ABSTRACT
It is currently unknown how the islet transcriptional pattern changes as glucose metabolism deteriorates and progresses to fulminant type 2 diabetes (T2D). In this study, we hypothesized that islets from donors with elevated HbA1c levels, but not yet diagnosed with T2D, would show signs of cell stress on a transcriptional level. Laser capture microdissection and qPCR arrays including 330 genes related to mitochondria, oxidative stress, or the unfolded protein response were used to extract and analyze islets from organ donors with HbA1c <5.5% (37 mmol/mol), elevated HbA1c (6.0–6.5% (42–48 mmol/mol)), high HbA1c (>6.5% (48 mmol/mol)) or established T2D. Principal component analysis and hierarchical clustering based on the expression of all 330 genes displayed no obvious separation of the four different donor groups, indicating that the inter-donor variations were larger than the differences between groups. However, 44 genes were differentially expressed (P < 0.05, false discovery rate <30%) between islets from donors with HbA1c <5.5% (37 mmol/mol) compared with islets from T2D subjects. Twelve genes were differentially expressed compared to control islets in both donors with established T2D and donors with elevated HbA1c (6.0–6.5% (42–48 mmol/mol)). Overexpressed genes were related mainly to the unfolded protein response, whereas underexpressed genes were related to mitochondria. Our data on transcriptional changes in human islets retrieved by LCM from high-quality biopsies, as pre-diabetes progresses to established T2D, increase our understanding on how islet stress contributes to the disease development.
KEYWORDS: HbA1c, laser capture, transcriptome, type 2 diabetes
Introduction
In type 2 diabetes (T2D), impaired glucose metabolism develops gradually and overt hyperglycemia is often present for a period before diagnosis.1 Hypersecretion of insulin in the early stages of the disease progression suggests an initial crucial role of insulin resistance in peripheral tissues.1 In later stages, a reduction in insulin secretion is observed,1 leading to fulminant T2D and eventually, in some patients, loss of endogenous insulin production.1,2
Recent studies have compared the transcription profiles of islets isolated by enzymatic digestion from organ donors with T2D and non-diabetic controls3,4 and found a number of genes with significantly different expression regulating vital functions of the endocrine pancreas. However, these studies also demonstrate that the islet isolation procedure and subsequent in vitro culture per se affect the transcriptional profiles.3 Indeed, of the 444 genes identified with significantly different expression in isolated islets from subjects with T2D when compared to non-diabetic subjects, only 19 could be verified to differentiate in laser capture microdissected (LCM) islets from patients with T2D undergoing pancreatectomy.3 Likely, the use of biopsies from patients undergoing pancreatectomy due to malignancies and incurable pancreatitis, i.e. conditions well-known to also affect the endocrine function of non-affected parts of the pancreas,5 also affects the islet transcriptional profile. Therefore, transcriptome analysis of islets extracted by LCM from frozen pancreata of brain dead organ donors with progressive impairments of their glucose metabolism presumably represent the best tissue specimens available to examine disease-modified expression patterns.
Part of the insulin insufficiency in T2D has been attributed to a reduced beta cell mass.6,7 However, Marselli et al. recently reported only a minor reduction of beta cells8 and similar results have been reported also in other studies where electron microscopy has been used to assess beta cell number.9 Moreover, endocrine mass measured by endocrine PET tracers in vivo, is not decreased in subjects with T2D in relation to reduced c-peptide levels.2 Taken together, this suggests that the frequently reported reduced number of insulin positive cells in T2D may be derived from exhausted and degranulated or partly dedifferentiated beta cells rather than through actual loss of cells.2,10-12
The endoplasmic reticulum (ER) and mitochondrial function are important for insulin secretion. The beta cell normally has a well-developed ER13 to handle the abundant production of insulin (preproinsulin). A high synthesis of proteins leads to accumulation of unfolded proteins and ER stress. The cell may compensate for the increased amount of unfolded proteins through the unfolded protein response (UPR), where upregulation of chaperones, foldases, increased ER volume and reduced protein synthesis occur. Increased ATP/ADP ratio generated through oxidative phosphorylation in the mitochondria is part of a cascade that downstream results in insulin release from the beta cell.14 Interestingly, both the ER and mitochondria appear to be functionally and morphologically altered in T2D15 and on a transcriptional level, islets obtained from subjects with established T2D exhibit some signs of ER stress,13 oxidative stress16 and mitochondrial dysfunction.15,17
It is currently unknown how the islets vary in their transcriptional pattern as normoglycemia progresses to fulminant T2D. In this study, we hypothesized that donors with elevated HbA1c levels, but not yet diagnosed with T2D, would show signs of cell stress on a transcriptional level. With the aim of characterizing the expression of stress-related genes, laser capture microdissection and qPCR arrays including 330 genes related to mitochondria, oxidative stress, or the UPR were used to extract and analyse islets in a cross-sectional study including organ donors with normal HbA1c (<5.5%, 37 mmol/mol), elevated HbA1c (6.0–6.5%, 42–48 mmol/mol), high HbA1c (>6.5%, 48 mmol/mol) or established T2D.
Results
Dynamic glucose-stimulated insulin secretion from islets in the different donor groups
Islets from all donors in all groups responded to high glucose by increased insulin secretion and the stimulation index ranged from 1.8 to 23.8 (Table 1). In absolute values, the insulin secretion tended to be higher from islets isolated from donors with elevated HbA1c levels without a clinical T2D diagnosis (semi-diabetic and undiagnosed T2D), both when perifused with low and with high glucose concentrations, when compared with islets from control donors and islets from donors diagnosed with T2D (Figure 1A). However, the dynamic stimulation index was lower in all donor groups with elevated HbA1c, or diagnosed with T2D, compared to the non-diabetic controls (Figure 1B).
Table 1.
Group characteristics of the donors successfully used for LCM and qPCR arrays.
| Control | Semi-diabetic | Undiagnosed T2D | Established T2D | |
|---|---|---|---|---|
| Mean HbA1c, %, (range) | 5.3 (5.2–5.4) | 6.2 (6.0–6.3) | 7.0 (6.6–7.9) | 7.3 (6.7–8.4)* |
| Mean HbA1c, mmol/mol, (range) | 34 (33–36) | 44 (42–45) | 53 (49–63) | 56 (50–68)* |
| Number of donors, n | 7 | 6 | 6 | 7 |
| Male, n | 4 | 3 | 3 | 5 |
| Female, n | 3 | 3 | 3 | 2 |
| Mean age, yrs, (range) | 64 (46–70) | 59 (51–69) | 64 (49–76) | 70 (65–81) |
| Mean BMI, kg/m2, (range) | 24.6 (22.6–26.3) | 26.1 (21.5–32.4) | 30.0 (27.7–34.0) | 27.5 (24.5–32.8) |
| Mean SI, high/low, (range) | 11.7 (3.6–23.8) | 6.8 (2.9–11.1) | 7.9 (1.8–18.0) | 5.2 (1.9–8.6) |
| GADA postive, n | 0 | 0 | 0 | 1 |
| IA2A positive, n | 0 | 0 | 0 | 0 |
HbAc from two donors with established T2D was not available;
SI = stimulation index, IA2A = tyrosine phosphatase IA2 antibodies, GADA = Glutamic acid decarboxylase antibodies.
Figure 1.
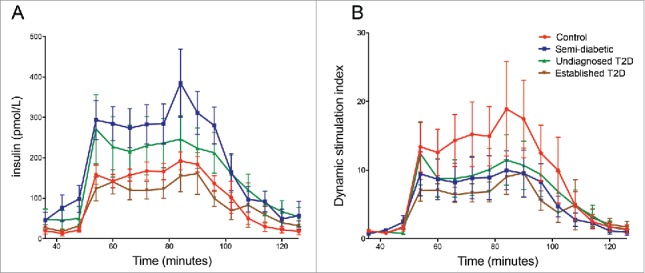
Glucose-stimulated insulin secretion in isolated islets assessed in a dynamic perifusion system. Twenty handpicked islets were sequentially perifused with low (1.67 mM) glucose from minute 1–48, high (20 mM) glucose from minute 48–90, and then low glucose again from minute 90–120. Fractions were collected at 6-min intervals, and the secreted insulin was measured by ELISA. In (A) the concentration of insulin in the collected fractions is shown whereas the dynamic stimulation index is displayed in (B). The dynamic stimulation index at each time point was calculated by normalizing the insulin concentration in each fraction to the mean basal insulin secretion at low glucose from the same islet sample.
Large inter-donor variations in expression of stress-related genes
Principal component analysis based on the expression of all 330 analysed genes displayed no obvious separation of the four different donor groups (Figure 2A). Heat map and hierarchical clustering analysis of genes and samples did not identify clusters based on donor groups (Figure 2B).
Figure 2.
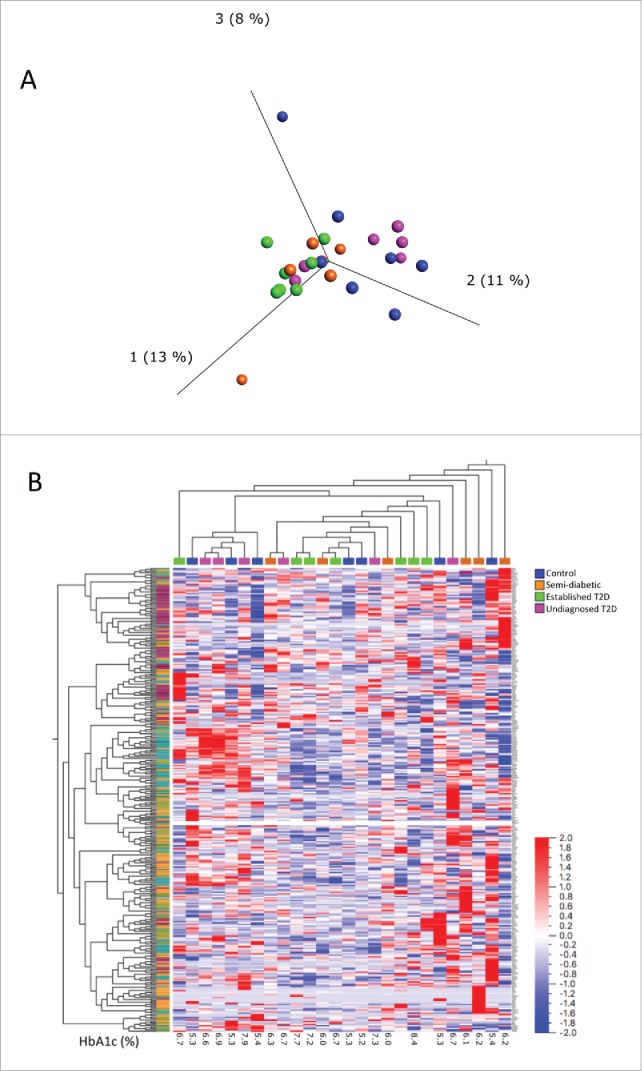
Principal component analysis (A) and heat map (B) of all 330 analysed genes in islets from the four different donor groups. In the principal component analysis, the samples are colour coded by donor age as displayed in the legend. In the heat map (B), samples and genes are ordered by hierarchical clustering (average linkage method). Genes are labeled by the pathway-focused array on which they were analyzed (purple is unfolded protein response, olive green is mitochondria, turquoise is mitochondria energy metabolism, and orange is oxidative stress).
Differential expression compared to islets from control donors
44 of the 330 analysed genes were differently expressed (p < 0.05, FDR <0.30) in islets from donors with T2D compared with islets from the control group (Figure 3 and ESM Table S1). Islets from donors not diagnosed with T2D but with high HbA1c levels had fewer genes that were differentially expressed compared to the controls and there was only a limited overlap with the genes differentially expressed in the donors with established T2D (Figure 3A). P values, false discovery rate and fold difference for each differentially expressed gene in each donor group compared to the controls are given in ESM Table S1–3.
Figure 3.
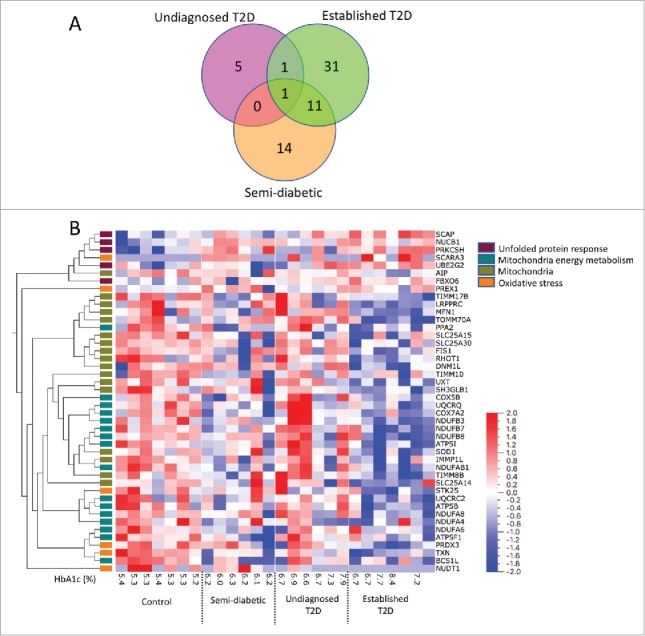
Differentially expressed genes in the different donor groups compared to controls. In (A) a Venn diagram shows the number of differentially expressed genes (p<0.05) in each donor group compared to the control group. In (B) a heat map and hierarchical clustering of the 42 genes differentially expressed in islets from donors with established T2D compared with islets from control donors (p < 0.05) is shown. Samples are ordered by group and genes by hierarchical clustering based on their expression in all samples across all four donor groups (average linkage method). Genes are labelled by the pathway-focused array on which they were analyzed. The false discovery rate is <0.30 and individual FDR Q values are shown in Supplementary Table S1.
Hierarchical clustering of the 44 genes differentially expressed in T2D donors compared to the control group, based on the expression in all samples across all four donor groups, showed that genes involved in the UPR and mitochondria-related genes formed separate clusters. Genes related to oxidative stress were spread in different clusters (Figure 3B). Heat map and hierarchical clustering of 35 genes that were differently expressed between groups (multi-group comparison by Kruskal-Wallis, p < 0.05) is shown in ESM Figure 1 and ESM Table S4.
Volcano plots of the expression of each gene in each donor group compared to the control donors (Figure 4) show a general under-expression of mitochondria-related genes in semi-diabetic donors (Figure 4A) and in donors with established T2D (Figure 4C). The majority of genes related to the unfolded protein response had higher expression in all donor groups compared to the controls (Figure 4A-C). Genes related to oxidative stress were found both over and underexpressed compared to the controls.
Figure 4.
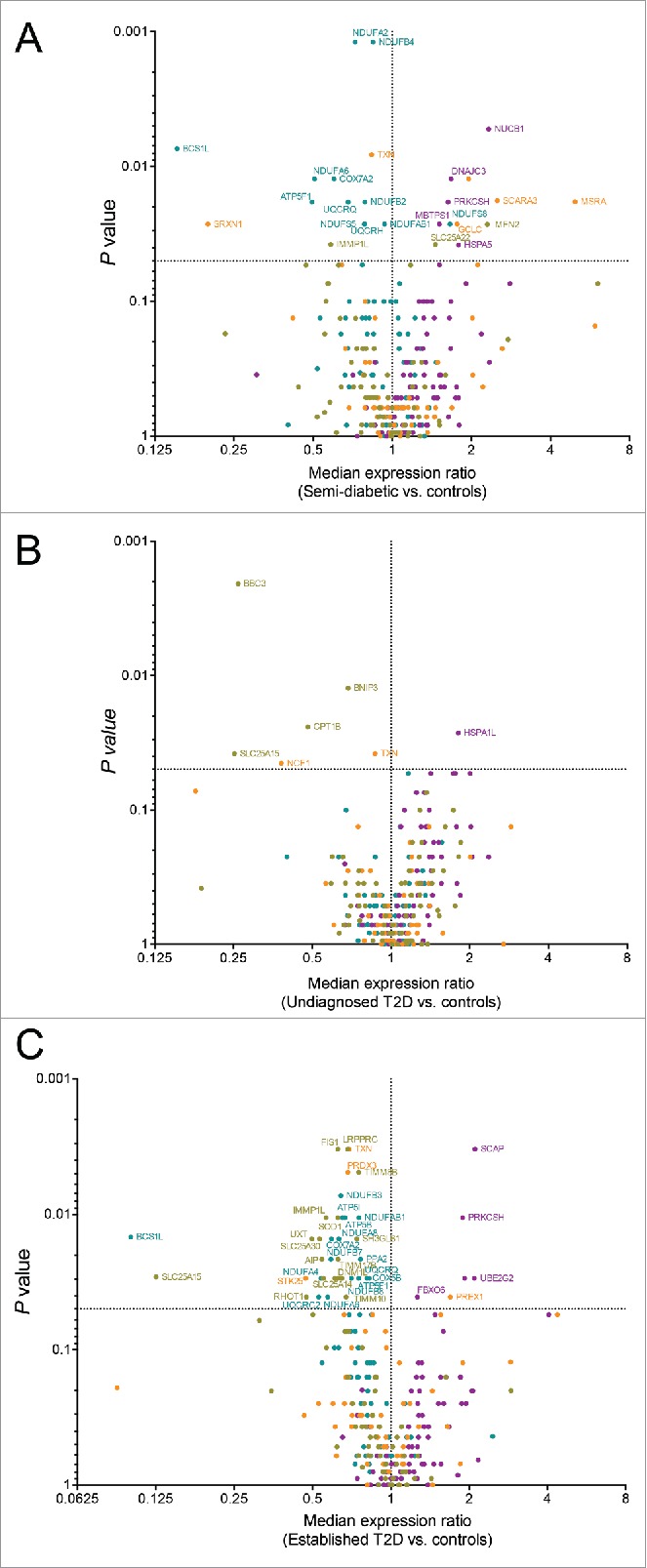
Expression analysis of 330 stress related genes in islets from subjects with semi-diabetes (A), undiagnosed diabetes (B), or established type 2 diabetes (C). The expression of each gene was normalized to the expression of the reference genes (ACTB, GAPDH, and RPLP0) and a rank-based volcano plot comparing the median expression of each gene in islets of the respective condition vs. control islets is shown. Genes are color labelled by the pathway-focused array on which they were analyzed (purple is unfolded protein response, olive green is mitochondria, turquoise is mitochondria energy metabolism, and orange is oxidative stress). Dotted lines mark no change (vertical), and P = 0.05 (horizontal). Genes with P < 0.05 are labeled with their gene symbol. P values were calculated for each gene using the Mann-Whitney signed rank test.
Discussion
This stratified study on subjects with increasing deterioration in their glucose metabolism found 44 out of 330 genes to be differentially expressed in islets from subjects with T2D when compared to control islets. Overexpressed genes were mainly related to the UPR whereas underexpressed genes were mainly related to mitochondrial function. In contrast, a study including laser capture microdissected islets from 10 controls and 10 donors with T2D did not find differential expression of genes related to mitochondria or ER stress.16 This may in part be due to the larger number (approximately 38,500) of genes analysed and the GeneChip array approach used. To avoid some of the inherent problems associated to examining a large data size on a small sample size, we selected four pre-designed qPCR arrays including groups of genes important for three different aspects of beta cell stress; UPR, mitochondrial function, and oxidative stress.18
Six of the seven genes that were overexpressed in our study do not seem to be affected by 24 h high glucose in vitro.19 Of the 37 genes found to be underexpressed in our study, none are downregulated by high glucose in vitro.19 In fact, 18 of these were slightly upregulated under this condition in islets isolated from non-diabetic donors. It is possible that acute exposure to high glucose induces upregulation of these genes (that are mainly related to mitochondrial function) but that the prolonged exposure to high glucose in the donors with T2D leads to their downregulation. Indeed, none of these genes were upregulated in islets from hyperglycaemic donors cultured in vitro with high glucose.19
The present study is the so far largest study based on LCM conducted on human type 2 diabetic pancreases procured as for clinical transplantation; and the first of its kind performed on biopsies obtained from subjects in progression towards T2D. Retrieval of islets through LCM techniques circumvents the introduction of confounding factors inherent in the analysis of isolated islets, e.g. islet isolation (hypoxia, enzyme digestion and exposure to hyperosmolar conditions during density gradient separation) and in vitro islet culture.3,20,21 We recently reported that islets retrieved from subjects with T2D are often infiltrated by a large number of immune cells22 and this possible important confounding factor has not previously been accounted for. By following a protocol previously developed and utilized to identify and capture CD45+ immune cell aggregates, the risk of analysing the transcription profile of immune cells rather than endocrine cells was minimized. The limited number of donor pancreata in the current study is due to the scarce access to high quality human biopsies from organ donors without any known pancreatic abnormalities or diseases, e.g. malignancies, pancreatitis or neuro-endocrine tumours. However, the use of pancreatic tissue from organ donors without pancreatic disease is important as both established diabetes and impaired glucose tolerance are frequently occurring in subjects with pancreatic cancer or chronic pancreatitis3; this beta cell dysfunction seems dependent on mechanism(s) separate from those in T2D.3 Indeed, none of the herein identified 44 genes differentially expressed in donors with established T2D when compared with non-diabetic subjects were among the 19 genes validated by Solimena et al on surgical specimens obtained after pancreatectomies.3
Herein, donors were categorized based on HbA1c, reflecting the metabolic control in the donors during their last 1–2 months. Donors without a clinical diagnosis of T2D but with an HbA1c between 6.0 and 6.5% (42–48 mmol/mol) are likely in a stage of prediabetes and donors with HbA1c >6.5% (48 mmol/mol) can be classified as having T2D according to the WHO criteria. We also included a group of donors with established T2D noted in their medical records. These donors have most likely been given standard of care treatment, but, as we do not have ethical permission to examine the medical records of the donors, their anti-diabetic treatment is unknown. Assessment of glucose-stimulated insulin secretion from the islets suggested that, in absolute numbers, the insulin secretion is increased from donors with elevated HbA1c, both during low and high glucose concentration, possibly reflecting the hyperinsulinemia required to compensate for increased insulin resistance in these donors. However, the stimulation index (ratio of secreted insulin during exposure to low vs. high glucose) was decreased in donors with elevated HbA1c and in donors with T2D compared to the control donors, demonstrating impaired islet function in these three groups.
Our study identified 32 genes related to mitochondrial function that were downregulated in donors diagnosed with T2D, 7 of which were also significantly downregulated in semi-diabetic subjects. Interestingly, the expression level of the mitochondrial chaperone BCS1 (BCS1L) was >10-fold downregulated in both donors with HbA1c 6.0–6.5 % (42–48 mmol/mol) and in donors with T2D. Supporting the indication that mitochondrial genes generally are underexpressed in T2D islets, MacDonald et al. reported reduced expression of genes involved in glucose-stimulated insulin secretion in islets isolated from donors diagnosed with T2D.17 In addition, the mitochondria in beta cells appear to be functionally and morphologically differentiated in T2D.15,23 Collectively, it becomes increasingly convincing that the mitochondria are affected on a transcriptional, functional, and morphological level in T2D. Additionally, our data suggest that genes related to mitochondrial function are affected already in subjects with increased HbA1c.
The mRNA levels of several central proteins in the UPR (BiP/HSPA5, XBP-1/XBP1 and CHOP/DDIT3) have previously been reported to be unaltered in T2D islets during in vitro euglycemia, but increased during hyperglycemic in vitro conditions.13 HSPA5 and XBP1 were also induced by high glucose in vitro in islets isolated from donors without T2D.19 In our study, HSPA5 was slightly overexpressed in islets from donors with HbA1c between 6 and 6.5 % (42–48 mmol/mol), but not in the other groups, compared to non-diabetic controls. No difference in the expression of XBP1 or DDIT3 was found between groups, which is in agreement with earlier reports of laser-captured islets from donors with T2D.16 However, other genes part of the UPR were significantly overexpressed, and most genes in the UPR array had a higher median expression in islets from donors with elevated HbA1c or diagnosed with T2D compared with islets from subjects with HbA1c <5.5% (37 mmol/mol), suggesting that the UPR indeed is induced as pre-diabetes progresses to T2D. Two genes in the UPR, NUCB1 and PRKCSH, were overexpressed both in donors with diagnosed T2D and donors with elevated HbA1c compared with control donors. None of these are induced by culture of human islets under hyperglycaemic conditions,19 suggesting that their induction is not a secondary event to the high blood glucose in the donors with elevated HbA1c. Potentially most relevant in relation to our data, the ER has been reported to be altered functionally and morphologically in T2D.13,15,23 Taken together, the reduced c-peptide in the circulation of T2D patients may be due to an inability of beta cells to synthesise and release insulin as a consequence of ER stress and dysfunctional mitochondria.
Genes related to oxidative stress were found both upregulated and downregulated in our study. In agreement with the study by Marselli et al.16 the antioxidant thioredoxin (TXN) was highly expressed in the islets and downregulated in islets from donors with T2D. Interestingly, this gene was underexpressed also in donors with elevated HbA1c levels in our study whereas it was not affected by culture of islets obtained from non-diabetic subjects in high glucose,19 suggesting that loss of antioxidant function may be associated with T2D development. Also, other antioxidants were downregulated in our study (SRXN1 and PRDX3) whereas some other genes associated with oxidative stress were upregulated (PREX1, MSRA, SCARA3, GCLC).
Islets from semi-diabetic donors, without diagnosis of T2D, had fewer genes (26/330) than islets from donors with established T2D, that were differentially expressed compared with controls. This argues for that the semi-diabetic islets on a transcriptional level have started differentiating to the phenotype seen in islets from T2D donors and that these islets exhibit a cellular stress response. It is unknown if the altered gene expression profiles in islets obtained from semi-diabetic and established T2D donors are permanent or reversible. The inability of T2D islets to reverse hyperglycemia, even several weeks after transplantation, in nude mice24 suggests that the beta cells have become irreversibly dysfunctional. On the other hand, life style changes by pre-diabetic individuals can lead to improved beta cell function, lowered risk to establish T2D and reversion to euglycemia.1,12,25-27 This suggests that islets from pre-diabetic subjects can regain function. Islets from undiagnosed T2D donors are expected to exhibit a more severe cellular stress response than semi-diabetic donors if elevated HbA1c is the stressor. However, other factors that we unfortunately cannot account for in order to protect the integrity of the deceased patient, such as the blood glucose levels the days before death, intensive care treatment and anti-diabetic treatment likely also affect the islet transcriptome. The group with established T2D had the most pronounced cellular stress response in our study, possibly due to the duration of the disease or direct effects excreted by therapeutic drugs, e.g. metformin. Metformin and sulfonylureas are known to have significant effects on the islet transcriptome.28,29
Taken together, despite large inter-donor variations in the expression of stress-related genes, 44 genes were identified to be differentially expressed on a group level in donors with established T2D. Our data on transcriptional changes in human islets retrieved by LCM from high-quality biopsies, as pre-diabetes progresses to established T2D, increase our understanding of how islet stress contributes to the disease development.
Materials and methods
Ethics statement
The consent to use pancreatic tissue from deceased organ donors for research purposes was obtained verbally from the deceased person's next of kin by the physician in charge or obtained from an online database and was fully documented in accordance with Swedish law and regional standard practices. All the tissue included in this study was procured, stored and analyzed in accordance with written approval from the Regional Ethics Committee in Uppsala (Dnr: 2015/444).
Organ donors and tissue preparation
This research was based on the study of frozen pancreatic biopsies from deceased organ donors procured within the Nordic Network for Islet Transplantation. Pancreatic biopsies from 32 subjects with different HbA1c levels were included in the study and divided into four study groups. The groups were defined as control donors (HbA1c <5.5%, 37 mmol/mol), semi-diabetic donors (HbA1c 6.0–6.4 %, 42–48 mmol/mol), undiagnosed T2D donors (HbA1c >6.5 %, 48 mmol/mol) and patients with established T2D (HbA1c not taken into consideration), based on the WHO guidelines for definition and diagnosis of T2D.30
Eleven donors with an HbA1c >6.5 (48 mmol/mol) without a T2D diagnosis were available for study. Of these, 8 were included. The donors in the control group and the semi-diabetic group were age- and gender-matched to the donors with undiagnosed T2D; the next available donor with the same sex and age +/− 5 years was included. Control donors were not included if they had a BMI >28 kg/m2. The group of donors with established T2D consisted of 8 donors diagnosed with T2D where ongoing use of oral anti-diabetics was noted in the donor register. Due to poor tissue quality, eight donors were subsequently excluded from the study. Group characteristics for the 26 donors used for LCM and qPCR arrays are shown in Table 1.
The pancreatic biopsies were taken one at a time from −80°C storage and processed through a Leica CM1860 UV cryostat (Leica, Wetzlar, Germany) where the tissue was sectioned in −22°C into 10 μm thick sections consecutively placed on either a Superfrost Ultra Plus object glass (Thermo Fisher Scientific, MA, USA) for immunohistochemistry (IHC) staining or on an Arcturus PEN Membrane Glass Slide (Thermo Fisher Scientific, Gothenburg, Sweden) that within 2 hours had been UV treated for 12 minutes. The glasses and slides were thereafter immediately stored in −80°C awaiting LCM. In total, 16 sections from each donor were cut in series, with 10 sections being put on PEN membranes and 6 sections being put on object glass slides as described previously31 IHC was performed utilizing a standardized protocol (DAKO EnVision G/2 Double Stain Visualization kit) staining for insulin (DAKO Ab: AO564, Agilent, CA, USA) and CD45 (DAKO Ab:2B11 PD7/26).
Histology
The content of fibrosis, adipocytes, and the density of leucocytes were examined on pancreatic sections stained for insulin and CD45. Pancreata with inter- and extra-lobular fibrotic tissue, with adipocytes and a massive pancreatic leucocyte infiltration could be discovered in two cases. One case belonged to the semi-diabetic group and the other belonged to the undiagnosed T2D group. Islets situated within fibrotic regions or infiltrated with immune cells were not selected in the LCM procedure and the analysed islets from both of these cases had similar gene expression profiles as islets from other donors within the respective groups (data not shown). Among the remaining control, semi-diabetic and undiagnosed T2D cases, there was no/very little fibrotic tissue, and a homogenous distribution of pancreata from each group contained a slightly elevated number of adipocytes or leucocytes. However, a majority of the pancreata from donors with established T2D contained adipocytes as well as fibrotic tissue. Only islets embedded in exocrine parenchyma, with no or few leucocytes within the islets, were extracted by LCM.
Glucose-stimulated insulin secretion
Glucose-stimulated insulin secretion (GSIS) was assessed in a dynamic perifusion system, Suprafusion 1000 (BRANDEL, Gaithersburg, MD). One day after isolation, twenty islets were handpicked under a light microscope and placed in the incubation chamber of the perifusion system. The islets were sequentially perifused (200 μL/min) with low (1.67 mM) glucose from minute 1–48, high (20 mM) glucose from minute 48–90, and then low glucose again from minute 90–120. Fractions were collected at 6-min intervals, and the secreted insulin was measured by ELISA (Mercodia, Uppsala, Sweden). The dynamic stimulation index at each time point was calculated by normalizing the insulin concentration in each fraction to the mean basal insulin secretion at low glucose from the same islet sample.
Laser capture micro dissection and RNA extraction
Samples were removed from −80°C one at a time and were immediately thawed and dehydrated utilizing a standard protocol (75% EtOH for 30 s at −20°C, followed by 95% EtOH for 60s, 100% EtOH for 60s, and 4 minutes Xylene at room temperature). LCM was performed on an Arcturus XT LCM instrument. The Islets of Langerhans were localized through islet auto-fluorescence and their localization was confirmed using the corresponding object glasses stained for CD45 and insulin in order to avoid islets with an ongoing intra-insular infiltration. The islets were captured on Arcturus CapSure HS LCM Caps (Thermo Fisher Scientific) and the caps were subsequently incubated for 30 min at 42°C in 20 μl RLT Buffer Plus (Qiagen, Hilden, Germany) containing 1% beta-mercaptoethanol. All samples were then stored in −80°C prior to RNA extraction. RNA extraction was performed using a Qiagen RNeasy Plus Micro kit (Qiagen) according to the manufacturer's instructions.
qPCR analysis
cDNA synthesis from the RNA extracted from the islet tissue and subsequent pre-amplification and expression analysis were performed using kits from Qiagen according to the manufacturer's instructions as described previously.32 Four different pre-designed primer mixes (PBH-087Z, PBH-008Z, PBH-065Y and PBH-089Z, Qiagen) containing 84 genes each were used to pre-amplify the cDNA. The arrays chosen for this analysis were Human Mitochondria (art. no. PAHS-087Z), Human Mitochondrial Energy metabolism, (art. no. PAHS-008Z), Human Oxidative Stress (art. no. PAHS-065Y) and Unfolded Protein Response (art. no. PAHS-089Y). For the six genes that appeared on more than one array, mean expression was calculated and used for further analyses. In total, the expression of 330 different genes were analyzed. The reference genes ACTB, GAPDH, and RPLP0 were chosen for normalization and the expression level of each gene was calculated with the 2−dCq method. Genes with a quantification cycle (Cq) value >35 were regarded as non-detected.
Statistics and bioinformatics
Principal component analysis, hierarchical clustering (average linkage method), and statistical analyses were performed with Omics Explorer version 3.3 software with interface to R (Qlucore, Lund, Sweden). Non-parametric testing was used for two-group (Mann-Whitney) and multi-group (Kruskal-Wallis) comparisons. False discovery rate (q values) was calculated using the Benjamini-Hochberg method. P values were calculated for each gene in each array using a Mann-Whitney signed rank test, and a rank-based volcano plot was created based on the raw p values and the fold expression relative to the median of the controls.
Funding Statement
This study was supported by grants from the Swedish Medical Research Council (65X-12219-15-6, K2015-54X-12219-19-4), the Novo Nordic Insulin Fund, the Åke Wiberg Foundation, the Tore Nilsson Foundation, Magnus Bergvall's Foundation, the Ernfors Family Fund, Barndiabetesfonden, the Swedish Diabetes Association, the Diabetes Wellness Foundation, and the Juvenile Diabetes Foundation International.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank everyone in the Nordic Network for Clinical Islet Transplantation involved in the procurement of pancreases and islets, and Sofie Ingvast for excellent technical assistance. We also give our deepest gratitude to all organ donors.
References
- 1.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–90. doi: 10.1016/S0140-6736(12)60283-9. PMID:22683128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlbom L, Espes D, Lubberink M, Martinell M, Johansson L, Ahlstrom H, Carlsson PO, Korsgren O, Eriksson O. [11C]5-hydroxy-tryptophan PET for assessment of islet mass during progression of type 2 diabetes. Diabetes. 2017;66(5):1286–92. doi: 10.2337/db16-1449. PMID:28246291. [DOI] [PubMed] [Google Scholar]
- 3.Solimena M, Schulte AM, Marselli L, Ehehalt F, Richter D, Kleeberg M, Mziaut H, Knoch KP, Parnis J, Bugliani M, et al.. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia. 2017; doi: 10.1007/s00125-017-4500-3. PMID:29185012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taneera J, Fadista J, Ahlqvist E, Atac D, Ottosson-Laakso E, Wollheim CB, Groop L. Identification of novel genes for glucose metabolism based upon expression pattern in human islets and effect on insulin secretion and glycemia. Hum Mol Genet. 2015;24(7):1945–55. doi: 10.1093/hmg/ddu610. PMID:25489054. [DOI] [PubMed] [Google Scholar]
- 5.Permert J, Larsson J, Westermark GT, Herrington MK, Christmanson L, Pour PM, Westermark P, Adrian TE. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994;330(5):313–8. doi: 10.1056/NEJM199402033300503. PMID:8277951. [DOI] [PubMed] [Google Scholar]
- 6.Bonner-Weir S, O'Brien TD. Islets in type 2 diabetes: in honor of Dr. Robert C. Turner. Diabetes. 2008;57(11):2899–904. doi: 10.2337/db07-1842. PMID:18971437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7].Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10. doi: 10.2337/diabetes.52.1.102. PMID:12502499. [DOI] [PubMed] [Google Scholar]
- 8.Marselli L, Suleiman M, Masini M, Campani D, Bugliani M, Syed F, Syed F, Martino L, Focosi D, Scatena F, et al.. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia. 2014;57(2):362–5. doi: 10.1007/s00125-013-3098-3. PMID:24233056. [DOI] [PubMed] [Google Scholar]
- 9.Dayeh T, Volkov P, Salo S, Hall E, Nilsson E, Olsson AH, Kirkpatrick CL, Wollheim CB, Eliasson L, Rönn T, et al.. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet. 2014;10(3):e1004160. doi: 10.1371/journal.pgen.1004160. PMID:24603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24(5):366–71. doi: 10.1007/BF00251826. PMID:6347784. [DOI] [PubMed] [Google Scholar]
- 11.Spijker HS, Song H, Ellenbroek JH, Roefs MM, Engelse MA, Bos E, Bos E, Koster AJ, Rabelink TJ, Hansen BC, et al.. Loss of beta-cell identity occurs in Type 2 diabetes and is associated with islet amyloid deposits. Diabetes. 2015;64(8):2928–38. doi: 10.2337/db14-1752. PMID:25918235. [DOI] [PubMed] [Google Scholar]
- 12.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–34. doi: 10.1016/j.cell.2012.07.029. PMID:22980982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50(12):2486–94. doi: 10.1007/s00125-007-0816-8. PMID:17906960. [DOI] [PubMed] [Google Scholar]
- 14.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–43. doi: 10.1038/nature12985. PMID:24429632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48(2):282–9. doi: 10.1007/s00125-004-1627-9. PMID:15654602. [DOI] [PubMed] [Google Scholar]
- 16.Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, Marchetti P, Weir GC. Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS ONE. 2010;5(7):e11499. doi: 10.1371/journal.pone.0011499. PMID:20644627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald MJ, Longacre MJ, Langberg EC, Tibell A, Kendrick MA, Fukao T, Ostenson CG. Decreased levels of metabolic enzymes in pancreatic islets of patients with type 2 diabetes. Diabetologia. 2009;52(6):1087–91. doi: 10.1007/s00125-009-1319-6. PMID:19296078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J. Clin Invest. 2006;116(7):1802–12. doi: 10.1172/JCI29103. PMID:16823478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottosson-Laakso E, Krus U, Storm P, Prasad RB, Oskolkov N, Ahlqvist E, Fadista J, Hansson O, Groop L, Vikman P. Glucose-induced changes in gene expression in human pancreatic islets: causes or consequences of chronic hyperglycemia. Diabetes. 2017;66(12):3013–28. doi: 10.2337/db17-0311. PMID:28882899. [DOI] [PubMed] [Google Scholar]
- 20.Goto M, Eich TM, Felldin M, Foss A, Kallen R, Salmela K, Tibell A, Tufveson G, Fujimori K, Engkvist M, et al.. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78(9):1367–75. doi: 10.1097/01.TP.0000140882.53773.DC. PMID:15548977. [DOI] [PubMed] [Google Scholar]
- 21.Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, et al.. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–40. doi: 10.2337/dc15-1988. PMID:27208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg M, Seiron P, Ingvast S, Korsgren O, Skog O. Insulitis in human diabetes: a histological evaluation of donor pancreases. Diabetologia. 2017;60(2):346–53. doi: 10.1007/s00125-016-4140-z. PMID:27796420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masini M, Martino L, Marselli L, Bugliani M, Boggi U, Filipponi F, Marchetti P, De Tata V. Ultrastructural alterations of pancreatic beta cells in human diabetes mellitus. Diabetes Metab Res Rev. 2017;33(6). doi: 10.1002/dmrr.2894. PMID:28303682. [DOI] [PubMed] [Google Scholar]
- 24.Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, et al.. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53(3):624–32. doi: 10.2337/diabetes.53.3.624. PMID:14988246. [DOI] [PubMed] [Google Scholar]
- 25.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–81. doi: 10.1016/S0140-6736(11)60614-4. PMID:21705072. [DOI] [PubMed] [Google Scholar]
- 26.Taylor R, Barnes AC. Translating aetiological insight into sustainable management of type 2 diabetes. Diabetologia. 2018;61(2):273–283. doi: 10.1007/s00125-017-4504-z. PMID:29143063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steven S, Hollingsworth KG, Small PK, Woodcock SA, Pucci A, Aribasala B, Al-Mrabeh A, Batterham RL, Taylor R. Calorie restriction and not glucagon-like peptide-1 explains the acute improvement in glucose control after gastric bypass in Type 2 diabetes. Diabet Med. 2016;33(12):1723–31. doi: 10.1111/dme.13257. PMID:27589584. [DOI] [PubMed] [Google Scholar]
- 28.Del Guerra S, D'Aleo V, Lupi R, Masini M, Bugliani M, Boggi U, Filipponi F, Marchetti P. Effects of exposure of human islet beta-cells to normal and high glucose levels with or without gliclazide or glibenclamide. Diabetes Metab. 2009;35(4):293–8. doi: 10.1016/j.diabet.2009.01.004. PMID:19502091. [DOI] [PubMed] [Google Scholar]
- 29.Kwon MJ, Chung HS, Yoon CS, Ko JH, Jun HJ, Kim TK, Lee SH, Ko KS, Rhee BD, Kim MK, et al.. The effects of glyburide on apoptosis and endoplasmic reticulum stress in INS-1 cells in a glucolipotoxic condition. Diabetes Metab J. 2011;35(5):480–8. doi: 10.4093/dmj.2011.35.5.480. PMID:22111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Guidelines Approved by the Guidelines Review Committee Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 31.Lundberg M, Krogvold L, Kuric E, Dahl-Jorgensen K, Skog O. Expression of Interferon-stimulated genes in insulitic pancreatic islets of patients recently diagnosed with type 1 diabetes. Diabetes. 2016;65(10):3104–10. doi: 10.2337/db16-0616. PMID:27422384. [DOI] [PubMed] [Google Scholar]
- 32.Krogvold L, Wiberg A, Edwin B, Buanes T, Jahnsen FL, Hanssen KF, Larsson E, Korsgren O, Skog O, Dahl-Jørgensen K. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia. 2016;59(3):492–501. doi: 10.1007/s00125-015-3820-4. PMID:26602422. [DOI] [PubMed] [Google Scholar]


