Supplemental Digital Content is available in the text.
Key Words: 5T4 tumor antigen, chimeric antigen receptor (CAR), ovarian cancer, immunotherapy
Abstract
Chimeric antigen receptor (CAR) T cells represent a novel targeted approach to overcome both quantitative and qualitative shortfalls of the host immune system relating to the detection and subsequent destruction of tumors. The identification of antigens expressed specifically on the surface of tumor cells is a critical first step in the ability to utilize CAR T cells for the treatment of cancer. The 5T4 is a tumor-associated antigen which is expressed on the cell surface of most solid tumors including ovarian cancer. Matched blood and tumor samples were collected from 12 patients with ovarian cancer; all tumors were positive for 5T4 expression by immunohistochemistry. Patient T cells were effectively transduced with 2 different anti-5T4 CAR constructs which differed in their affinity for the target antigen. Co-culture of CAR T cells with matched autologous tumor disaggregates resulted in antigen-specific secretion of IFN-gamma. Furthermore, assessment of the efficacy of anti-5T4 CAR T cells in a mouse model resulted in therapeutic benefit against established ovarian tumors. These results demonstrate proof of principle that 5T4 is an attractive target for immune intervention in ovarian cancer and that patient T cells engineered to express a 5T4-specific CAR can recognize and respond physiologically to autologous tumor cells.
Genetic modification of T cells to express chimeric antigen receptors (CARs) can produce effector populations with defined antigen specificities that function independently of both the natural T-cell receptor and major histocompatibility complex restriction. In cancer patients, a paucity of effector T cells specific for antigens expressed by the tumor is one factor that limits the therapeutic potential of the host immune system to eradicate the malignancy. The ability to redirect the specificity of a patient’s T cells to recognize an antigen expressed on their tumor enables the possibility of being able to deliver a therapeutically relevant dose of antigen-specific T cells. In its simplest form (so called first-generation CARs), an immunoglobulin-derived single-chain variable fragment (scFv) specific for the antigen of choice is fused to the CD3ζ signaling domain of the T-cell receptor complex to facilitate T-cell activation. Later generation CARs include ≥1 costimulatory domains such as CD28 or 4-1BB, which promote expansion and survival of the CAR T cells in vivo.1 Recent trials of CAR T cells in patients with hematological malignancies have demonstrated impressive clinical responses.2,3 For example, several groups have reported response rates of >80% using CAR T cells targeting the CD19 antigen, which is expressed on several B-cell malignancies including B-acute lymphoblastic leukemia.4,5 Indeed, on 30th August the FDA-approved Kymriah (tisagenlecleucel) for pediatric and young adult patients with a form of acute lymphoblastic leukemia and on 18 October, FDA cleared Kite Pharma’s Yescarta (axicabtagene ciloleucel) for the treatment of adults with relapsed or refractory large B-cell lymphoma. Trials are ongoing to determine if these responses can be replicated in solid tumors; however, to date the data emerging from studies in solid tumors have been less compelling.6 The reason for the difference in clinical responses is likely to be related to the unique challenges posed by solid tumors, such as the requirement for the CAR T cells to home to the tumor site and the presence of immunosuppressive cells within the tumor microenvironment.7 Despite these issues, a growing number of clinical trials are being initiated which target antigens expressed on the surface of solid tumors, for example HER2, CEA, EGFR, Mesothelin, and GD2.6,8 Currently, there is no consensus on the best CAR construct to use for the treatment of solid tumors. Although there is evidence that the addition of costimulatory domains (eg, CD28 or 4-1BB) to first-generation CARs enhanced cytotoxicity and T-cell persistence,9,10 more head-to-head comparisons are needed before clear conclusions can be drawn.
As more clinical data become available in solid tumors, there have been some concerns relating to the safety of CAR T cells caused by on-target, off-tumor effects; indeed, in some trials patient deaths were reported, which were thought to be associated with T-cell infiltration and destruction of normal tissues expressing the target antigen.11
In addition to the expression profile of the target antigen, the affinity of the antibody used to generate the CAR is another factor that could affect safety. Several publications have reported that the affinity of the scFv could affect functional responses of the CAR T cells. For example, it has been shown that a high-affinity CAR-targeting folate receptor β exhibited enhanced antitumor activity both in vitro and in vivo compared with a lower affinity CAR.12 However, other researchers have demonstrated that by decreasing the affinity of antibodies with known off-tumor toxicity issues (eg, ErbB2, EGFR), they were able to maintain the therapeutic index while decreasing reactivity against normal tissues expressing the target antigen.13,14
One tumor-associated antigen which could be an attractive target for a CAR T cell in solid tumors is 5T4. The 5T4 oncofetal antigen was first identified by searching for surface molecules shared between human trophoblasts and cancer cells with the rationale that they may function to allow survival of the fetus as a semiallograft in the mother, or a tumor in its host. The 5T4 is a 72-kDa transmembrane protein expressed on the placenta and a wide range of human carcinomas including ovarian, prostate, renal, non–small cell lung cancer, head and neck, mesothelioma, and colorectal cancers, but rarely on normal tissues.15 The human gene for 5T4 encodes a 42 kDa transmembrane protein core which contains several leucine-rich repeats that are associated with protein-protein interactions.16 The extracellular part of the molecule contains leucine-rich repeats in 2 domains separated by a short hydrophilic sequence; there is a transmembrane domain and a short cytoplasmic sequence. When human 5T4 was overexpressed in murine fibroblasts the cells became more spindle shaped and had reduced adherence,17 whereas in normal epithelial cells there was E-cadherin down regulation, increased motility, and cytoskeletal disruption.18 The cytoskeletal disruption through 5T4 overexpression is dependent on the 5T4 cytoplasmic domain, which interacts with TIP2/GIPC, known to mediate links to the actin cytoskeleton.19 These studies were the first to indicate a possible association of 5T4 expression with cancer spread. On the basis of these properties, it is perhaps not surprising that expression of 5T4 has been associated with poor prognosis in non–small cell lung cancer, colorectal, gastric, and ovarian cancers.20–24 The restricted expression of 5T4 on normal tissues and its high prevalence on many common human carcinomas make it an attractive target for cancer immunotherapy.
Ovarian cancer remains the most lethal gynecologic malignancy with a 5-year survival of 40% for patients with advanced disease.25 Most patients with advanced disease will have an initial favorable response to cytoreductive surgery and platinum-based chemotherapy; however, ultimately 80% of patients relapse within 18 months of completion of first-line treatment.26 Advances in traditional cytotoxic chemotherapy such as intraperitoneal administration, dose-dense schedules, and the addition of targeted therapies including bevacizumab have improved progression-free survival but have failed to have a significant effect on overall survival rates. There is extensive evidence that ovarian cancer is under immune surveillance. Multiple studies have demonstrated a positive correlation between the number of CD3+ tumor-infiltrating lymphocytes and overall survival.27,28 Similarly, high frequencies of immune effector cells such as CD8+ T cells and natural killer cells have also been shown to correlate with positive clinical outcomes.29,30 Such observations suggest that immune-targeted approaches in ovarian cancer could deliver therapeutic benefit. Initial CAR T-cell studies targeting ovarian cancer focused upon exploiting the folate receptor as a target. However, despite extensive in vitro and in vivo proof of principle studies showing activity of the Folate receptor-specific CAR,31–33 no significant clinical activity was observed.34 This was subsequently hypothesized to be due to the short-term persistence of the first-generation CAR construct used in this study. A potential advantage of a cavity cancer such as ovarian cancer is the opportunity for intraperitoneal delivery of CAR T cells. Localized delivery of CAR T cells has been tested in mesothelioma, where intrapleural administration of CAR T cells was found to be more efficacious than systemic delivery. Indeed, Adusumilli et al35 demonstrated that 30-fold fewer CAR T cells were required to induce long-term complete remissions when administered intrapleurally compared with IV delivery. Although the intrapleural cavity is significantly different to the peritoneal cavity, parallels can still be drawn.
The 5T4 is known to be highly expressed in ovarian cancer and its expression correlates with more advanced stage of disease (FIGO stages III and IV) and with poorly differentiated tumors. Furthermore, patients whose tumors express 5T4 seem to have a worse progression-free and overall survival.23 Given the expression profile of 5T4, it is an attractive target for immune therapeutic intervention in ovarian cancer. Here we report on the functional activity of anti-5T4 CAR T cells against ovarian tumors both in vitro and in vivo and question whether the affinity of the 5T4-specific scFv affects the functional activity of the CAR T cell.
MATERIALS AND METHODS
5T4 Antibodies and Generation of 5T4 CAR Constructs
Two 5T4-specific monoclonal antibodies were used in this study. The murine IgG1 5T4-specific mAb H8 was raised against a purified extract of human syncitiotrophoblast membrane.36 The second 5T4-specific murine mAb (2E4) was raised against purified recombinant 5T4 protein (lacking the transmembrane region). The affinity of the 2 antibodies was determined by Biacore using the Biacore T200m, a series S sensor chip CM5 (GE Healthcare), running buffer consisting of 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 150 mM NaCl, 3 mM EDTA, 0.005% Tween 20, pH 7.4 and run at a temperature of 25°C. The CAR constructs containing scFv domains against 5T4 linked to CD8 hinge and transmembrane domain and 4-1BB and CD3 zeta intracellular signaling domains, were synthesized and subcloned into minimal HIV-1 based lentiviral vectors.
Collection of Matched Tumor and Blood Sample
Tumor and blood samples were collected from patients undergoing surgery for ovarian cancer at St Mary’s Hospital, Manchester, UK. Samples were collected through the Central Manchester University Hospital NHS Foundation Trust Biobank with appropriate ethical approval (14/NW/1260/10) and informed consent.
Immunohistochemistry
Tumors from patients with ovarian cancer were formalin-fixed and paraffin-embedded (FFPE). Tissue sections (4-µm thick) were cut from FFPE blocks and used for assessment of 5T4 expression. Automated staining was performed using the Leica Bond Max (Leica Biosystems, Wetzlar, Germany). Heat induced epitope retrieval was performed at pH6 for 20 minutes. Tissue sections were incubated with a casein block for 30 minutes, to prevent nonspecific binding of the primary antibody. Slides were incubated for 1 hour at room temperature with the 5T4 antibody (mouse monoclonal, anti-human 5T4 antibody; R&D Systems) at a concentration of 6.25 μg/mL. Antibody detection was performed using the Refine Detection Kit (Leica Biosystems). Slides were counterstained for hematoxylin. Negative (IgG1 isotype control) and positive (placenta) controls were used. Stained sections were evaluated for the proportion and intensity of staining using the modified H-score.37
Disaggregation of Tumor Samples
Ovarian tumor samples were disaggregated into single-cell suspensions using the GentleMACS dissociator and human tumor dissociation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) as previously described.38 Following disaggregation, the suspension was passed through a 100 μm strainer and washed in T-cell media [RPMI-1640,500 mL (Lonza, Slough, UK) supplemented with 10% heat inactivated fetal calf serum (FCS), 2 mM l-glutamine, 25 mM HEPES (all Life Technologies, Paisley, UK), and 100 IU/mL penicillin/100 μg/mL Streptomycin (Sigma-Aldrich, Dorset, UK)]. Cells were counted using trypan blue (Sigma-Aldrich). Cells were then cryopreserved in 90% FCS/10% dimethyl sulfoxide at a minimum density of 2×107 cells/mL.
Cell Culture
OVCAR-3 cells were obtained from ATCC (Manassas, VA, USA) and transfected with a retroviral vector to express luciferase. The SKOV-3/Luc cell lines were obtained from Cell Biolabs Inc. (San Diego, CA) in 2015. The OVCAR-3 and SKOV-3 cells were cultured as adherent monolayers in RPMI-1640 or Dulbecco’s modified eagle medium with 0.1 mM MEM nonessential amino acids, supplemented with 10% FCS, 2 mM l-glutamine and 1% Pen-Strep respectively (all Gibco, Paisley, Scotland). Both cell lines were routinely tested for mycoplasma contamination before use. All experiments were performed within 8 weeks of thawing early passage cells.
Flow Cytometry
To determine 5T4 expression on tumor cells, 1×105 cells were labeled in 100 μL fluorescence-activated cell sorting (FACS) buffer [1% FCS in phosphate-buffered saline (PBS)] containing EpCAM (PE, clone 9C4; Biolegend) and 5T4 (clone H8 or 2E4) antibodies for 30 minutes at 4°C. To determine transduction efficiency for 5T4 CAR, 1×105 cells were stained with biotin-SP AffiniPure goat anti-mouse IgG F(ab′) 2 fragment-specific antibody (Jackson Immuno Research Laboratories, PA) in human AB serum buffer (PBS containing 1% human AB serum) for 30 minutes at 4°C. Cells were then washed and stained with CD3 (APC, clone UCHT1, Biolegend), CD4 (BV785, clone OKT4, Biolegend), CD8 (APC-H7, clone SK1, BD Biosciences, UK), and Streptavidin (PE, BD Biosciences) diluted in FACS buffer for 30 minutes. Samples were washed and then fixed in 1% paraformaldehyde (Sigma-Aldrich) in PBS. Analysis was performed on the LSR Fortessa (BD Biosciences). Data were analyzed using FlowJo v. 7.6.2 software (Tree Star Inc., Ashland, OR).
Cell Seeding, T-Cell Expansion, and Transduction
Peripheral blood mononuclear cells were isolated from heparinized blood samples by centrifugation on Lymphoprep density gradient media (Stemcell technologies, Cambridge, UK), washed in T-cell media and stored at 2×107 cells/mL in 90% FCS/10% dimethyl sulfoxide. After thawing, 1.5×106 cells were added to wells of a 24-well plate and mixed with anti-CD3/anti-CD28 Dynabeads (Life Technologies) at a 1:1 ratio, 100 IU/mL of IL-2 (Novartis), and the appropriate lentiviral vector (3 μL of H8-CAR, 4 μL 2E4-CAR or no vector in mock wells). Fresh media and IL-2 were supplemented every 2–3 days. Cells were counted on alternate days from day 3, and cell concentration was maintained at 0.5×106 cells/mL. Dynabeads were removed from the culture by magnetic separation on day 9. T cells were expanded for 14 days in total.
In Vitro Testing of 5T4 CAR T Cells
The 5T4 CAR T cells were co-cultured with autologous tumor or with 2 ovarian cancer cell lines known to express 5T4 (SKOV-3 and OVCAR-3 cells), at a 1:1 ratio (1×105 T cells and 1×105 autologous tumor cells) in a 96-well plate. Simultaneously, T cells were cultured alone (negative control) and with 50 ng/mL phorbol 12-myristate 13-acetate and 1 μg/mL ionomycin (positive control). The cultures were incubated at 37°C for 24 hours, before the supernatant was harvested. The supernatant was analyzed for IFNγ and IL-2 production using IFNγ and IL-2 enzyme-linked immunosorbent assay kits (both Diaclone, France), according to the manufacturer’s instructions.
In Vivo Testing of 5T4 CAR T Cells
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD scid gamma, NSG) mice were obtained from JAX labs and bred in-house at the Cancer Research UK Manchester Institute, UK. In vivo studies were carried out under the 1986 ASPA Act and EU Directive 2010/63 under UKCCCR guidelines, approved by a local ethical committee and performed under a UK Home Office license. Mice were housed in Tecniplast 1284 IVC cages holding a maximum of 7 animals on aspenchips-2 bedding with sizzlenest nesting material and a cardboard tunnel on a 12/12 light/dark cycle under specific pathogen free facilities. Mice received filtered water and were fed ad-lib on Teklad Global 19% protein extruded rodent diet. For the initial in vivo testing of the 5T4 CARs, SKOV-3, or OVCAR-3 ovarian cancer cells (both expressing the marker luciferase) were injected by the intraperitoneal route into recipient NSG (NOD/SCID IL-2Rγ−/−) mice and 7 days later, CAR T cells (100 μL volume) were infused by the IV route. Tumor burden was assessed via bioluminescence imaging using the In-Vivo Xtreme II system (Bruker, UK) on day 6 (1 d before T-cell transfer) and then at regular times thereafter over a 100-day period until the mice were sacrificed.
Statistical Analysis
Data were analyzed for significance using a 2-way analysis of variance with Sidak’s correction (GraphPad Prism 7, GraphPad Software, La Jolla, CA). For the in vivo assays, the significance of the survival advantage of the mice receiving the different CAR T cells or Mock T cells was determined using the Log-rank (Mantel-cox) test. The value for which P<0.05 was considered significant. All error bars represent the mean and SD unless otherwise noted in the figure legends. In vitro assays were performed in triplicate.
RESULTS
Construction and Expression of 5T4-specific CARs
Analysis of the binding affinity of 2 5T4-specific monoclonal antibodies by Biacore demonstrated affinities of 5.2×10−9 KD (H8 mAb) and 3.4×10−8 KD (2E4 mAb) for the 5T4 protein (data not shown). Two second generation CAR constructs were synthesized which contained scFvs derived from H8 or 2E4, the EF1α promoter, the CD3ζ signaling domain and the 4-1BB costimulatory domain (Fig. 1A). The two constructs are denoted H8-CAR and 2E4-CAR. Both CAR constructs effectively transduced human T cells in a vector dose-dependent manner (supplementary Fig. 1A, Supplemental Digital Content 1, http://links.lww.com/JIT/A483). Cell growth and viability of transduced cells was not affected relative to comparable nontransduced T cells (supplementary Fig. 1B, Supplemental Digital Content 1, http://links.lww.com/JIT/A483). Analysis of the transduction of T cells recovered from healthy donors or from patients with ovarian cancer showed no significant differences in transduction levels with either the H8-CAR or 2E4-CAR (Figs. 1B, C, respectively). The median percentage transduction of patient’s T cells for both the higher affinity (H8) and lower affinity (2E4) CARs was very similar at 28.1% (23.1% to 44.7%) and 28.3% (16.6% to 44.6%), respectively. However, in T cell recovered from patients, the relative transduction efficiency of CD8+ T cells was significantly higher compared with CD4+ T cells for both H8 and 2E4-CAR constructs (Figs. 1D, E; P<0.05 and P<0.0001, respectively). In contrast, the opposite was the case in T cells taken from healthy donors, in whom the transduction of CD4+ T cells was more efficient than CD8+ T cells for both H8-CAR and 2E4-CAR constructs (Figs. 1F, G; P<0.05 and P<0.01, respectively).
FIGURE 1.
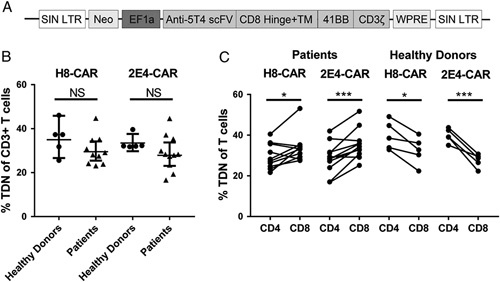
5T4 CAR construct and transduction efficiency. A, Anti-5T4 CAR construct shown in the integrated form. B, Percentage of CD3 T cells from healthy donors and patients transduced with H8-CAR and 2E4-CAR. C, Percentage of patient-derived and healthy donor-derived CD4 and CD8 T cells transduced with H8-CAR and 2E4-CAR. The Student t test, *P<0.05; **P<0.01; ***P<0.001. CAR indicates chimeric antigen receptor, LTR, long terminal repeat; Neo, Neomycint; NS, not significant; SIN, self-inactivating; WPRE, Woodchuck Hepatitis Virus posttranscriptional regulatory element.
5T4 Expression on Ovarian Tumor Biopsies
Matched blood and tumor samples were collected from 12 patients with ovarian cancer (Table 1). The 5T4 expression was determined by immunohistochemistry on FFPE sections and by flow cytometry on tumor disaggregates (Fig. 2). All 12 tumor biopsies were positive for 5T4 expression by immunohistochemistry, and clearly demonstrated a membranous pattern of staining although the intensity and proportion of staining varied between patient samples (Fig. 2A). The 5T4 expression on the tumor disaggregates (Figs. 2B, C) and ovarian cancer lines (SKOV-3 and OVCAR-3; data not shown) were also assessed by flow cytometry. Among all cell types present within the tumor disaggregates 25.12% (±24.89%) were EpCAM+ tumor cells (supplementary Fig. 2A, Supplemental Digital Content 1, http://links.lww.com/JIT/A483). Hematopoietic cells (CD45+) accounted for a lower proportion (mean of 12.61%). Overall, <20% of cells were double positive for 5T4 and EpCAM (Fig. 2B). However, as a percentage of tumor cells (EpCAM+) present, ∼50% expressed 5T4, with the exception of MOC 45 and MOC 52, which had around 20% positivity for 5T4 (Fig. 2C). Both SKOV-3 and OVCAR-3 cell lines had high levels of 5T4 expression (>90% and >70% positive, respectively; data not shown). The magnitude of 5T4 expression on tumor biopsies determined by H-score following immunohistochemistry and by mean fluorescence intensity (MFI) on tumor disaggregates determined by flow cytometry is shown in Figure 2D. MFI was calculated by geometric mean of 5T4 expression on the EpCAM positive (EpCAM+) population. It is interesting to note that, there was no correlation between 5T4 expression and immune infiltration (supplementary Fig. 2B, Supplemental Digital Content 1, http://links.lww.com/JIT/A483).
TABLE 1.
Patient Characteristics
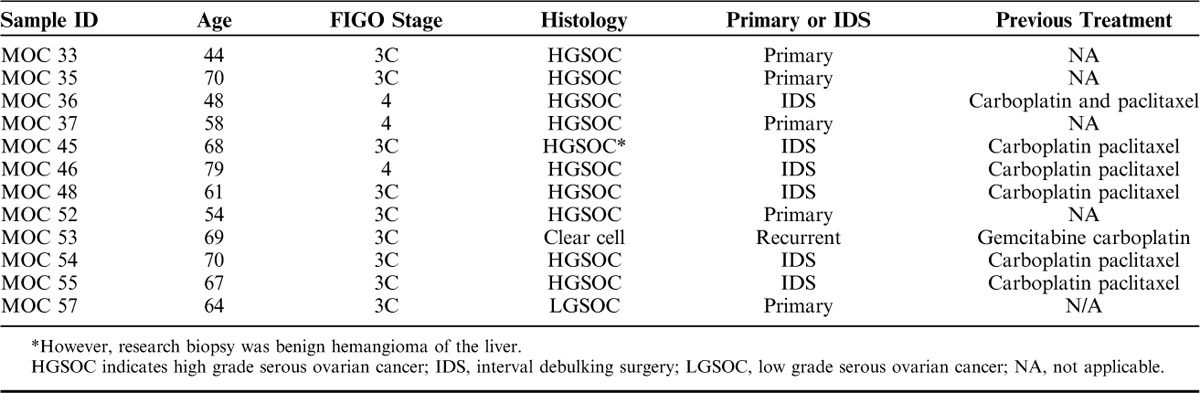
FIGURE 2.
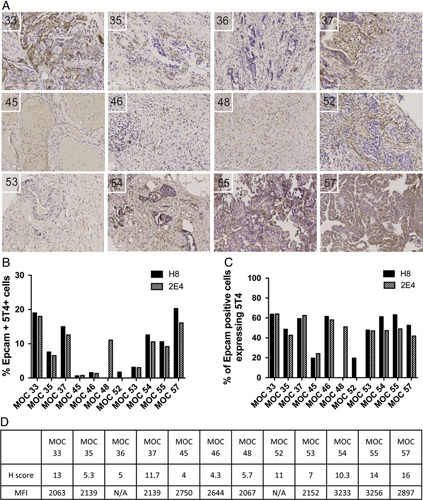
5T4 expression on FFPE sections of tumor biopsies from patients with ovarian cancer. A, Light microscopy (×20 magnification) of FFPE sections after staining with a mouse anti-human 5T4 monoclonal antibody and hematoxylin. B, Percentage of EpCAM+ 5T4+ cells in the tumor disaggregates as determined by flow cytometry. C, Percentage of EpCAM positive tumor cells expressing 5T4. D, 5T4 expression on tumor samples as determined by H-score and mean fluorescence intensity (MFI). FFPE indicates formalin-fixed and paraffin-embedded.
Functional Activity of CAR T 5T4 Cells In Vitro
To determine the relative functional reactivity of the higher (H8) and lower affinity (2E4) CAR T cells against 5T4 ovarian cell lines and primary tumor cells, CAR T cells were co-cultured overnight with target cells and cytokine secretion measured. Peripheral blood mononuclear cells from MOC 52 did not expand; therefore no assays were performed with T cells from this patient. Figure 3A shows that patient-derived peripheral T cells transduced with either the H8-CAR or 2E4-CAR both produce high levels of IFNγ in response to co-culture with SKOV-3 or OVCAR-3 ovarian cancer cell lines. Where a comparison was possible, the higher affinity H8-CAR produced significantly more IFNγ than the lower affinity 2E4-CAR in 9 of 10 patients (Fig. 3A).
FIGURE 3.
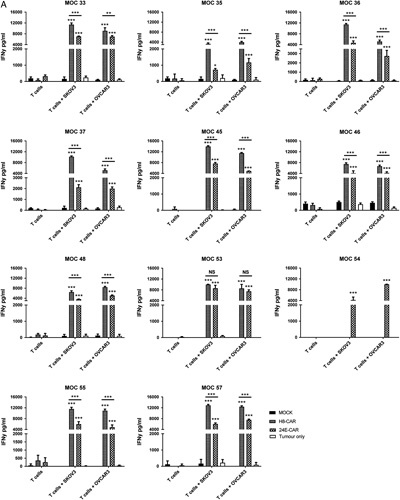
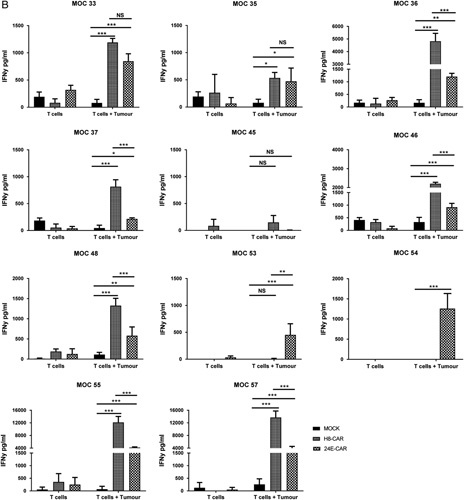
IFNγ production by 5T4 CAR T cells in response to immortalized ovarian cell lines expressing 5T4 and autologous tumor cells. Peripheral T cells were successfully transduced from 11 patients. T cells were transduced with the H8-CAR or 2E4-CAR or no CAR (Mock) vector. T cells (1×105) were co-cultured for 24 hours with 1×105 SKOV-3, OVCAR-3 (A) and primary autologous tumor cells (B). After 24 hours, supernatant was collected and IFNγ quantitified by enzyme-linked immunosorbent assay. Error bars represent the mean and SD of triplicate results. Two-way analysis of variance with Sidak’s correction; *P<0.05, **P<0.01, ***P<0.001. CAR indicates chimeric antigen receptor; INFγ, interferon-γ; NS, not significant.
When co-cultured with autologous tumor cells, 10 of the 11 patient-derived CAR T cells (no T cells were available from patient MOC 52) generated significantly higher levels of IFNγ compared with mock-transduced T cells (Fig. 3B). In the majority of patients (6/10), cells transduced with the higher affinity H8-CAR construct produced significantly more IFNγ than the 2E4-CAR construct (Fig. 3B). For MOC 53 only the lower affinity 2E4-CAR T cells produced significantly higher levels of IFNγ than mock-transduced T cells and MOC 54 only had 2E4-CAR T cells available for functional assessment. The MOC 45 was the only CAR T cell that failed to produce significant levels of IFNγ with either of the CAR constructs upon co-culture with autologous tumor. However, this is perhaps not surprising as the biopsied sample was shown to be a benign hemangioma of the liver. When these cells were assessed, the number of EpCAM+ cells were extremely low (<1%; Fig. 3B) and of those cells, <20% were 5T4+.
FIGURE 3.
(Continued)
With regard to IL-2 secretion, most patient-derived CAR T cells produced moderate levels of IL-2 (mean of 344±56.8 pg/mL for H8-CAR and 131±35.4 pg/mL for 2E4-CAR) when co-cultured with SKOV-3 and OVCAR-3 cells (supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/JIT/A483), however minimal IL-2 was produced in response to autologous tumor. Analogous to the results seen for IFNγ production, the higher affinity H8-CAR secreted more IL-2 than the lower affinity 2E4-CAR in the majority of donors.
Numerous variables may affect the quantity of cytokine secreted by the CAR T cells. These include the affinity of the CAR, the number and phenotype of T cells expressing the CAR, the number of tumor cells expressing the target antigen as well as the density of the antigen. Where cell lines were co-cultured with CAR T cells, the number of tumor cells and the magnitude of 5T4 expression is consistent and the main variables relate to the CAR T cells. Preliminary analysis of correlates between levels of IFNγ or IL-2 production and variables relating to CAR T cells showed no significant associations with the total number of CD4+ CAR+ T cells, but a significant correlation between the number of CD8+ CAR+ T cells and IFNγ (but not IL-2) production for both CAR constructs (supplementary Table 1A, Supplemental Digital Content 1, http://links.lww.com/JIT/A483; data only shown for IFNγ). Where CAR T cells were co-cultured with autologous tumor, the number of variables was greater as the patient tumor disaggregates contained different numbers of 5T4+ tumor cells and had different levels of 5T4 expression. In this scenario, the number of CAR+ T cells (total, CD4+ or CD8+) did not show significant correlates with IFNγ production but was significant for CD8+CAR+ T cells and IL-2 (P=0.02; data not shown). However, when the 5T4 expression level was quantified either using H-score (immunohistochemistry) or MFI (FACS analysis) there was a significant correlation with IFNγ and IL-2 production for both H8-CAR and 2E4-CAR constructs (supplementary Table 1B, Supplemental Digital Content 1, http://links.lww.com/JIT/A483). Although this analysis involved small numbers and needs validating in a larger cohort, the results putatively suggest a link between antigen expression level and CAR functionality.
Functional Activity of 5T4 CAR T Cells In Vivo
Having demonstrated that CAR T cells derived from patients were able to respond specifically to autologous tumor in vitro, the ability of 5T4 CAR T cells to treat ovarian cancer was then tested in vivo using a NSG mouse model. Mice were challenged via the intraperitoneal route with 2.5×106 SKOV-3 cells (expressing luciferase) on day 0 and were treated with CAR T cells on day 7. Preliminary experiments using the H8-CAR demonstrated that a dose of 2×107 CAR T cells delivered IV resulted in long-term treatment of SKOV-3 tumors (data not shown). Subsequent experiments aimed to establish the minimum dose of 5T4 CAR T cells required to demonstrate efficacy. NSG mice bearing 7 day established peritoneal tumors were treated with a dose-escalation schedule ranging from 0.03×107 to 1×107 total T cells; this equated to 0.23×105 to 7.6×105 5T4-specific CAR T cells. The in-life assessment of tumor growth and survival of treated and control animals are shown in Figures 4A and B, respectively. Animals treated with 1×107 and 0.3×107 total T cells showed a significant survival advantage relative to mice treated with 1×107 mock-transduced T cells or saline (both P<0.05). All animals treated with 1×107 total T cells (7.6×105 CAR T cells) remained alive beyond 100 days.
FIGURE 4.
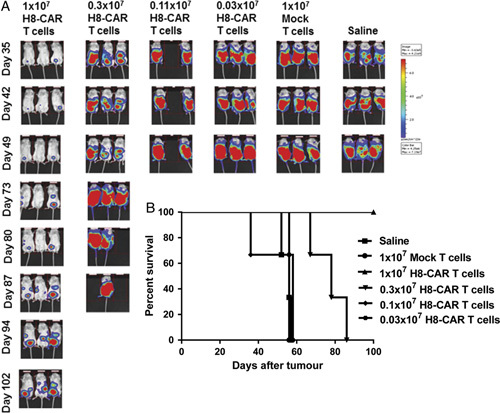
Dose escalation of 5T4 CAR T cells in NSG ovarian cancer model. NSG mice were challenged with 2.5×106 SKOV-3 tumor cells on day 0 and 7 days later were treated with either ascending doses of H8-CAR T cells or saline. A, In-life bioluminescence images of NSG mice treated with ascending doses of H8-CAR T cells are shown over time alongside control animals. B, Kaplan-Meier survival curves of NSG mice receiving ascending doses of H8-CAR T cells. CAR indicates chimeric antigen receptor.
Further work aimed to assess the efficacy of CAR T cells delivered via systemic (IV) or local (intraperitoneal) routes. A suboptimal dose of CAR T cells was selected for testing in order to be able to detect any potential enhancement of efficacy. Administration of 1×106 T cells (of which 2.6×104 were CAR positive) via the IP route of administration resulted in a significant survival advantage (median 102 d) relative to mock-transduced controls (median 64 d; supplementary Fig. 4, Supplemental Digital Content 1, http://links.lww.com/JIT/A483). However, 1×106 T cells administered via the IV route did not result in a significant survival advantage (median 57 d). Therefore, there is a suggestion that localized delivery of CAR T cells could be more efficacious than systemic administration in this model.
Functional Activity of Higher Versus Lower Affinity 5T4 CAR T Cells In Vivo
Following demonstration that 5T4-specific CAR T cells are functionally active against 5T4 expressing tumor cells in vivo, a comparison of two 5T4-specific CARs of different affinities was performed. Both the higher affinity (H8-) and lower affinity (2E4-) CAR T cells (1×107) resulted in a statistically significant increase in survival of SKOV-3 tumor bearing mice compared with mice treated with mock-transduced T cells (P=0.002 and P=0.044, respectively). Furthermore, there was a significant increase in survival of mice treated with H8-CAR T cells compared with 2E4-CAR T cells (median survival of 100 d and 68 d, respectively; P=0.008, Fig. 5). A similar experiment was performed in NSG mice challenged with OVCAR-3 cells. In this model, 3 of 4 animals remained alive in the H8 group and 4 of 5 animals in the 2E4 group at day 100, whereas all 5 animals in the mock-transduced group had died (data not shown).
FIGURE 5.
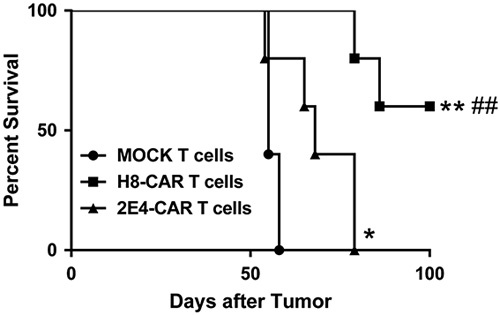
Comparison of higher versus lower affinity CAR constructs. NSG mice were inoculated with ovarian cancer cell lines on day 0. Seven days later mice were treated with either the higher affinity H8-CAR or the lower affinity 2E4-CAR T cells. Kaplan-Meier survival curves of NSG mice bearing SKOV-3 tumors and treated with 1×107 CAR T 5T4 cells. Log-rank (Mantel-Cox) test; *P<0.05, **P<0.01 compared with Mock T cells, ##P<0.01 compared with 2E4-CAR T cells. CAR indicates chimeric antigen receptor.
Meta-analysis
On the basis of the multiple experiments performed using the H8-CAR construct in the NSG SKOV-3 mouse model, a meta-analysis was performed to assess the dose of 5T4 CAR T cells (delivered via the IV route) required to deliver a statistically significant increase in overall survival relative to the mock-transduced group (supplementary Fig. 5, Supplemental Digital Content 1, http://links.lww.com/JIT/A483). It was clear that in all experiments a dose of ≥1×105 CAR T cells is required to deliver a significant survival advantage. Experiments in which animals received <1×105 CAR T cells did not have a significant survival advantage. In humans, this would be equivalent to an approximate dose of 4×106 CAR T cells/kg bodyweight.
DISCUSSION
CAR T-cell therapy has shown great promise in the treatment of B-cell malignancies,2,3 but clinical benefit in the treatment of solid tumors has been far less compelling. One of the difficulties in targeting solid tumors is a paucity of suitable targets. In hematological malignancies, the expression of the target antigen (eg, CD19) is usually homogenous and present on the majority, if not all, of the tumor population. Although CD19 is also expressed on normal B-cells, they are dispensable and patients can be treated with immunoglobulin to ensure sufficient immune competence. However, the majority of proteins targeted in solid cancers are known to be expressed on normal tissue39 and this has resulted in some unexpected toxicities during clinical testing.40 The 5T4 is an oncofetal antigen that is expressed on most solid tumors, but rarely expressed on normal tissues, thus making it a promising target for CAR T-cell therapy. Indeed, previous studies using different therapeutic approaches (a vaccine targeting 5T4, a 5T4-targeted antibody super-antigen and a 5T4-targeted antibody-drug conjugate) have all shown a good safety profile in preclinical and clinical testing with no reported autoimmune reactions.15
Here, we have confirmed previous reports that the tumor-associated antigen 5T4 is highly expressed in ovarian cancer.23 Although there was heterogeneity of 5T4 expression within individual patient biopsies and between different donors, overall ∼50% of EpCAM+ tumor cells expressed 5T4. Following demonstration of 5T4 expression in ovarian cancer, we developed 2 CAR constructs using the scFv from 2 5T4-specific monoclonal antibodies (H8 and 2E4) which have different affinities for 5T4. Second generation CARs were constructed, which linked the scFv to the 4-1BB and CD3ζ signaling domains. Consistent transduction rates were seen in T cells derived from healthy donors and patients with ovarian cancer, for both constructs. It is interesting to note that, we noted higher transduction efficiencies for CD8+ T cells compared with CD4+ T cells in patient donors. It is recognized that anti-CD3/anti-CD28 Dynabeads induce more rapid cell cycling in CD8+ T cells compared with CD4+ T cells.41 It is not clear why this phenomenon was only observed in the patient donors, and as such this warrants further investigation. Nevertheless, we have shown for the first time that CAR T cells from patients with ovarian cancer were able to produce IFNγ and IL-2 when co-cultured with 5T4+ autologous tumor cells and ovarian cancer cell lines. This follows previous studies which have shown that T cells expressing a first-generation CAR targeting 5T4 were able to produce IFNγ and lyse autologous renal cancer cells42 and target 5T4 expressing tumor cell lines.43
Both the high-affinity H8-CAR and the lower affinity 2E4-CAR T cells produced IFNγ and IL-2 when co-cultured with 5T4+ ovarian cancer cell lines and autologous tumor but the higher affinity H8-CAR produced more IFNγ and IL-2 across the majority of donors. Furthermore, IFNγ production correlated with the level of expression of 5T4 on the surface of the tumor cells, but not with the number of 5T4+ tumor cells. Previous work comparing higher and lower affinity CAR constructs demonstrated that a higher affinity scFv (2.48 nm KD) targeting the tumor antigen folate receptor β resulted in significantly enhanced cytokine secretion in vitro, activation marker expression, cell lysis, and in vivo antileukemic activity compared with the use of a lower affinity scFv (54.3 nm KD).12 Our results largely agree with Lynn et al,12 in that the higher affinity CAR resulted in greater levels of cytokine secretion and a better therapeutic effect in vivo. However, the specific role of scFv affinity remains to be fully understood. Early studies suggested that high-affinity scFv could enable CAR T cells to recognize cells with high and low levels of target expression.44 However, the relative effect of extracellular spacer regions, costimulatory domains, and the specific properties of the target antigen itself is not understood, meaning that there is currently a lack of generic rules to guide CAR engineering. Hence, empirical testing of individual CAR constructs remains critical to add to the body of knowledge in the field.
Having demonstrated that T cells from ovarian cancer patients could successfully be transduced with 5T4 CAR constructs and were reactive against their autologous tumors, we set out to test the efficacy of the 5T4 CAR T cells in models of ovarian cancer (SKOV-3 and OVCAR-3) in immunodeficient NSG mice. In the SKOV-3 model, the high-affinity H8-CAR T cells were more effective than the lower affinity 2E4-CAR T cells but this was not duplicated in the OVCAR-3 model.
In the assessment of efficacy in an in vivo model of ovarian cancer, it was demonstrated that as few as 105 CAR T cells delivered via the IV route were able to mediate significant antitumor effects; this corresponds to a human dose of 4×106 CAR T cells/kg. Although doses of <105 CAR+ T cells delivered IV did not result in significant therapeutic benefit, a dose of 2.6×104 CAR+ T cell did deliver a significant survival advantage when delivered via the intraperitoneal route. Therefore, in patients with ovarian cancer, delivery of CAR T cells via the intraperitoneal route may potentially be more efficacious and this warrants further investigation.
Several trials of CAR T-cell therapy are underway in ovarian cancer identifying the unmet need for novel therapeutic strategies in this disease setting.45,46 Our study suggests that 5T4 is a valid target in ovarian cancer. Furthermore, we have shown that polyclonal lymphocytes isolated from the peripheral blood of patients with ovarian cancer, can be redirected to effectively target tumor cells expressing 5T4. Taken together, our data supports further investigation of a 5T4 CAR T-cell approach in a clinical trial setting.
CONFLICTS OF INTERESTS/FINANCIAL DISCLOSURES
Supported by Oxford BioMedica.
D.B., R.H., G.K., and Y.L.: wish to disclose that they are employees of Oxford BioMedica, the developer of CAR T 5T4. D.E.G.: is a founder and shareholder of Cellular Therapeutics Ltd.
The remaining authors have declared that there are no financial conflicts of interest with regard to this work.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
Footnotes
G.L.O., V.E.S., and M.K. contributed equally.
REFERENCES
- 1.Zhang C, Liu J, Zhong JF, et al. Engineering CAR-T cells. Biomark Res. 2017;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil DN, Smith EL, Brentjens RJ, et al. The future of cancer treatment: immunomodulation, CARs, and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whilding LM, Maher J. CAR T-cell immunotherapy: the path from the by-road to the freeway? Mol Oncol. 2015;9:1994–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei G, Ding L, Wang J, et al. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp Hematol Oncol. 2017;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geyer MB, Brentjens RJ. Review: current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy. 2016;18:1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat Med. 2016;22:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilham DE, Debets R, Pule M, et al. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012;18:377–384. [DOI] [PubMed] [Google Scholar]
- 8.Yong CSM, Dardalhon V, Devaud C, et al. CAR T-cell therapy of solid tumors. Immunol Cell Biol. 2017;95:356–363. [DOI] [PubMed] [Google Scholar]
- 9.Finney HM, Lawson AD, Bebbington CR, et al. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 10.Hombach A, Wieczarkowiecz A, Marquardt T, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–6131. [DOI] [PubMed] [Google Scholar]
- 11.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynn RC, Feng Y, Schutsky K, et al. High-affinity FRbeta-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Jiang S, Fang C, et al. Affinity-Tuned ErbB2 or EGFR chimeric antigen receptor t cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso HG, Hurton LV, Najjar A, et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75:3505–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern PL, Harrop R. 5T4 oncofoetal antigen: an attractive target for immune intervention in cancer. Cancer Immunol Immunother. 2017;66:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers KA, Rahi-Saund V, Davison MD, et al. Isolation of a cDNA encoding 5T4 oncofetal trophoblast glycoprotein. An antigen associated with metastasis contains leucine-rich repeats. J Biol Chem. 1994;269:9319–9324. [PubMed] [Google Scholar]
- 17.Carsberg CJ, Myers KA, Evans GS, et al. Metastasis-associated 5T4 oncofoetal antigen is concentrated at microvillus projections of the plasma membrane. J Cell Sci. 1995;108(pt 8):2905–2916. [DOI] [PubMed] [Google Scholar]
- 18.Carsberg CJ, Myers KA, Stern PL. Metastasis-associated 5T4 antigen disrupts cell-cell contacts and induces cellular motility in epithelial cells. Int J Cancer. 1996;68:84–92. [DOI] [PubMed] [Google Scholar]
- 19.Awan A, Lucic MR, Shaw DM, et al. 5T4 interacts with TIP-2/GIPC, a PDZ protein, with implications for metastasis. Biochem Biophys Res Commun. 2002;290:1030–1036. [DOI] [PubMed] [Google Scholar]
- 20.Starzynska T, Rahi V, Stern PL. The expression of 5T4 antigen in colorectal and gastric carcinoma. Br J Cancer. 1992;66:867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzynska T, Marsh PJ, Schofield PF, et al. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69:899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starzynska T, Wiechowska-Kozlowska A, Marlicz K, et al. 5T4 oncofetal antigen in gastric carcinoma and its clinical significance. Eur J Gastroenterol Hepatol. 1998;10:479–484. [DOI] [PubMed] [Google Scholar]
- 23.Wrigley E, McGown AT, Rennison J, et al. 5T4 oncofetal antigen expression in ovarian carcinoma. Int J Gynecol Cancer. 1995;5:269–274. [DOI] [PubMed] [Google Scholar]
- 24.Naganuma H, Kono K, Mori Y, et al. Oncofetal antigen 5T4 expression as a prognostic factor in patients with gastric cancer. Anticancer Res. 2002;22(2B):1033–1038. [PubMed] [Google Scholar]
- 25.CRUK. 2014 29th October 2015. Ovarian Cancer Statistics. Available at: www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer. Accessed October 29, 2015.
- 26.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. [DOI] [PubMed] [Google Scholar]
- 28.Hwang WT, Adams SF, Tahirovic E, et al. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garzetti GG, Cignitti M, Ciavattini A, et al. Natural killer cell activity and progression-free survival in ovarian cancer. Gynecol Obstet Invest. 1993;35:118–120. [DOI] [PubMed] [Google Scholar]
- 30.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. [DOI] [PubMed] [Google Scholar]
- 31.Kershaw MH, Westwood JA, Hwu P. Dual-specific T cells combine proliferation and antitumor activity. Nat Biotechnol. 2002;20:1221–1227. [DOI] [PubMed] [Google Scholar]
- 32.Hwu P, Yang JC, Cowherd R, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Res. 1995;55:3369–3373. [PubMed] [Google Scholar]
- 33.Parker LL, Do MT, Westwood JA, et al. Expansion and characterization of T cells transduced with a chimeric receptor against ovarian cancer. Hum Gene Ther. 2000;11:2377–2387. [DOI] [PubMed] [Google Scholar]
- 34.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20pt 1):6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leake R, Barnes D, Pinder S, et al. Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J Clin Pathol. 2000;53:634–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldan V, Griffiths R, Hawkins RE, et al. Efficient and reproducible generation of tumour-infiltrating lymphocytes for renal cell carcinoma. Br J Cancer. 2015;112:1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol. 2013;31:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Kurlander RJ. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: differing impact on CD8 T cell phenotype and responsiveness to restimulation. J Transl Med. 2010;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffiths RW, Gilham DE, Dangoor A, et al. Expression of the 5T4 oncofoetal antigen in renal cell carcinoma: a potential target for T-cell-based immunotherapy. Br J Cancer. 2005;93:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guest RD, Hawkins RE, Kirillova N, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28:203–211. [DOI] [PubMed] [Google Scholar]
- 44.Chmielewski M, Hombach A, Heuser C, et al. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004;173:7647–7653. [DOI] [PubMed] [Google Scholar]
- 45.Koneru M, O'Cearbhaill R, Pendharkar S, et al. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandalaft LE, Powell DJ, Jr, Coukos G. A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med. 2012;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.


