancer vaccination, maraba virus, Listeria monocytogenes
Abstract
Anticancer vaccination is becoming a popular therapeutic approach for patients with cancers expressing common tumor antigens. One variation on this strategy is a heterologous virus vaccine where 2 viruses encoding the same tumor antigen are administered sequentially to prime and boost antitumor immunity. This approach is currently undergoing clinical investigation using an adenovirus (Ad) and the oncolytic virus Maraba (MRB). In this study, we show that Listeria monocytogenes can be used in place of the Ad to obtain comparable immune priming efficiency before MRB boosting. Importantly, the therapeutic benefits provided by our heterologous L. monocytogenes-MRB prime-boost strategy are superior to those conferred by the Ad-MRB combination. Our study provides proof of concept for the heterologous oncolytic bacteria-virus prime-boost approach for anticancer vaccination and merits its consideration for clinical testing.
Oncolytic virotherapy targets cancers by several ways.1 An important part of the treatment is mediated by direct oncolysis and relies on the specific replication of the oncolytic virus (OV) in tumor cells. The induction of antitumor immunity by the virus is also believed to be an important facet of OV therapy that confers long-term benefits.2 Following the same idea, anticancer vaccines have shown great success in various clinical trials, notably in several anti-idiotype vaccine studies for patients with B-cell non-Hodgkin lymphoma,3 synthetic human papilloma virus type 16 E6-E7 long peptide immunization for gynecologic cancers4 as well as gp100 vaccines with IL-2 administration for metastatic melanoma patients,5 all of which demonstrate the tremendous potential of this strategy. To further improve on this aspect, OVs encoding tumor antigens can be used in vaccination strategies.6 Optimal results were obtained by using 2 different viruses to prime and boost immunity. This strategy is currently being tested using an adenovirus (Ad) and Maraba (MRB) encoding the tumor antigen MAGE-A3 in patients with solid tumors (NCT02285816 and NCT02879760). Although this approach provides protection in mouse tumor models,7,8 Ad is only effective at priming the immune response when administered intramuscularly and has no direct oncolytic effects. In contrast, MRB kills cancer cells in addition to boosting antitumor immunity. We sought to determine if an alternative priming agent that can be administered intratumorally and trigger inflammation locally would improve efficacy. We tested the bacteria Listeria monocytogenes (LM) based on its well-established vaccination potential9,10 as well as the previous reports of the bacteria directly infecting tumor cells.11 We found the magnitude of the immune response induced by both prime-boost combinations to be comparable, but the therapeutic benefits provided by the LM-MRB strategy to be improved compared with Ad-MRB, with the mice showing smaller tumors and prolonged survival.
MATERIAL AND METHODS
LM, Ad, and MRB Propagation
LM was cultured in brain-heart infusion media, Ad and MRB were expanded on HEK 293T and Vero cells, respectively. The MRB virus used in this study is the double mutant MG1 and has been described previously.12
Flow Cytometry
Splenocytes were stimulated for 6 hours with the Ova peptide SIINFEKL (Biomer Technology) and golgi-plug (BD Biosciences) was added after 1 hours. Antibodies were purchased from BD Biosciences. The samples were analyzed using an LSR Fortessa flow cytometer.
Enzyme-linked immunospot
Splenocytes were seeded into IFNγ enzyme-linked immunospot (ELISPOT) plates (Mabtech) and the assay was performed following the manufacturer’s protocol.
Histologic Analysis
Tumors were fixed in formalin and special stainings were performed by the University of Ottawa Pathology core. For caspase-3 staining (Cell signaling technology), the samples were rehydrated through graded alcohol. Heat-mediated antigen retrieval was performed using citrate buffer (sodium citrate 10 mM, pH 6).
High mobility group box 1 protein and Lactate dehydrogenase Assays
Serum was collected 48 hours after treatment by saphenous bleed. The high mobility group box 1 protein (HMGB1) and lactate dehydrogenase (LDH) concentrations were determined using a mouse enzyme-linked immunosorbent assay kit (Antibodies online) and an LDH assay (Abcam), respectively, following manufacturers’ protocols.
In Vivo Studies
All experiments were performed in accordance with the institutional guidelines of animal care and veterinary services. Tumor volume=(length×width2)/2.
RESULTS
LM and Ad Have Comparable Immune priming Activity
In this study, we used LM and Ad variants encoding Ovalbumin (Ova) to compare the vaccination potential of both agents in our heterologous prime-boost setting. First, we compared the antiOva immune response induced by Ad and LM in an ELISPOT assay following the treatment regimen illustrated in Figure 1A. Our results show the induction of an important antigen-specific response 7 days after vaccination using both priming agents (Supplemental Fig. 1A, Supplemental Digital Content 1, http://links.lww.com/JIT/A488). In contrast, vaccination with empty LM did not induce Ova-specific immunity. Flow cytometry analysis confirmed that 10%–15% of the cytotoxic T cells were responsive to Ova (Fig. 1B), and a significant proportion of these cells produced both IFNγ and TNFα (Fig. 1C). We next tested LM as a priming agent in the prime-boost setting and found that LM-Ova priming could efficiently be combined with MRB-Ova boosting (Supplemental Fig. 1B, Supplemental Digital Content 1, http://links.lww.com/JIT/A488). Impressively, we observed that 30% of the cytotoxic T cells from the LM-MRB group were responsive to Ova upon vaccination (Fig. 1D, E). Consistent with previous reports,8 MRB was not able to induce an antigen-specific immune response in absence of previous priming (LM+MRB-Ova group). Importantly, we observed no significant difference in the response to vaccination using LM-Ova or Ad-Ova. Taken together, these results show that LM is as efficient as Ad at priming antitumor immunity in the heterologous prime-boost setting.
FIGURE 1.
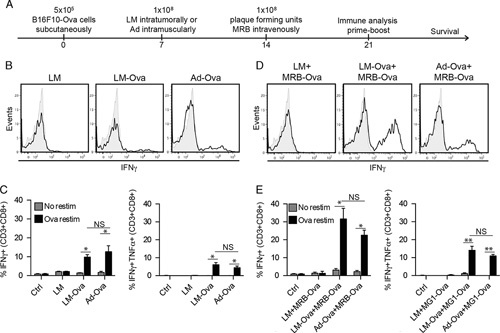
LM and Ad induce comparable immune responses. A, Treatment schedule used in this study. Flow cytometry analysis (n=3–5) of splenocytes restimulated ex vivo with Ova peptide 7 days post prime (B and C) or 7 days post prime and boost (D and E). Unpaired 2-tailed t test with the Welch correction (*P<0.05, **P<0.01). Ad indicates adenovirus; LM, Listeria monocytogenes; MRB, Maraba; NS, not significant; Ova, ovalbumin.
The LM-MRB Prime-boost is an Improved Therapeutic Strategy
We next wanted to confirm that LM could replicate in our tumor models. To do so, we performed a histologic analysis of B16F10-Ova melanoma tumors 24 hours after treatment. As expected, we were able to observe the bacteria in treated tumors by Gram staining (Fig. 2A). It is interesting to note that, our hematoxylin and eosin staining revealed that most of the tumor surface was necrotic and bloody upon LM treatment (Fig. 2B). In order to further assess the cytotoxic effect of intratumoral LM treatment, we performed immunohistochemical staining for cleaved caspase-3 on tumor sections 48 hours after treatment. Consistent with the hematoxylin and eosin staining (Fig. 2B), most of the tumor surface stained positive for caspase-3. Furthermore, the levels of LDH (Fig. 2D) and HMGB1 (Fig. 2E), 2 markers of necrosis, were elevated in the serum of LM-injected, tumor-bearing mice. Taken together, these results indicate that the bacteria treatment indeed contributes to tumor killing.
FIGURE 2.
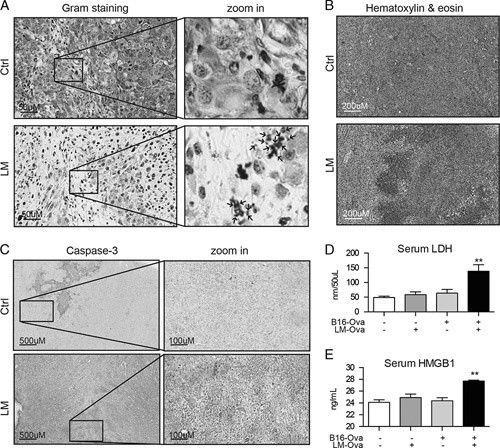
LM replicates in tumors. The Gram (A) and Hematoxylin and eosin stainings (B) of B16F10-Ova tumors 24 hours after injection. C, Immunohistochemistry analysis of caspase-3 cleavage in B16F10-Ova tumors 48 hours after LM treatment. Serum from the same animals were collected and LDH (D) and HMGB1 (E) concentrations were measured. Unpaired 2-tailed t test with the Welch correction (**P<0.01). LM indicates Listeria monocytogenes; Ova, ovalbumin; HMGB1, high mobility group box 1 protein; LDH, Lactate dehydrogenase.
In order to determine if our LM-MRB prime-boost could efficiently control tumor growth, we treated and measured B16F10-Ova tumors as depicted in Figure 1A. Our results clearly show that while single MRB or LM treatments only slightly affect tumor growth, the LM-MRB prime-boost was very efficient at controlling most of the tumors (Fig. 3A). We then compared our bacteria-virus prime-boost approach to the current Ad-MRB vaccination strategy and found that the LM-MRB prime-boost provided improved therapeutic benefits, with the mice showing smaller tumors and prolonged survival compared with the Ad-MRB group (Figs. 3B, C). To determine if the LM-MRB prime-boost could provide long-term protection, we rechallenged animals which had been previously cured of B16F10-Ova tumors for 123–314 days. Animals were challenged with E0771 cells, to which they were naïve, or B16F10-Ova cells. Our results show that while all long-term survivors displayed E0771 tumors 14 days posttumor challenge, all of the B16F10-Ova tumors were rejected. Taken together, our results show that the LM-MRB prime-boost efficiently controls established tumors and provides long-term protection.
FIGURE 3.
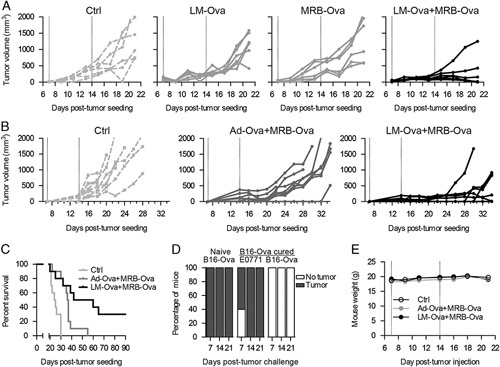
The LM-MRB prime-boost improves efficacy. A, Tumor growth analysis of B16F10-Ova tumor-bearing animals that received LM, MRB, or LM and MRB (n=6) or (B) Ad and MRB or LM and MRB (n=10). C, Survival curves obtained from (B). D, Long-term survivors (n=5) (123 or 314 d) from LM-Ova-MRB-Ova prime-boost experiments were rechallenged with E0771 cells and B16F10-Ova cells on opposite flanks. The graph shows the percentage of mice that displayed tumors of each type. E, Mouse weight over time (n=10). Ad indicates adenovirus; LM, Listeria monocytogenes; MRB, Maraba; Ova, ovalbumin.
To determine if our approach caused adverse effects, we monitored the animals from each group. None of the animals displayed any sign of discomfort over the course of the experiment and we found no drop in body weight, indicating that our LM-MRB combination was well tolerated (Fig. 3D).
DISCUSSION
In this study, we developed a novel oncolytic bacteria-virus prime-boost approach for anticancer vaccination. Our results clearly show the induction of antitumor immunity to levels that are comparable with those induced by the current Ad-MRB prime-boost approach. As we expected, the therapeutic benefits provided by our treatment strategy on established tumors are superior compared with the Ad-MRB prime-boost. We believe that this improved efficacy is the result of the direct killing of tumor cells by the bacteria, as well as the local inflammation in the tumor microenvironment triggered upon injection. Moreover, LM-based vaccines induce both cellular a humoral immunity13 and although we did not investigate this possibility, the generation of Ova-specific antibodies might have contributed to the improved tumor control observed for the LM-MRB group.
Our study provides proof of concept for the combination of bacteria and viruses in vaccination approaches. Although we focused our work on LM, other bacteria like Salmonella have been previously described as good immune priming agents and are also able to replicate and kill tumor cells directly.14,15 Alternatively, an OV that could efficiently prime the immune response against a tumor antigen could be a suitable candidate. For instance, Newcastle disease virus, Herpes simplex virus and vaccinia virus are all OVs that have been shown to be efficient anticancer vaccination agents6 and could therefore be used in heterologous virus prime-boost regiments.
Given that both LM and the Ad-MRB anticancer vaccination strategies are already being evaluated clinically, our LM-MRB prime-boost approach has the potential for rapid clinical translation.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by the Terry Fox Foundation and the Canadian Institute for Health Research (CIHR).
J.C.B. is a founder of Turnstone Biologics and have equity in the company. A.S.A. received an Ontario Graduate Scholarship. D.G.R. and M.-C.B.-D. were supported by the CIHR. The remaining authors have declared that there are no financial conflicts of interest with regard to this work.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
Footnotes
D.G.R. and N.T.M. contributed equally.
REFERENCES
- 1.Breitbach CJ, Lichty BD, Bell JC. Oncolytic viruses: therapeutics with an identity crisis. EBioMed. 2016;9:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichty BD, Breitbach CJ, Stojdl DF, et al. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–567. [DOI] [PubMed] [Google Scholar]
- 3.Park HJ, Neelapu SS. Developing idiotype vaccines for lymphoma: from preclinical studies to phase III clinical trials. Br J Haematol. 2008;142:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Poelgeest MIE, Welters MJP, van Esch EMG, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aitken A, Roy D, Bourgeois-Daigneault M-C. Taking a stab at cancer; oncolytic virus-mediated anti-cancer vaccination strategies. Biomedicines. 2017;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridle BW, Clouthier D, Zhang L, et al. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8(+) T-cell responses to anticancer vaccines. Oncoimmunology. 2013;2:e26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pol JG, Zhang L, Bridle BW, et al. Maraba virus as a potent oncolytic vaccine vector. Mol Ther. 2014;22:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangney M, Gahan CGM. Listeria monocytogenes as a vector for anti-cancer therapies. Curr Gene Ther. 2010;10:46–55. [DOI] [PubMed] [Google Scholar]
- 10.Wood LM, Paterson Y. Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quispe-Tintaya W, Chandra D, Jahangir A, et al. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:8668–8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brun J, McManus D, Lefebvre C, et al. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol Ther. 2010;18:1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood LM, Guirnalda PD, Seavey MM, et al. Cancer immunotherapy using Listeria monocytogenes and listerial virulence factors. Immunol Res. 2008;42:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall DM, Srikanth C V, McCormick BA. Targeting tumors with salmonella Typhimurium-potential for therapy. Oncotarget. 2010;1:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toussaint B, Chauchet X, Wang Y, et al. Live-attenuated bacteria as a cancer vaccine vector. Expert Rev Vaccines. 2013;12:1139–1154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.


