ABSTRACT
Collagens are the most abundant fibrous protein in the human body and constitute the main structural element of the extracellular matrix. It provides mechanical and physiological support for cells. In the pancreas, collagen VI content is more than double that of collagen I or IV. It is a major component of the islet-exocrine interface and could be involved in islet-cell survival. To test the impact of collagen VI on human encapsulated pancreatic islets-cells, we tested the effects of exogenous collagen type VI on in vitro functional survival of alginate encapsulated human islet-cells. Concentrations tested ranged from 0.1 to 50 µg/ml. Islets in capsules without collagen type VI served as control. Islet-cell interaction with collagen type VI at concentrations of 0.1 and 10 µg/ml, promoted islet-cell viability (p<0.05). Although no improvement in glucose induced insulin secretion (GSIS) was observed, islets in capsules without incorporation of collagen type VI showed more dysfunction and oxygen consumption rates was improved by inclusion of collagen type VI. Our results demonstrate that incorporation of collagen type VI in immunoisolated human islets supports in vitro viability and survival of human pancreatic islets.
KEYWORDS: alginate capsules, collagen, glucose induced insulin secretion, oxygen consumption rate, pancreatic islets, viability
Introduction
Islet transplantation is a promising method to cure patients with type I diabetes. Transplantation of allogeneic pancreatic islets can theoretically regulate glucose levels from a minute-to-minute level,1,2 preventing the development of hypoglycemia and diabetic complications.3 An advantage of implanting pancreatic islets over transplantation of the whole pancreas is that isolated islets can be modulated before transplantation to reduce the risk of graft rejection.4,5 Moreover, islet transplantation is a minimal invasive surgical procedure with short hospitalization periods and can be repeated in cases of graft failure with minor adverse effects.1,6 Isolated islets can also be enveloped in immunoisolating capsules that are impermeable to immunoglobulins and cells of the immune system but allow diffusion of nutrients, glucose, and insulin.6-8 This could potentially lead to avoidance of the need to administer immunosuppressive drugs, which cause unwanted side-effects.9,10 This technology of immunoisolation is subject of intense research, as it would make islet-transplantation available for a larger group of patients with type I diabetes.6,11
The proof of principle application of encapsulated islets for the treatment of type I diabetes has been shown in several studies in both experimental animals and in humans,12,13 however, islet graft survival is still never permanent and in most cases limited to several months.14 A factor considered to influence the duration of graft survival is the loss of interaction with the extracellular matrix (ECM) in isolated islets.2,15-17 As a consequence of islet-isolation, the ECM molecules that surround the islets and interconnect the endocrine cells in the pancreatic islets are damaged by application of collagenases.2,16,18,19 This enzymatical isolation process impacts laminins as well as collagen type I, III, IV, V and VI in islets.20 Moreover, recently it has been shown that after isolation with ECM-degrading collagenases, the whole microvasculature of the islet is destroyed,20 and that pancreatic islet-cells undergo different cell death processes such as anoikis, necroptosis, and necrosis.18,19,21,22
A strategy of supplementation of islets with ECM molecules has been shown to enhance functional survival of encapsulated pancreatic islets.23-25 Incorporation of ECM guides cellular development by mimicking the biochemical composition of ECM in the target organ, its fibrillar structure, and its viscoelastic properties.26 Examples of molecules that are considered to enhance survival of islets are laminins and collagens.27 Laminin may benefit islet-survival by modulating the expression of specific transcription factors and hormones such as Pdx-1, insulin 1 and insulin 2, glucagon, somatostatin, and GLUT-2.28 Moreover, different adhesive laminin sequences such as IKVAV, VAYI, and IKLLI, YIGSR, PDSGR, located in α1 and β1chain have been shown to influence pancreatic islet-cell function.29,30
The most essential collagens used as supplement or adjuvant for cellular functions in biomedical applications are collagens type I and IV.31,32 Collagen is present and located in the islet–exocrine interface and basement membrane of islets where it regulates fibronectin assembly by restraining cell–fibronectin interactions.33-35 In contrast to collagen type IV, effects of collagen type VI on islet-survival and function has not been studied. Collagen type VI is composed of three polypeptide chains that form a short triple helix. These polypeptide chains are named α1 (VI), α2 (VI), and α3 (VI), and are encoded by three genes.36 The importance of collagen type VI for tissue homeostasis is illustrated by the observation that a deficiency of collagen type VI results in death of muscle cells causing muscular dystrophy.37 Since collagen type VI is abundantly present in the pancreas,38,39 we investigated the effect of exogenous collagen VI on islet-cell survival and function.
The goal of this study was to investigate the effect of collagen type VI on the functional survival of human pancreatic islets in immunisolating alginate-based capsules. Functional survival of islets was studied by determining the glucose stimulated insulin secretion (GSIS) and the oxygen consumption rate (OCR) in islets encapsulated in alginate with or without collagen VI.
Materials and methods
Human islets culture
Human islets were obtained from Prodo Laboratories Inc. (Irvine, USA). A dithizone (Merck, USA) staining was performed before shipment to determine the purity. Islets with a purity over 80% were shipped to the Groningen University Medical Center (Groningen, The Netherlands). Immediately after arrival, islets were washed before handpicking and cultured in CMRL medium (Gibco, USA), supplemented with 10% fetal calf serum (FCS), 1% penicillin/streptomycin (Gibco, USA), 1% glutamax (Gibco, USA), 2% HEPES (Gibco, USA), and 8.3 mM D-glucose (Sigma-Aldrich, USA. Before encapsulation, islets were both micro- and macroscopically inspected. After encapsulation islets were tested for glucose stimulated insulin secretion (GSIS) and subjected to live-dead staining kit for mammalian cells to confirm viability and function. Human islets were cultured in a humidified 5% CO2 incubator at 37°C.
Microencapsulation
Capsules were composed of purified 3.4% intermediate-G alginate (44% G-chains, 56% M-chains, 23% GG-chains, 21% GM-chains, 37% MM-chains), mixed with collagen type VI (Abcam, UK) by physical entrapment in concentrations of 0.1, 10, and 50 µg/ml. Capsules without type VI collagen served as controls. An air-driven droplet generator was used for the encapsulation of human islets.40,41 Before encapsulation, per donor, islets were split up in different appropriate portions and mixed with 3.4% ultrapure alginate containing either 0.1, 10, or 50 µg/ml collagen VI. The alginate was purified as previously described42 and tested for presence of endotoxins.43,44 Islets were mixed in a ratio of 1000 islets/ml alginate and transferred into droplets45,46 and collected in 100 mM CaCl2. Droplets were gelled in 100 mM CaCl2 solution for at least 10 minutes.47 The diameters of the droplets were between 500–650 µm. All droplets were washed with KRH buffer containing 2.5 mM CaCl2 for 2 minutes. Subsequently, encapsulated islets were cultured in CMRL 1066 (Gibco, USA) supplemented with 8.3 mM D-glucose, penicillin/streptomycin (1%) (Gibco, USA), and 10% fetal calf serum (FCS) (Gibco, USA) at 37 °C, 5% CO2 till further use.48-50
Glucose-stimulated insulin secretion of encapsulated islets
Human pancreatic islets were tested for GSIS at days 3, 5, and 7. The production of insulin was measured in response to low and high glucose solutions. Twenty-five encapsulated islets were transferred to glass incubation tubes. The first incubation consisted of a low glucose concentration solution in KRH (2.75 mM) for 1 hour, followed by a high glucose concentration buffer in KRH (16.7 mM) for 1 hour, and another 1-hour incubation in 2.75 mM glucose in KRH. At the end of each incubation, the incubation media were removed and frozen for insulin measurement via Enzyme-Linked Immunosorbent Assay (ELISA) (Mercodia AB, Sweden) using a spectrophotometric plate reader as previously described.41 Finally, insulin concentrations were calculated through the interpolation of sample absorbance values from the standard curves.
DNA content of islets was quantified with a fluorescent Quant-iT PicoGreen double-strand DNA (dsDNA) assay kit (Invitrogen, United States). The insulin secretory responses were expressed as a nanogram of insulin.ml−1. μDNA−1. hour−1.
Live/dead cell viability assay
Viability of the encapsulated islets was tested using a LIVE/DEAD Cell Viability/Cytotoxicity Assay Kit (Thermo Scientific). Encapsulated islets were incubated in culture media with Calcein AM (1 mM) and Ethidium homodimer (EthD) (2 mM) (concentration according to manual) for 30 minutes in the dark. After the incubation period, islets were washed with 25 mM KRH buffer, pH 7.4 prior to imaging.
A helium-neon and an argon-krypton laser were used in combination with a confocal microscope (Leica) to quantify islet-cell viability at days 3, 5, and 7. The data was analyzed in ImageJ (v1.48b; National Institutes of Health), and viable or dead cells was expressed as the percentage the total number of stained cells. Imaris 664 version 7.6.4 software was applied.45
Islet oxygen consumption
The oxygen consumption rate (OCR) was measured using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience, USA).51 This experiment was performed at 72 hours after encapsulation and for one-time point only as the assay requires the use of high numbers of islets. To perform OCR, human islets were retrieved from the capsules by dissolving the capsules in 25 mM citrate solution at 37°C. The islets were transferred to 6-well plates, and fresh medium was added before putting the plate at culture conditions. After that, human islets were transferred to the microplate reader provided with the XF24 Islet FluxPak (Seahorse Bioscience, USA). For the correct measurement, at least 80 islets were inserted in every well of the XF24 islet capture microplate. Experiments were performed in triplo. The islets were centered in the wells and a screen was fixed upon each islet-containing well. The medium was removed and the islets were washed 3 times with MA medium (XF assay media) and incubated for 60 minutes at 37°C. After addition of 500 µl of MA medium, the plate was placed into the XF24 Extracellular Flux Analyzer. At the end of the experiment, islets were transferred to Eppendorf cups and stored at −20°C for DNA content analysis. OCR data was normalized for DNA content to allow for comparison of different experimental conditions and adjust for the variation in islets numbers. Oxygen consumption rate was expressed as minutes OCR/DNA (pmol O2·min–1·mg–1 DNA) and analyzed by Seahorse XF24 software. An initial drift in OCR was typically observed in the first 1–2 measurements. Analysis started at an interval of 5–8 minutes after steady state was reached.
Statistical analysis
Results were expressed as a mean ± standard error of the mean (SEM). A Two-way ANOVA followed by Tukey's secondary tests for significance was used for GSIS, stimulation index and live-dead staining data. Paired t-test was applied in order to evaluate the OCR. The analysis was performed using GraphPad Prism 5.0 (GraphPad Software, USA). A value of p < 0.05 was considered statistically significant.
Results
Effect of collagen type VI on GSIS on encapsulated human islets
We investigated the effect of collagen type VI on islet-cell functionality and survival, as collagen type VI is an ECM component and basement membrane (BM) anchoring molecule in human islets.35,52 To this end, collagen VI was incorporated into islet-containing alginate microcapsules. We tested the effects of 0.1, 10, and 50 µg/ml on GSIS of human islets encapsulated in alginate at days 3, 5, and 7. The GSIS data for encapsulated islets with and without collagen type VI is shown in Fig. 1. GSIS was not statistically influenced by collagen type VI addition, but there was a trend toward higher GSIS in encapsulated islets with 10 μg/ml and 50 μg/ml at day 7.
Figure 1.
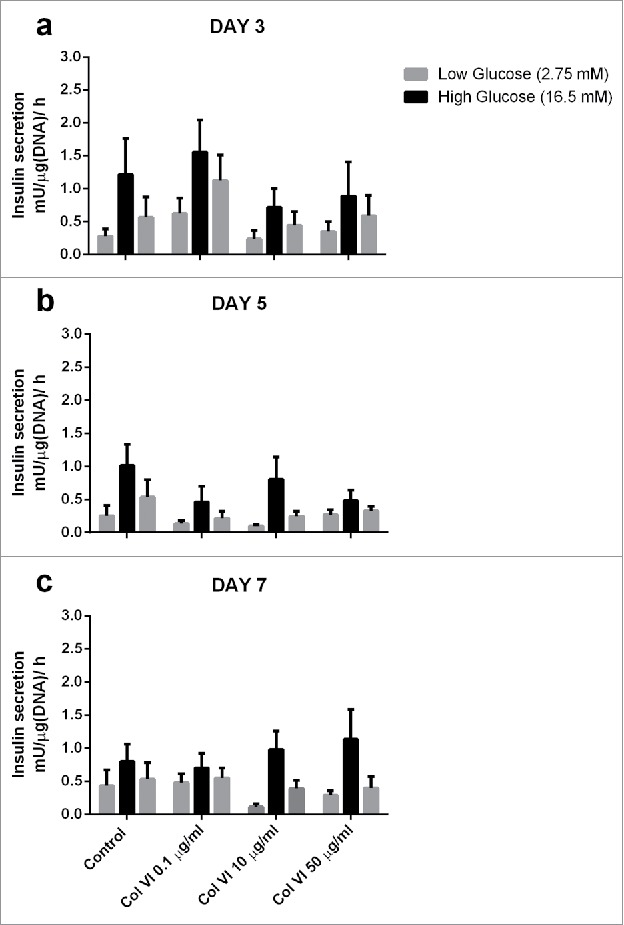
Glucose induced insulin secretion (GSIS) of human islets encapsulated in alginate-based capsules supplemented with collagen VI. Concentrations of 0.1, 10 and 50 µg/ml collagen VI tested at day 3 (a), 5 (b) and 7 (c). The bars from left to right indicate insulin production upon incubation with low (2.75 mM), high (16.7 mM), and low glucose solution of sets of 25 pancreatic human islets for each condition. Values represent mean ± SEM (n = 4, different donors).
Interestingly, stimulation index of pancreatic islets entrapped with 10 µg/ml collagen type VI was significantly higher (p < 0.05) than that of control groups (Fig. 2). The glucose stimulation index significantly increased 1.8 and 2.3 time-fold at day 5 and 7 for islets encapsulated in alginate with 10 µg/ml collagen type VI (p < 0.05).
Figure 2.
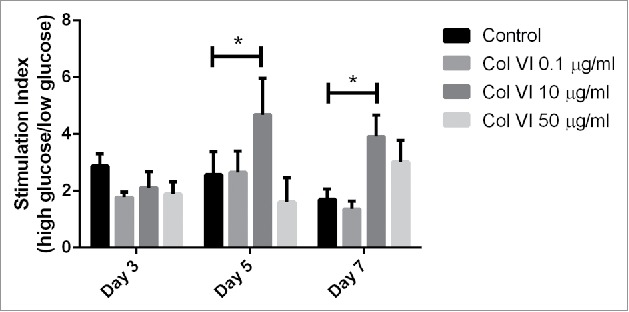
The figure shows the stimulation index (SI) of encapsulated islet in response to low (2.75 mM), and high (16.7 mM) glucose. Results from human islets encapsulated in alginate-based capsules supplemented with collagen VI at concentrations of 0.1, 10 and 50 µg/ml collagen VI tested at day 3, 5 and 7. Error bars represent standard error. Statistical significance is indicated by stars (p < 0.05).
Collagen type VI improves cell viability of human islets
Despite the absence of statistically significant effects on GSIS, we did find significant effects of collagen type VI incorporation on the viability of encapsulated pancreatic islets, as shown in Fig. 3b. From day 3 of culture and onwards, cell-survival enhancing effects of collagen type VI were observed. At day 5, all the tested groups containing collagen type VI had strong and statistically significant effects on islet-cell viability (p < 0.01). The percentage of surviving cells was 87.7 ± 6.1%, 86.5 ± 2.4% and 85.6 ± 1.9% (p < 0.01), when collagen type VI at 0.1, 10 and 50 µg/ml were added to the alginate matrix respectively. Moreover, a significant increase of 11% on islet viability was observed in encapsulated islets entrapped with 10 μg/ml collagen type VI at day 7. Although, no differences between higher collagen VI concentrations at any time-point was observed, in general, the strongest and most persistent effects were observed with encapsulated islets containing 10 µg/ml collagen type VI (p < 0.05).
Figure 3.
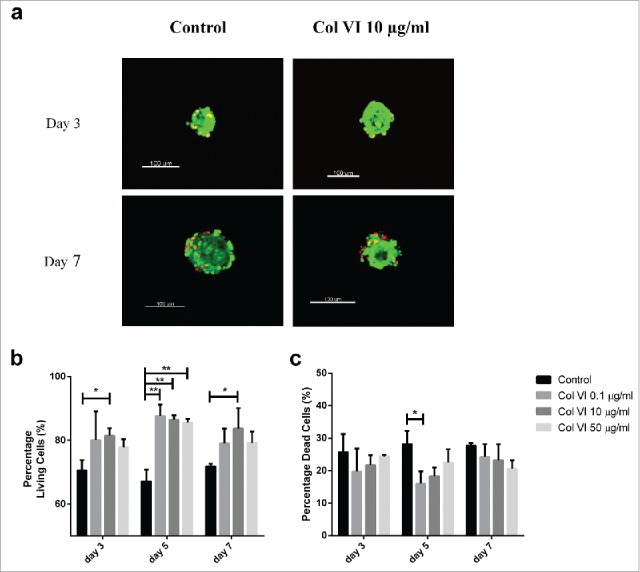
Live/dead staining of human islet-cells encapsulated in alginate-based capsules containing either 0.1, 10, and 50 µg/ml collagen type VI. Illustration of islets in control condition (in alginate capsule without collagen), and a capsule containing 10 µg/ml collagen VI (a). Islet-cell viability was enhanced by inclusion of collagen VI in the immunoprotective capsule at all-time points tested (b). Encapsulated human islets were stained with ethidium homodimer-1 to quantify the percentage of dead cells at days 3, 5, and 7 of culture (c). Alginate capsules without ECM components served as control. Values represent mean ± SEM (n = 4, different donors). *, and ** indicates statistical significant differences (p < 0.05), and (p < 0.01) when compared to control islets respectively (Col VI, collagen type VI).
Interestingly, islet-containing capsules with collagen type VI showed a diminished cell death trend compared to controls during the culture days (Fig. 3c). Although, not statistically significant at day 3, all tested groups containing collagen type VI demonstrated a trend to inhibition of cell death. At day 5, pancreatic islets entrapped with 0.1 µg/ml collagen type VI have shown a 16 ± 7.6% on death cells rate, presenting a decrease of 12.2% when compared to capsules without collagen type VI (p < 0.05).
Oxygen consumption rate analysis
The effect of collagen VI on oxygen consumption rate (OCR) of islet-cells was tested only at 72h of culture because we needed to use relatively high amounts of islets. The addition of collagen type VI does enhance OCR but this only reached statistically significant differences when applying 0.1 μg/ml (p < 0.01) and 10 μg/ml (p < 0.05). The oxygen consumption rate gives an indication of the viability and functionality of the islets and correlates with graft survival after implantation.53,54 As shown in Fig. 4, islets encapsulated in 0.1 µg/ml collagen type VI had an OCR of 769.07 ± 82.9 pmol O2·min–1·mg–1 DNA which was nearly two-fold higher than the OCR of the control group (p < 0.01). OCR of islets entrapped with 10 μg/ml collagen type VI was 1.5 fold higher than the control group (p < 0.05). Additionally, there was a trend towards higher OCR using higher concentrations of collagen type VI.
Figure 4.
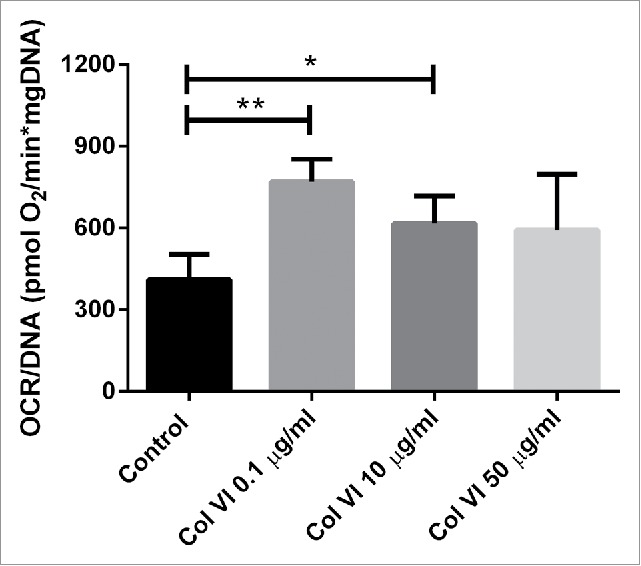
Oxygen consumption rate (OCR) of human islets encapsulated in alginate capsules containing a combination of 0.1, 10 or 50 µg/ml after 72 hours in culture. OCR was expressed after correction for DNA content (OCR/DNA). Values represent mean ± SEM. *, and ** indicates statistical significant differences (p < 0.05), and (p < 0.01) when compared to control islets respectively (n = 4, different donors).
Discussion
Various ECM molecules have shown to enhance the survival of islets. Examples of these molecules are laminin and several collagens.27 Collagens type I and type IV have been studied extensively for their role in enhancing survival of pancreatic islet-cells2,55 but collagen type VI has not been studied yet. Collagen type VI is expressed in human connective tissues, and in some specialized regions such as the pancreas islet–exocrine interface,35 but its presence in the basement membrane is less recognized.52 Just like other collagen types in islets, collagen VI is structurally damaged and digested during the enzymatic isolation process of islets from the pancreas.20 To the best of our knowledge, this study is the first to demonstrate the beneficial effects of collagen type VI on functional survival of human islets. Collagen type VI can provide advantages over other types of ECM, as collagen type VI is the predominant constituent subtype immediately surrounding islets in the pancreas.38 Previous studies have shown that collagen type VI is present in the peri-islet capsule in the porcine pancreas,33 whereas collagen type I, III, and IV are expressed predominantly in the peri-islet region.56
Although not statistical significant, effects on GSIS were found. We observed significant survival enhancing effect of up to 20% when collagen type VI was incorporated in the alginate-based capsules. In addition, beneficial, enhancing effects were observed on OCR. The latter is an important measure for viability after implantation as higher OCR values correlate with higher graft survival rates after implantation.54 OCR represents the metabolic responsiveness of the islets, and correlates better with functionality of the graft than GSIS.54 Notably, our results are obtained with islet-preparations with minimal donor-variations in viability. Results may be different with islet-sources of lower quality or preparations where larger donor-variability is present. In all cases however collagen VI may enhance viability.
Higher concentrations of collagen type VI were associated with a negative impact on survival of islet-cells. A similar concentration dependent effect on islet-OCR has been reported for collagen type IV.2 This can be explained by the fact that supraphysiological collagen concentrations diminish metabolic capacity such as insulin release.2,23,57 Negative effects of too high collagen concentrations have also been reported in cancer studies. Tumor-promoting effects of supraphysiological high concentrations of collagen type VI were observed,58 suggesting that increased concentrations enhance cell survival at the expense of cell functioning. Thus, increasing the concentration of type VI collagen beyond the tissue-concentration could induce toxicity and negatively impact islet-cell survival.2,57-59 The effect of collagen VI on islet-cell survival is different from that of collagen IV. Collagen IV impacted both GSIS and viability while collagen VI only impacts viability. Collagen IV enhanced GSIS in a concentration dependent fashion and was most effective at a concentration of 50 μg/ml.2,25 The positive effect on viability was similar for the two collagen types.
We recently have shown that inclusion of ECM is an efficacious strategy to promote islet-cell survival after isolation of human islets from the pancreas.2,57 Here we have focused on the efficacy of collagen type VI. We show it has a positive impact on islet-cell viability. It did not influence GSIS but did enhance cell-survival and OCR. Moreover, as collagen VI specifically stimulates the cell death inhibitor β1 integrin it is probably this interaction that is partly responsible for the beneficial effect of collagen VI on islet-cell survival. However, as ECM act in concert with other collagens and laminins it might also be that addition of collagen VI to the intracapsular environment contributes to re-assembly of the ECM islet-network and by that contributes to prevention of cell death. A third explanation is that islet-isolation is associated with release of deleterious cytokines that damage islet-cells in an auto- or paracrine fashion.18 Addition of ECM might reduce cytokine susceptibility of islets57 and thereby contribute to better survival of islet-cells.57 Which ECM components is most effective in supporting these beneficial effects is still subject of investigation.2,57,59 In previous studies, we have shown that the interaction and beneficial effects of ECM of islets is highly ECM dependent and even concentration dependent.2,57 Our current study extends these investigations and show that collagen VI contributes to survival of islet-cells after isolation from the pancreas.
Conclusions
Not all but only a few ECM molecules support function and survival of human pancreatic islet-cells in alginate-based microcapsules applied for immunoprotection of islets.2 Here we show that collagen VI is one of these survival promoting ECM molecules. Incorporation of 0.1 to 10 µg/ml collagen type VI in the alginate-capsule microenvironment promotes islet-cell viability. The present data underscore that the intracapsular environment should receive more attention in effort to support longevity of encapsulated islet.
Funding Statement
The authors gratefully acknowledge the financial support of Erasmus Mundus Lindo (scholarship ML12FD0331) and the Juvenile Diabetes Research Foundation (Grant No. 2013–2953).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.de Vos P, Spasojevic M, Faas MM. Treatment of diabetes with encapsulated islets. Adv Exp Med Biol. 2010;670:38–53. doi: 10.1007/978-1-4419-5786-3_5 [DOI] [PubMed] [Google Scholar]
- 2.Llacua A, de Haan BJ, Smink SA, de Vos P. Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J Biomed Mater Res A. 2016;104:1788–96. doi: 10.1002/jbm.a.35706 [DOI] [PubMed] [Google Scholar]
- 3.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–17. doi: 10.1016/j.biomaterials.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 4.Noguchi H, Miyagi-Shiohira C, Kurima K, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M. Islet Culture/Preservation Before Islet Transplantation. Cell Med. 2015;8:25–9. doi: 10.3727/215517915X689047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noguchi H, Naziruddin B, Jackson A, Shimoda M, Ikemoto T, Fujita Y, Chujo D, Takita M, Kobayashi N, Onaca N, et al.. Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation. Transplantation. 2010;89:47–54. doi: 10.1097/TP.0b013e3181be3bf2 [DOI] [PubMed] [Google Scholar]
- 6.de Vos P, Bucko M, Gemeiner P, Navratil M, Svitel J, Faas M, Strand BL, Skjak-Braek G, Morch YA, Vikartovská A, et al.. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials. 2009;30:2559–70. doi: 10.1016/j.biomaterials.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 7.Paredes Juarez GA, Spasojevic M, Faas MM, de Vos P. Immunological and technical considerations in application of alginate-based microencapsulation systems. Front Bioeng Biotechnol. 2014;2:26. doi: 10.3389/fbioe.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos P, van Hoogmoed CG, van Zanten J, Netter S, Strubbe JH, Busscher HJ. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials. 2003;24:305–12. doi: 10.1016/S0142-9612(02)00319-8 [DOI] [PubMed] [Google Scholar]
- 9.Bottino R, Trucco M. Clinical implementation of islet transplantation: A current assessment. Pediatr Diabetes. 2015;16:393–401. doi: 10.1111/pedi.12287 [DOI] [PubMed] [Google Scholar]
- 10.Vaithilingam V, Tuch BE. Islet transplantation and encapsulation: an update on recent developments. Rev Diabet Stud. 2011;8:51–67. doi: 10.1900/RDS.2011.8.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2014;67–68:35–73. doi: 10.1016/j.addr.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 12.Bhujbal SV, Paredes-Juarez GA, Niclou SP, de Vos P. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J Mech Behav Biomed Mater. 2014;37:196–208. doi: 10.1016/j.jmbbm.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 13.Kollmer M, Appel AA, Somo SI, Brey EM. Long-Term Function of Alginate-Encapsulated Islets. Tissue Eng Part B Rev. 2015;22(Issue):34–46. [DOI] [PubMed] [Google Scholar]
- 14.de Vos P, de Haan BJ, de Haan A, van Zanten J, Faas MM. Factors influencing functional survival of microencapsulated islet grafts. Cell Transplant. 2004;13:515–24. doi: 10.3727/000000004783983738 [DOI] [PubMed] [Google Scholar]
- 15.Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, Fontaine MJ. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012;33:6691–7. doi: 10.1016/j.biomaterials.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. The J Endocrinol. 1999;163:181–90. doi: 10.1677/joe.0.1630181 [DOI] [PubMed] [Google Scholar]
- 17.Lucas-Clerc C, Massart C, Campion JP, Launois B, Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9–20. doi: 10.1016/0303-7207(93)90046-M [DOI] [PubMed] [Google Scholar]
- 18.de Vos P, Smink AM, Paredes G, Lakey JR, Kuipers J, Giepmans BN, de Haan BJ, Faas MM. Enzymes for Pancreatic Islet Isolation Impact Chemokine-Production and Polarization of Insulin-Producing beta-Cells with Reduced Functional Survival of Immunoisolated Rat Islet-Allografts as a Consequence. PloS One. 2016;11:e0147992. doi: 10.1371/journal.pone.0147992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irving-Rodgers HF, Choong FJ, Hummitzsch K, Parish CR, Rodgers RJ, Simeonovic CJ. Pancreatic islet basement membrane loss and remodeling after mouse islet isolation and transplantation: impact for allograft rejection. Cell Transplant. 2014;23:59–72. doi: 10.3727/096368912X659880 [DOI] [PubMed] [Google Scholar]
- 20.Shima H, Inagaki A, Imura T, Yamagata Y, Watanabe K, Igarashi K, Goto M, Murayama K. Collagen V Is a Potential Substrate for Clostridial Collagenase G in Pancreatic Islet Isolation. J Diabetes Res. 2016;2016:4396756. doi: 10.1155/2016/4396756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg L, Wang R, Paraskevas S, Maysinger D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126:393–8. doi: 10.1016/S0039-6060(99)70183-2 [DOI] [PubMed] [Google Scholar]
- 22.Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis. 1999;20:749–55. doi: 10.1093/carcin/20.5.749 [DOI] [PubMed] [Google Scholar]
- 23.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng JY, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue Eng Part B Rev. 2011;17:235–47. doi: 10.1089/ten.teb.2011.0004 [DOI] [PubMed] [Google Scholar]
- 25.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14:1959–68. doi: 10.1089/ten.tea.2007.0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14:551–8. doi: 10.1016/j.copbio.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 27.Huang G, Greenspan DS. ECM roles in the function of metabolic tissues. Trends Endocrinol Metab. 2012;23:16–22. doi: 10.1016/j.tem.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leite AR, Correa-Giannella ML, Dagli ML, Fortes MA, Vegas VM, Giannella-Neto D. Fibronectin and laminin induce expression of islet cell markers in hepatic oval cells in culture. Cell Tissue Res. 2007;327:529–37. doi: 10.1007/s00441-006-0340-z [DOI] [PubMed] [Google Scholar]
- 29.Nomizu M, Kuratomi Y, Malinda KM, Song SY, Miyoshi K, Otaka A, Powell SK, Hoffman MP, Kleinman HK, Yamada Y. Cell binding sequences in mouse laminin alpha1 chain. J Biol Chem. 1998;273:32491–9. doi: 10.1074/jbc.273.49.32491 [DOI] [PubMed] [Google Scholar]
- 30.Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004–11. doi: 10.1016/j.biomaterials.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 31.Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, et al.. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem. 2004;279:53762–9. doi: 10.1074/jbc.M411202200 [DOI] [PubMed] [Google Scholar]
- 33.White SA, Hughes DP, Contractor HH, London NJ. An investigation into the distribution of different collagen types within adult and juvenile porcine pancreata. J Mol Med. 1999;77:79–82. doi: 10.1007/s001090050306 [DOI] [PubMed] [Google Scholar]
- 34.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab. 2008;10(Suppl 4):119–27. doi: 10.1111/j.1463-1326.2008.00955.x [DOI] [PubMed] [Google Scholar]
- 35.Hughes SJ, Clark A, McShane P, Contractor HH, Gray DW, Johnson PR. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81:423–6. doi: 10.1097/01.tp.0000197482.91227.df [DOI] [PubMed] [Google Scholar]
- 36.Chu ML, Zhang RZ, Pan TC, Stokes D, Conway D, Kuo HJ, Glanville R, Mayer U, Mann K, Deutzmann R, et al.. Mosaic structure of globular domains in the human type VI collagen alpha 3 chain: similarity to von Willebrand factor, fibronectin, actin, salivary proteins and aprotinin type protease inhibitors. EMBO J. 1990;9:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernardi P, Bonaldo P. Dysfunction of mitochondria and sarcoplasmic reticulum in the pathogenesis of collagen VI muscular dystrophies. Ann NY Acad Sci. 2008;1147:303–11. doi: 10.1196/annals.1427.009 [DOI] [PubMed] [Google Scholar]
- 38.Hughes SJ, McShane P, Contractor HH, Gray DW, Clark A, Johnson PR. Comparison of the collagen VI content within the islet-exocrine interface of the head, body, and tail regions of the human pancreas. Transplant Proc. 2005;37:3444–5. doi: 10.1016/j.transproceed.2005.09.027 [DOI] [PubMed] [Google Scholar]
- 39.Meyer T, Chodnewska I, Czub S, Hamelmann W, Beutner U, Otto C, Thiede A, Ulrichs K. Extracellular matrix proteins in the porcine pancreas: a structural analysis for directed pancreatic islet isolation. Transplant Proc. 1998;30:354. doi: 10.1016/S0041-1345(97)01302-X [DOI] [PubMed] [Google Scholar]
- 40.Wolters GH, Fritschy WM, Gerrits D, van Schilfgaarde R. A versatile alginate droplet generator applicable for microencapsulation of pancreatic islets. J Appl Biomater. 1991;3:281–6. doi: 10.1002/jab.770030407 [DOI] [PubMed] [Google Scholar]
- 41.de Haan BJ, Faas MM, de Vos P. Factors influencing insulin secretion from encapsulated islets. Cell Transplant. 2003;12:617–25. doi: 10.3727/000000003108747226 [DOI] [PubMed] [Google Scholar]
- 42.Tam SK, de Haan BJ, Faas MM, Halle JP, Yahia L, de Vos P. Adsorption of human immunoglobulin to implantable alginate-poly-L-lysine microcapsules: effect of microcapsule composition. J Biomed Mater Res A. 2009;89:609–15. doi: 10.1002/jbm.a.32002 [DOI] [PubMed] [Google Scholar]
- 43.Dusseault J, Tam SK, Menard M, Polizu S, Jourdan G, Yahia L, Hallé JP. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J Biomed Mater Res A. 2006;76:243–51. doi: 10.1002/jbm.a.30541 [DOI] [PubMed] [Google Scholar]
- 44.Paredes-Juarez G, de Haan B, Faas M, de Vos P. A Technology Platform to Test the Efficacy of Purification of Alginate. Materials. 2014;7:2087. doi: 10.3390/ma7032087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spasojevic M, Paredes-Juarez GA, Vorenkamp J, de Haan BJ, Schouten AJ, de Vos P. Reduction of the inflammatory responses against alginate-poly-L-lysine microcapsules by anti-biofouling surfaces of PEG-b-PLL diblock copolymers. PloS One. 2014;9:e109837. doi: 10.1371/journal.pone.0109837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vos P, De Haan BJ, Van Schilfgaarde R. Upscaling the production of microencapsulated pancreatic islets. Biomaterials. 1997;18:1085–90. doi: 10.1016/S0142-9612(97)00040-9 [DOI] [PubMed] [Google Scholar]
- 47.Klokk TI, Melvik JE. Controlling the size of alginate gel beads by use of a high electrostatic potential. J Microencapsul. 2002;19:415–24. doi: 10.1080/02652040210144234 [DOI] [PubMed] [Google Scholar]
- 48.Holmes MA, Clayton HA, Chadwick DR, Bell PR, London NJ, James RF. Functional studies of rat, porcine, and human pancreatic islets cultured in ten commercially available media. Transplantation. 1995;60:854–60. doi: 10.1097/00007890-199510270-00016 [DOI] [PubMed] [Google Scholar]
- 49.Lee RH, Carter J, Szot GL, Posselt A, Stock P. Human albumin preserves islet mass and function better than whole serum during pretransplantation islet culture. Transplant Proc. 2008;40:384–6. doi: 10.1016/j.transproceed.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 50.Smelt MJ, Faas MM, de Haan BJ, de Vos P. Pancreatic beta-cell purification by altering FAD and NAD(P)H metabolism. Exp Diabetes Res. 2008;2008:165360. doi: 10.1155/2008/165360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malmgren S, Nicholls DG, Taneera J, Bacos K, Koeck T, Tamaddon A, Wibom R, Groop L, Ling C, Mulder H, et al.. Tight coupling between glucose and mitochondrial metabolism in clonal beta-cells is required for robust insulin secretion. J Biol Chem. 2009;284:32395–404. doi: 10.1074/jbc.M109.026708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groulx JF, Gagne D, Benoit YD, Martel D, Basora N, Beaulieu JF. Collagen VI is a basement membrane component that regulates epithelial cell-fibronectin interactions. Matrix Biol. 2011;30:195–206. doi: 10.1016/j.matbio.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 53.Sweet IR, Gilbert M, Scott S, Todorov I, Jensen R, Nair I, Al-Abdullah I, Rawson J, Kandeel F, Ferreri K. Glucose-stimulated increment in oxygen consumption rate as a standardized test of human islet quality. Am J Transplant. 2008;8:183–92. [DOI] [PubMed] [Google Scholar]
- 54.Cornolti R, Figliuzzi M, Remuzzi A. Effect of micro- and macroencapsulation on oxygen consumption by pancreatic islets. Cell Transplant. 2009;18:195–201. doi: 10.3727/096368909788341252 [DOI] [PubMed] [Google Scholar]
- 55.Riopel M, Wang R. Collagen matrix support of pancreatic islet survival and function. Front Biosci. 2014;19:77–90. doi: 10.2741/4196 [DOI] [PubMed] [Google Scholar]
- 56.Meyer T, Czub S, Chodnewska I, Beutner U, Hamelmann W, Klock G, Zimmermann U, Thiede A, Ulrichs K. Expression pattern of extracellular matrix proteins in the pancreas of various domestic pig breeds, the Goettingen Minipig and the Wild Boar. Ann Transplant. 1997;2:17–26. [PubMed] [Google Scholar]
- 57.Llacua LA, de Haan BJ, de Vos P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J Tissue Eng Regen Med. 2018;12:460–7. [DOI] [PubMed] [Google Scholar]
- 58.Chen P, Cescon M, Bonaldo P. Collagen VI in cancer and its biological mechanisms. Trends Mol Med. 2013;19:410–7. doi: 10.1016/j.molmed.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 59.Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018. doi: 10.1007/s00125-017-4524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


