Abstract
Background
The Food and Drug Administration’s 2004 antidepressant warning was followed by decreases in antidepressant prescribing for youth. This was due to declines in all types of depression treatment, not just the intended changes in antidepressant prescribing patterns. Little is known about how these patterns varied by race/ethnicity.
Method
Data are Medicaid claims from four U.S. states (2002–2009) for youth ages 5-17. Interrupted time series analyses measured changes due to the warning in levels and trends, by race/ethnicity, of three outcomes: antidepressant prescription fills, depression treatment visits, and incident fluoxetine prescription fills.
Results
Pre-warning, antidepressant fills were increasing across all racial/ethnic groups, fastest for White youth. Post-warning, there was an immediate drop and continued decline in the rate of fills among White youth, more than double the decline in the rate among Black and Latino youth. Pre-warning, depression treatment visits were increasing for White and Latino youth. Post-warning, depression treatment stabilized among Latinos but declined among White youth. Pre-warning, incident fluoxetine fills were increasing for all groups. Post-warning, immediate increases and increasing trends of fluoxetine fills were identified for all groups.
Conclusions
Antidepressant prescription fills declined most post-warning for White youth, suggesting that risk information may have diffused less rapidly to prescribers or caregivers of minorities. Decreases in depression treatment visits help to explain the declines in antidepressant prescribing and were largest for White youth. An increase in incident fluoxetine fills, the only medication indicated for pediatric depression at the time, suggests that the warning may have shifted prescribing practices.
Keywords: Child/Adolescent, Antidepressants, Depression, Minority Groups
Introduction
The Food and Drug Administration (FDA) issued a boxed warning (BW) for antidepressant medications in 2004 to address the risk of suicidal ideation in younger users after a series of related advisories and media reports. After the warning, significant declines were observed in pediatric antidepressant use (Olfson, Marcus et al. 2008, Busch, Frank et al. 2010). These declines were driven predominantly by declines in antidepressant prescribing for white youth and not racial/ethnic minority youth (DePetris and Cook 2013), suggesting that the BW did not influence antidepressant prescribing for minority youth and white youth equally. Treatment of pediatric depression also declined during this time period (Libby, Brent et al. 2007), suggesting that the BW may have had a chilling effect on provider willingness to treat pediatric depression. It is not known whether changes in pediatric depression treatment also differed by race/ethnicity following the BW. Disparities research in this area can help policymakers and clinicians anticipate how future medication warnings will affect medication and office-based treatment in vulnerable populations. Both major depressive disorder (MDD) (Merikangas, He et al. 2010) and suicidal ideation (Evans, Hawton et al. 2005) are common across all groups of youth, and so appropriate prescribing of antidepressants across racial/ethnic groups, and for particularly vulnerable groups such as those insured by Medicaid, remains a serious public health concern (Cummings and Druss 2011).
FDA warnings for other classes of psychotropic medications have been associated with differential prescribing effects across racial/ethnic groups. One example is the sharper decline in olanzapine prescription fills among White patients as compared to Hispanic patients after a 2003 FDA warning on the use of certain antipsychotic medications (Dusetzina, Busch et al. 2012, Dusetzina, Cook et al. 2013). Systematic reviews of FDA risk communications have observed decreases in antidepressant use and “spillover” decreases among older age groups not included in the warning, though subgroup analysis of racial/ethnic differences are not available (Dusetzina, Busch et al. 2012). As the FDA continues to issue antidepressant warnings, some with unintended consequences [e.g. unintended increases in hospitalizations after a 2011 FDA communication on recommended citalopram dosages (Rector, Adabag et al. 2016)], it is necessary to identify the impact of FDA warnings on treatment patterns for vulnerable patient groups.
Though the FDA warning included all antidepressants, fluoxetine was the only selective serotonin reuptake inhibitor (SSRI) that had an FDA indication for treatment of pediatric depression through 2009 (Hirsch, Katz 2003), in part due to evidence for best outcomes in this population (Whittington, Kendall et al. 2004). Though tricyclic antidepressants were also FDA approved for treating depression in children, these medications have a worse safety profile than SSRIs (Geller, Reising et al. 1999). Examining the warning’s impact on fluoxetine prescribing overall and by racial/ethnic group represents the chance to examine whether there was a shift towards an antidepressant with the best evidence at the time, and whether this shift occurred equally across racial/ethnic groups.
Using Medicaid data from 2002–2009, we examined how the BW influenced trends in racial/ethnic disparities in antidepressant prescription fills for youth, and to what extent changes in treatment of pediatric depression explain these trends. Incident prescription fills of fluoxetine were also specifically assessed to disentangle whether the warning shifted new prescriptions toward the only SSRI with formal FDA indication for treatment of pediatric depression. We studied youth insured by Medicaid because this group is more likely to include racial/ethnic minority youth (Saloner, Carson et al. 2014) and to use antidepressants relative to privately insured youth both before and after the FDA warning (DePetris and Cook 2013). Medicaid data offer a detailed record of health care visits, diagnoses, and prescription drug use over time and across settings for vulnerable youth (Crystal, Akincigil et al. 2007), thus making these data appropriate to a disparities analysis of antidepressant use. Medicaid coverage for youth in the four states studied in this paper is prevalent; with mean estimates in 2013 of approximately 42% (Kaiser Family Foundation).
We tested three hypotheses. First, we hypothesized that antidepressant prescription fills and depression treatment among Whites, compared to Black and Latino youth, would show the greatest decrease in rate and the greatest decline in trend (slope) in an interrupted time series (ITS) analysis. Second, we hypothesized that the larger declines in antidepressant prescription fills among white youth were driven by larger declines in depression treatment visits for white youth compared to other racial/ethnic groups. Third, we hypothesized that white youth would have an increased rate of incident prescription fills of fluoxetine compared to black and Latino youth.
Methods
Study Sample
Data are from the Centers for Medicare and Medicaid Services (CMS) Medicaid Analytic Extract (MAX) files for the states of California, Florida, New York, and North Carolina for the years 2002–2009. We combined Personal Summary, outpatient, inpatient, and pharmacy MAX files to form a dataset of individual-level demographic, eligibility, encounter, and pharmacy data for all youth age 5-17 years enrolled in Medicaid programs in these four states. The sample was restricted to non-Latino White, Black, and Latino youth with monthly Medicaid eligibility for a total sample of 10,919,491 Medicaid beneficiaries (3,300,509 White; 2,479,730 Black; and 5,139,252 Latino youth).
In order to conduct an interrupted time series (ITS) analysis, individual data were aggregated by month and by race/ethnicity for a sample of 249 observations (3 race/ethnicity X 96 months minus 13 months “phase-in,” described in Data Analysis). Thus, each of the 249 observations represents the mean rate of antidepressant prescription fills (or mean rate of treatment visits for pediatric depression, or mean rate of incident fluoxetine prescription fills) per month for each racial/ethnic group. Rates are measured as the number of antidepressant prescription fills per 10,000 youth per month. Data for person-months in which individuals were not Medicaid eligible were not included in the respective month and race/ethnicity aggregation.
Dependent variables
The three dependent variables of interest were rates of any antidepressant prescription fill, any treatment visit for pediatric depression, and incident prescription fill of fluoxetine. Antidepressant prescribing was defined as any antidepressant prescription fill in a given calendar month. This was determined by matching therapeutic class and medication name in pharmacy claims data to the National Drug Code directory (U.S. Food and Drug Administration). Any treatment visit for pediatric depression in a given month was defined by having a diagnosis of major depressive disorder or a related psychiatric disorder (ICD-9-CM codes 296.xx–300.xx or 311.xx) on a medical claim assigned by a clinician for treatment in outpatient, inpatient, or long-term care settings.
Incident prescribing of fluoxetine was defined as a new prescription fill for fluoxetine after a 90-day period with no office visit for mental health treatment nor any antidepressant prescription fills from any prescriber. This period of time has been used in prior health services research, and considered to be sufficiently long to indicate a new episode of mental health treatment (Keeler, Manning et al. 1988, Tansella, Micciolo et al. 1995, Cook, Zuvekas et al. 2014). We focused on incident fluoxetine prescribing because fluoxetine was the sole SSRI with an FDA indication for major depressive disorder in youth before 2009. Race/ethnicity was determined using Census categories of non-Latino White (White from here forward), non-Latino Black or African American (Black from here forward), and Latino or Hispanic (Latino from here forward).
Data Analysis
We used an interrupted time series (ITS) approach, estimating ordinary least square (OLS) regression models to compute predicted rates and slopes before and after the warning in October 2004 for antidepressant prescription fills, any treatment visit for pediatric depression, and incident prescribing of fluoxetine by month and race/ethnicity. ITS is a strong quasi-experimental research design (Penfold and Zhang 2013) that controls for baseline rates and slopes in outcomes before the implementation of the policy being studied (Wagner, Soumerai et al. 2002). By examining changes in outcomes in large populations before and after a specific time period, ITS differences out contextual variables whose effect does not vary greatly over time, such as state residence or proportion of Medicaid-insured racial/ethnic groups in each state. Therefore, the regression in this analysis controlled only for racial/ethnic group, the interaction between race/ethnicity group and post-BW indicator, the interaction between race/ethnicity and pre-BW time trend, and the interaction between race/ethnicity and post-BW time trend. Time-invariant effects are canceled out in the ITS design and therefore do not have to enter the regression as independent predictors.
A “phase-in” period (October 2003 to October 2004) was used to account for the FDA antidepressant advisories preceding the formal BW. The inclusion of a phase-in period, as used in other ITS analyses (Lu, Soumerai et al. 2010, Lu, Zhang et al. 2014), accommodates the potential confounding effects of earlier FDA advisories that could have impacted provider awareness of suicidality concerns and subsequent prescribing of antidepressants prior to the BW. Eight years of data (2002–2009) were used to improve our ability to detect autocorrelation and secular trends pre- and post-BW (Ramsay, Matowe et al. 2003).
ITS models adjusted for baseline trends and included a binary indicator to estimate the immediate level change in outcomes after the BW as well as a term indicating the slope change after the warnings. Coefficients for indicators of Black race and Latino ethnicity, and interactions between race/ethnicity and rate and slope indicators, were also included. Using linear combinations of these coefficients, we calculated the predicted racial/ethnic disparities in rates and slopes of antidepressant prescription fills, any treatment of pediatric depression, and incident fluoxetine prescription fills, and tested for their significance. Results are presented graphically in order to visually inspect the time series for differences before and after the BW in slope and rate (Wagner, Soumerai et al. 2002). The analysis tested for serial correlation between time periods using the Durbin-Watson statistic and adjusted for significant first order autoregressive parameters to control for correlation between consecutive months (Wagner, Soumerai et al. 2002). Models were adjusted for month fixed effects regardless of the year to control for variation in antidepressant prescribing related to seasonality.
Finally, we assessed changes in racial/ethnic group rates and slopes of antidepressant prescribing conditional on having a pediatric depression treatment visit. Results from this model, viewed alongside results from the model of any pediatric depression visit, provide evidence on whether overall declines in antidepressant prescribing were due to declines in depression visits, or declines in provider prescriptions among those with a visit.
Results
Table 1 describes unadjusted outcomes, demographics, and clinical characteristics of the study sample. Temporary Assistance for Needy Families was the most common reason for Medicaid eligibility across all three groups. On average, White youth were more likely to be diagnosed with MDD (0.8%) than Black (0.5%) and Latino (0.3%) youth, and had over twice the frequency of filling an antidepressant prescription in a given month (5.9%) compared to Black (2.6%) and Latino (1.6%) youth.
Table 1.
Unadjusted outcomes and socio-demographic characteristics of Medicaid-insured youth
| White (N= 3,300,509) |
Black (N= 2,479,730) |
Latino (N= 5,139,252) |
|
|---|---|---|---|
| % | % | % | |
| Prescription Fill | |||
| Any antidepressant prescription | 5.86 | 2.56 | 1.63 |
| Initiated antidepressant prescription | 4.93 | 2.26 | 1.47 |
| Terminated SSRI use | 0.34 | 0.12 | 0.08 |
| Sex | |||
| Male | 48.12 | 49.24 | 47.83 |
| Female | 51.88 | 50.76 | 52.17 |
|
| |||
| Age At First Medicaid Enrollment | |||
| 5-12 years | 67.79 | 71.84 | 71.41 |
| 13-17 years | 32.21 | 28.16 | 28.59 |
| Average Enrollment Time (in months) | 30 | 37 | 33 |
| Depression Diagnosis | |||
| Major Depressive Disorder | 0.80 | 0.48 | 0.25 |
| Other Depressive Disorders† | 1.38 | 0.86 | 0.42 |
| Co-morbid Disorders With Depression Diagnosis | |||
| Disruptive Behavior Disorder | 21.32 | 31.79 | 10.91 |
| Anxiety Disorder | 11.54 | 8.95 | 6.07 |
| Pervasive Developmental Disorder and Intellectual Disability | 0.89 | 1.12 | 0.35 |
| Other | 30.13 | 31.69 | 24.55 |
|
| |||
| Medicaid eligibility | |||
| Foster care | 8.02 | 6.03 | 2.64 |
| Temporary assistance for needy families | 19.27 | 29.63 | 21.84 |
| Supplemental Security Income | 3.70 | 6.89 | 2.38 |
| State Children’s Health Insurance Plan | 5.57 | 2.63 | 9.55 |
|
| |||
| State of residence | |||
| California | 37.16 | 25.11 | 70.03 |
| Florida | 23.23 | 27.53 | 12.57 |
| North Carolina | 14.58 | 18.55 | 2.37 |
| New York | 25.03 | 28.81 | 15.03 |
300.40 (neurotic depression); 309.10 (prolonged depressive reaction); 311.00 (depressive disorder, not otherwise specified)
Sample size for each racial/ethnic group is the total number of unique individuals during January 2002 to December 2009
Antidepressant Prescription Fills
Figure 1 displays the predicted rates (both with the BW and extrapolating slopes from the beginning of the “phase-in” period, as if the BW had not occurred) and observed rates of antidepressant prescription fills over time. In September 2003, immediately prior to the first FDA advisory applied to antidepressants as a class, White youth filled antidepressant prescriptions at a predicted rate of 289/10,000 youth, compared to 93/10,000 for Black and 55/10,000 for Latino youth. Table 2 shows the change in rate (level shift) before and after the phase-in period (October 2003 to October 2004), during which the FDA released its antidepressant advisories and BW. The level shift indicates a significant immediate decrease in antidepressant prescription fill rates for all three groups (Table 2). The decrease among White youth was greater than those for Blacks and Latinos (p<.001 for both comparisons). Regarding the change in slopes, rates of prescription fills before the BW (“slope before warning”) were increasing across all three racial/ethnic groups, with a significantly larger positive slope among White youth. Following the BW, the slopes reversed to negative for White and Black youth and flattened among Latino youth, with a significantly greater negative shift in slope among White youth (p<0.001 for both comparisons). White youth therefore experienced the biggest drop in rate and biggest slope decline of antidepressant prescription fills post-BW compared to Black and Latino youth.
Figure 1.
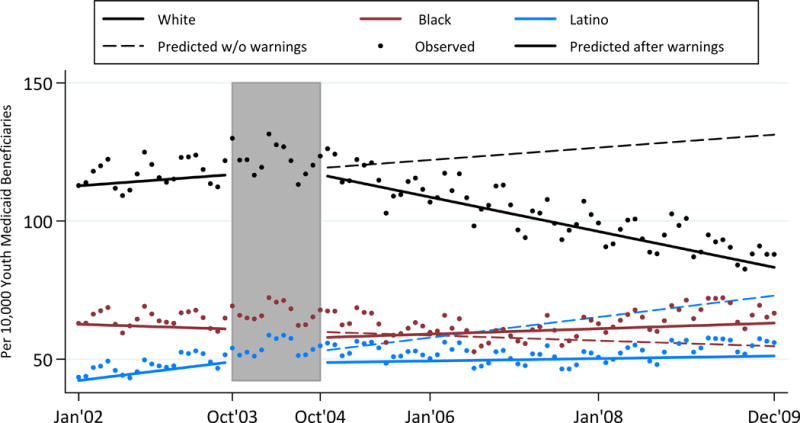
Rates of Antidepressant Prescription Fills Over Time
Shaded area represents the phase-in period from October 2003 to October 2004. The rate is per 10,000 youth Medicaid beneficiaries.
Table 2.
Predicted Changes in Rates and Slopes in Antidepressant Prescription Fills Before and After FDA Boxed Warning, by Race/Ethnicity (n=249)
| White | Black | Latino | |||
|---|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Different from White | Estimate (SE) | Different from White | |
|
| |||||
| Baseline rate Jan 2002† | 229.29 (5.86) | 66.16 (2.49) | *** | 29.17 (2.40) | *** |
|
| |||||
| Change in rate after warningΔ | −66.80 (10.94)*** | −29.12 (4.59)*** | *** | −20.76 (4.42)*** | *** |
|
| |||||
| Slope (Δ/month) before warning‡ | 2.46 (0.45)*** | 0.90 (0.19)*** | *** | 0.86 (0.18)*** | ** |
|
| |||||
| Slope (Δ/month) after warning‡ | −0.70 (0.10)*** | −0.11 (0.04)** | *** | −0.07 (0.04) | *** |
|
| |||||
| Change in slope | −3.16 (0.47)*** | −1.01 (0.20)*** | *** | −0.94 (0.19)*** | *** |
p<0.05,
p<0.01,
p<0.001
Rate is number of prescription fills/10,000 youth per month
Slope is monthly change in number of prescription fills/10,000 youth
Change in rate is level shift between October 2003 and October 2004
Treatment Visits for Pediatric Depressive Disorder
Figure 2 displays the predicted rates and observed rates of any treatment visits for depressive disorder over time. In September 2003, Medicaid beneficiaries ages 5-17 were treated at rates of 118 per 10,000 for White youth, 62 per 10,000 for Black youth and 50 per 10,000 for Latino youth. Between October 2003 and October 2004, depression treatment visits dropped at similar rates across all three groups. Regarding the change in slopes, before the warning, treatment visit rates were increasing for Latinos but flat for whites and Blacks. Rate trajectories diverged after the warning: White youth showed declines in any pediatric depression treatment visits (p<0.001, see Appendix Table 1); Black youth showed slightly increasing rates of any pediatric depression treatment over time (p<0.01); and Latino youth had steady rates of depression treatment. In summary, slopes were affected most markedly among White youth, who showed the largest downward shift in slope (p<0.001 compared to Black, and p<0.05 compared to Latino).
Figure 2.
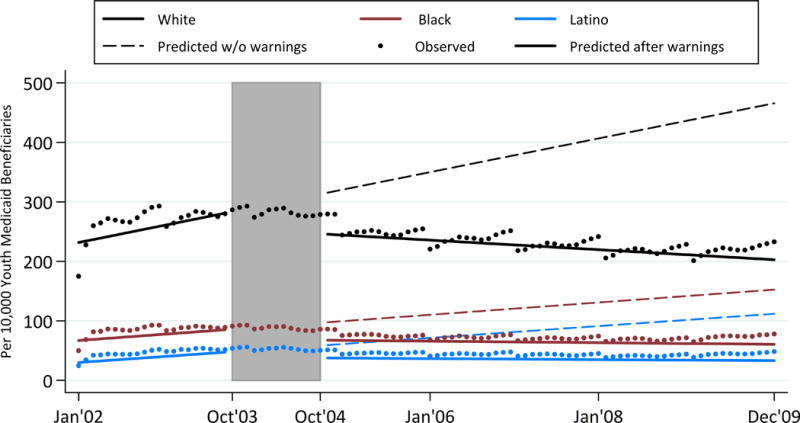
Rates of Pediatric Depression Treatment Over Time
Shaded area represents the phase-in period from October 2003 to October 2004. The rate is per 10,000 youth Medicaid beneficiaries.
Incident Prescription Fills of Fluoxetine
Figure 3 shows disparities in rates and slopes of incident fluoxetine prescription fills across the three patient groups among those patients who had no mental health visit, nor any antidepressant prescription fill, in the previous 90 days (see Appendix Table 2 for full results). The rates of model-predicted incident fluoxetine prescription fills in September 2003 ranged from 892/10,000 for Blacks, 950/10,000 for Whites, and 1,202/10,000 for Latinos. Rates of incident fluoxetine prescription fills increased significantly (all p<0.001) between October 2003 and October 2004 by a similar amount across groups. Regarding the change in slopes, rates of fluoxetine prescription fills were increasing for all youth at similar rates prior to the BW (all significant from zero at the p<0.05 level). After the BW, slopes of incident fluoxetine prescription fills remained positive for all groups, and highest for Black patients.
Antidepressant Prescription Fills among those with a Depressive Disorder
Appendix Figure 1 displays the predicted and observed rates of antidepressant prescription fills over time among only those youth with a depressive disorder in a given month. In September 2003, immediately prior to the first FDA advisory, White youth with any depressive disorder filled antidepressant prescriptions at a predicted rate of 4,007/10,000 youth. This was higher than rates for Black and Latino youth with any depressive disorder (2,633/10,000 and 2,488/10,000, respectively). The rate of antidepressant prescribing dropped from October 2003 to October 2004 at similar levels and then continued to decline for all three groups, most among Black youth (Appendix Table 3).
Discussion
This disparities analysis shows a greater immediate drop and greater decline in antidepressant prescription fills among White youth following the BW when compared to Black and Latino youth. One interpretation of these results is that information in the BW may have diffused less rapidly to the prescribers and/or caregivers of minority patients, leading to fewer changes in prescription fill patterns. These findings echo prior research showing decreases in antidepressant prescribing among White youth following the BW, in contrast to no change, or even increases, in prescribing among minority youth (DePetris and Cook 2013). Our results extend this prior work to a lower income, Medicaid-insured youth population, for whom quality of mental health care and racial/ethnic disparities are of particular importance.
By further comparing the overall rates of antidepressant prescription fills and the rates of antidepressant fills conditional on treatment for a depressive disorder, we identified that the drop in antidepressant prescription fills post-BW appears to be driven by the declining rate of treatment visits for pediatric depression. Disparities in prescribing observed in prior research (DePetris and Cook 2013) thus are likely explained by corresponding decreases in treatment visits for pediatric depression. This “chilling effect” of a federal medication advisory on practice patterns is an example of how warnings can have unintended consequences, similar to “spillover effects” in reduced prescribing of antidepressants to older groups (Dusetzina, Higashi et al. 2012). Future research should explore what conditions predict chilling effects following a medication warning; e.g., whether declines in rates of depression treatment visits are greater for primary care physicians compared to specialists, or whether declines in treatment are associated with clinician training, knowledge, or skills needed to identify and treat those conditions (Olson, Kelleher et al. 2001).
Black youth seemed relatively protected from this chilling effect, since their treatment visits increased slightly in the wake of the warning (Figure 2) as did incident fluoxetine prescriptions compared to Whites (Figure 3). This appears to be a rare example where depression visits increased following the antidepressant warning, as was recommended in the BW. From a disparities perspective, these outcomes are encouraging. Our current analysis is not able determine whether those increased visits followed new prescriptions or dose changes. Further qualitative research could explore how providers and families of under-served youth heard and reacted to the warning.
Across all groups, incident fluoxetine prescription fills jumped and continued increasing even as antidepressant prescription fills declined, suggesting that provider responses shifted toward an FDA-indicated medication for this population. Our hypothesis that White youth would see greater incident fluoxetine prescription fills was not supported. In the months after the BW, providers across all racial/ethnic groups prescribed an evidence-based option for incident medication treatments of depression, suggesting the BW may have been associated with beneficial effects on dissemination of best practices when new prescriptions were being recommended.
The mechanism underlying the differential decrease in antidepressant prescription fills post-BW cannot be identified in these data. However, our findings of a differential impact of the warning by race/ethnicity conveys the importance of the role of patient and caregiver decision-making, since they are ultimately responsible for filling prescriptions. Prescribing clinicians in Nebraska reported that 22% of caregivers refused antidepressants for their children after the FDA warning, as did 9% of pediatric patients (Bhatia, Rezac et al. 2008). Our results may partly be explained by white caregivers and patients being more likely to decline depression treatment.
Another explanation for racial/ethnic differences in prescription fills may be found in the declining rates of depression treatment visits after the warning. In our results, not only did antidepressant prescription fill rates drop more sharply for white youth than Black and Latino youth following the warning, so did visits for pediatric depression treatment. These findings can be viewed in light of evidence that suicide rates may have increased after the BW in relation to the decrease in pediatric depression treatment (Libby, Brent et al. 2007, Lu, Zhang et al. 2014). Minority youth, in particular Black youth, may have been somewhat protected from these negative consequences.
Given that lifetime prevalence rates are equal or even greater for Black and Latino youth as compared to whites (Merikangas, He et al. 2010), it does appear that depression management remains inadequate for racial/ethnic minorities. Both pre- and post-BW, minority youth attended fewer visits, providing less opportunity to discuss and act on FDA medication warnings. Other literature shows that minority youth were less likely to receive depression treatment from mental health specialists (Bach, Pham et al. 2004, Cummings and Druss 2011), and specialists were far less likely than family medicine clinicians and pediatricians to discontinue depression treatment post-BW (0.8%, 3.9% and 11.5%, respectively) (Bhatia, Rezac et al. 2008). Further research should clarify how boxed warnings affect both family preferences for treatment discipline (e.g. pursuing specialty mental health care) and antidepressant preferences.
Our findings might be used to develop policies that support informed decision making by the providers and caregivers of youth with mental health disorders, so that identification and treatment of such disorders do not decline in the wake of FDA warnings. The use of decision aids to enable shared decision-making (Shah, Montori et al. 2010) for depression treatment are available in English and Spanish and represent a practical policy tool to help minority families make informed decisions in collaboration with providers (Mayo Foundation for Medical Education and Research 2011). The FDA might consider which “change agents” are best suited to help the adoption of important medication warnings by providers taking care of minority patients (Haider and Kreps 2004), such as state public health departments or private foundations supporting shared decision-making. Decision aids and advocacy by change agents may complement the FDA’s efforts to address the “information asymmetries” that deprive patients and providers of medication information (Busch, Frank et al. 2010). This is especially true in child psychopharmacology, which has a much smaller evidence base available to guide drug-specific policy recommendations (Hammad, Neyarapally et al. 2013).
These data should be interpreted in the context of limitations associated with research using Medicaid data. Prescription fills in claims data are not equal to medication adherence, since caregivers may later decide not to give the medication and patients may decide to not take it. Therefore, the rates of prescription fills in our findings represent an “upper-bound estimate” of true antidepressant use, (Crystal, Akincigil et al. 2007). It is also not possible to determine whether decreases in antidepressant prescription fills were the result of changes in provider or caregiver/patient behavior, although the question of how comparative effectiveness evidence diffuses to both groups remains of interest. Our findings reflect the results of a decision-making process that involves, to varying degrees, prescriber, caregiver, and youth. While large sample sizes of all three racial/ethnic groups were analyzed, concerns have been raised regarding the accuracy of ethnicity reporting for these groups in claims data (Gomez, Kelsey et al. 2005), suggesting that some minorities may have been misclassified as white or missing from the data. This limitation could artificially narrow the disparities between the three racial/ethnic groups. Another limitation is that the data is limited to only four states, though it is important to note that these states represent approximately one third of all Medicaid beneficiaries in the United States (Horvitz-Lennon, Volya et al. 2014).
Conclusion
These limitations notwithstanding, we have described differences in the patterns of antidepressant prescription fills across racial/ethnic groups following the FDA’s boxed warning of 2004. Both antidepressant prescription fills and depression treatment declined most for White youth following the warning, suggesting that the warning had less of a negative impact on these outcomes among the providers and/or caregivers of Black and Latino youth. However, when initiating new treatment, providers increasingly chose an FDA-indicated, evidence-based treatment (fluoxetine) across all racial/ethnic groups, especially among Black youth. Our results provide greater detail of the impact FDA warnings have on prescription patterns among minority youth, who are vulnerable to under-treatment of depressive disorders.
Supplementary Material
Figure 3. Rates of Incident Fluoxetine Prescription Fills Over Time
Acknowledgments
This study was funded by the Agency for Healthcare Research and Quality. The grant number is 4 R01 HS021486-03.
Appendix Figure 1.
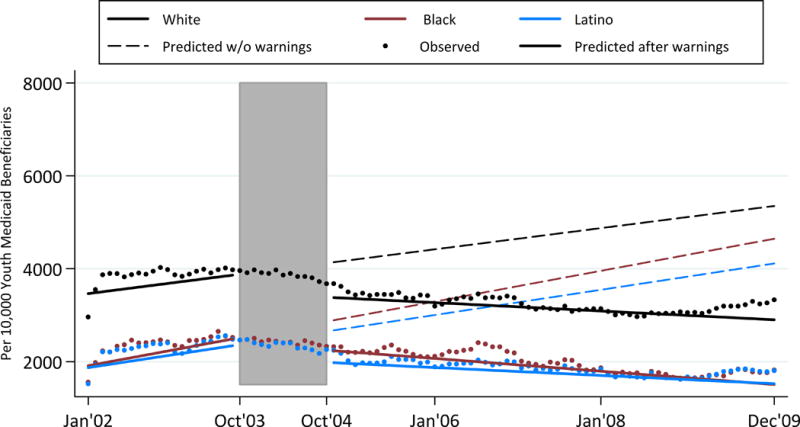
Rates of Antidepressant Prescription Fills Among Youth with a Depressive Disorder
Shaded area represents the phase-in period from October 2003 to October 2004. The rate is per 10,000 youth Medicaid beneficiaries who received treatment for depression.
Appendix Table 1.
Predicted Changes in Rates and Slopes in Treatment Visits for Depressive Disorder Before and After FDA Black Box Warning, by Race/Ethnicity (n=249)
| White | Black | Latino | |||
|---|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Different from White | Estimate (SE) | Different from White | |
|
| |||||
| Baseline Jan 2002 Rate† | 112.54 (2.47) | 62.71 (1.72) | *** | 41.94(1.46) | *** |
|
| |||||
| Change in rate after warningΔ | −2.40 (4.20) | −2.07 (2.95) | ns | −4.17 (2.53) | ns |
|
| |||||
| Slope (Δ/month) before warning‡ | 0.20 (0.18) | −0.08 (0.13) | ns | 0.32 (0.11)** | ns |
|
| |||||
| Slope (Δ/month) after warning‡ | −0.54 (0.04)*** | 0.09 (0.03)** | *** | 0.04 (0.02) | *** |
|
| |||||
| Change in slope | −0.74 (0.19)*** | 0.17 (0.13) | *** | −0.29 (0.11)* | * |
p<0.05,
p<0.01,
p<0.001
Rate is number of treatment visits/10,000 youth per month
Slope is monthly change in number of treatment visits/10,000 youth
Change in rate is level shift between October 2003 and October 2004
Appendix Table 2.
Predicted Changes in Rates and Slopes in Incident Prescription Fills of Fluoxetine Before and After FDA Boxed Warning, by Race/Ethnicity (n=240)
| White | Black | Latino | |||
|---|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Different from White | Estimate (SE) | Different from White | |
|
| |||||
| Baseline Jan 2002 Rate† | 765.19 (79.87) | 711.98(113.89) | ns | 893.89(109.59) | ns |
|
| |||||
| Change in rate after warningΔ | 658.57 (122.09)*** | 763.31(182.28)*** | ns | 840.25 (174.80)*** | ns |
|
| |||||
| Slope (Δ/month) before warning‡ | 10.56 (5.37)* | 10.37 (8.02)* | ns | 16.46 (7.69)* | ns |
|
| |||||
| Slope (Δ/month) after warning‡ | 2.93 (0.86)*** | 6.09(1.29)*** | * | 2.85 (1.24)* | ns |
|
| |||||
| Change in slope | −7.63 (5.45) | −4.28 (8.15) | ns | −13.60 (7.82) | ns |
p<0.05,
p<0.01,
p<0.001
Rate is number of incident fluoxetine prescription fills/10,000 youth per month
Slope is monthly change in number of prescription fills/10,000 youth
Change in rate is level shift between October 2003 and October 2004
Appendix Table 3.
Predicted Changes in Rates and Slopes in Antidepressant Prescription Fills Among Those Receiving Treatment for Depression and After FDA Boxed Warning, by Race/Ethnicity (n=249)
| White | Black | Latino | |||
|---|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Different from White | Estimate (SE) | Different from White | |
|
| |||||
| Baseline Jan 2002 Rate† | 3445.20 (86.20) | 1844.81 (68.10) | *** | 1848.30 (63.01) | *** |
|
| |||||
| Change in rate after warningΔ | −731.98 (146.81)*** | −614.44 (116.88)*** | ns | −665.91 (108.52)*** | ns |
|
| |||||
| Slope (Δ/month) before warning‡ | 19.86 (6.35)** | 28.76 (5.03)*** | ns | 23.59 (4.66)*** | ns |
|
| |||||
| Slope (Δ/month) after warning‡ | −7.84 (1.46)*** | −11.93 (1.15)*** | *** | −7.40 (1.06)*** | ns |
|
| |||||
| Change in slope | −27.70 (6.75)*** | −40.68 (5.33)*** | * | −30.99 (4.93)*** | ns |
p<0.05,
p<0.01,
p<0.001
Rate is number of prescription fills/10,000 youth with a diagnosis of depressive disorder per month
Slope is monthly change in number of prescription fills/10,000 youth
Change in rate is level shift between October 2003 and October 2004
Footnotes
All the authors declare no potential conflicts of interest at this time or in the past three years.
Contributor Information
Nicholas Carson, Center for Multicultural Mental Health Research, Cambridge Health Alliance & Harvard Medical School, 1035 Cambridge Street, Suite 26, Cambridge, MA 02141, Fax: (617) 806-8740, Office: (617) 617-5269.
Ana Progovac, Center for Multicultural Mental Health Research, Cambridge Health Alliance & Harvard Medical School
Ye Wang, Massachusetts General Hospital
Benjamin L. Cook, Center for Multicultural Mental Health Research, Cambridge Health Alliance & Harvard Medical School.
References
- Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351(6):575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Rezac AJ, Vitiello B, Sitorius MA, Buehler BA, Kratochvil CJ. Antidepressant prescribing practices for the treatment of children and adolescents. J Child Adolesc Psychopharmacol. 2008;18(1):70–80. doi: 10.1089/cap.2007.0049. [DOI] [PubMed] [Google Scholar]
- Busch SH, Frank RG, Leslie DL, Martin A, Rosenheck RA, Martin EG, Barry CL. Antidepressants and suicide risk: how did specific information in FDA safety warnings affect treatment patterns? Psychiatr Serv. 2010;61(1):11–16. doi: 10.1176/appi.ps.61.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook BL, Zuvekas SH, Carson N, Wayne GF, Vesper A, McGuire TG. Assessing racial/ethnic disparities in treatment across episodes of mental health care. Health Serv Res. 2014;49(1):206–229. doi: 10.1111/1475-6773.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal S, Akincigil A, Bilder S, Walkup JT. Studying Prescription Drug Use and Outcomes With Medicaid Claims Data: Strengths, Limitations, and Strategies. Medical Care. 2007;45(10):S58–S65. doi: 10.1097/MLR.0b013e31805371bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal S, Akincigil A, Bilder S, Walkup JT. Studying prescription drug use and outcomes with medicaid claims data: strengths, limitations, and strategies. Med Care. 2007;45(10 Supl 2):S58–65. doi: 10.1097/MLR.0b013e31805371bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JR, Druss BG. Racial/ethnic differences in mental health service use among adolescents with major depression. J Am Acad Child Adolesc Psychiatry. 2011;50(2):160–170. doi: 10.1016/j.jaac.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePetris AE, Cook BL. Differences in diffusion of FDA antidepressant risk warnings across racial-ethnic groups. Psychiatr Serv. 2013;64(5):466–471. 471 e461–464. doi: 10.1176/appi.ps.201200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePetris AE, Cook BL. Differences in Diffusion of FDA Antidepressant Risk Warnings Across Racial-Ethnic Groups. Psychiatric Services. 2013;64(5):466–471. doi: 10.1176/appi.ps.201200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusetzina SB, Busch AB, Conti RM, Donohue JM, Alexander GC, Huskamp HA. Changes in antipsychotic use among patients with severe mental illness after a Food and Drug Administration advisory. Pharmacoepidemiol Drug Saf. 2012;21(12):1251–1260. doi: 10.1002/pds.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusetzina SB, Cook BL, Busch AB, Alexander GC, Huskamp HA. Racial-ethnic differences in incident olanzapine use after an FDA advisory for patients with schizophrenia. Psychiatr Serv. 2013;64(1):83–87. doi: 10.1176/appi.ps.201200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusetzina SB, Higashi AS, Dorsey ER, Conti R, Huskamp HA, Zhu S, Garfield CF, Alexander GC. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med Care. 2012;50(6):466–478. doi: 10.1097/MLR.0b013e318245a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Hawton K, Rodham K, Deeks J. The prevalence of suicidal phenomena in adolescents: a systematic review of population-based studies. Suicide Life Threat Behav. 2005;35(3):239–250. doi: 10.1521/suli.2005.35.3.239. [DOI] [PubMed] [Google Scholar]
- Friedman RA. Antidepressants’ black-box warning–10 years later. N Engl J Med. 2014;371(18):1666–1668. doi: 10.1056/NEJMp1408480. [DOI] [PubMed] [Google Scholar]
- Geller B, Reising D, Leonard HL, Riddle MA, Walsh BT. Critical Review of Tricyclic Antidepressant Use in Children and Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(5):513–516. doi: 10.1097/00004583-199905000-00012. [DOI] [PubMed] [Google Scholar]
- Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S. Inconsistencies between self-reported ethnicity and ethnicity recorded in a health maintenance organization. Ann Epidemiol. 2005;15(1):71–79. doi: 10.1016/j.annepidem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Haider M, Kreps GL. Forty years of diffusion of innovations: utility and value in public health. J Health Commun. 2004;9(Suppl 1):3–11. doi: 10.1080/10810730490271430. [DOI] [PubMed] [Google Scholar]
- Hammad TA, Neyarapally GA, Iyasu S, Staffa JA, Dal Pan G. The future of population-based postmarket drug risk assessment: a regulator’s perspective. Clin Pharmacol Ther. 2013;94(3):349–358. doi: 10.1038/clpt.2013.118. [DOI] [PubMed] [Google Scholar]
- Hirsch GS. Antidepressants (SSRIs) and their use in children and adolescents. Retrieved May 29, 2014, from http://www.aboutourkids.org/articles/antidepressants_ssris_their_use_in_children_adolescents.
- Horvitz-Lennon M, Volya R, Donohue JM, Lave JR, Stein BD, Normand SL. Disparities in quality of care among publicly insured adults with schizophrenia in four large U.S. states, 2002–2008. Health Serv Res. 2014;49(4):1121–1144. doi: 10.1111/1475-6773.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation Health Insurance Coverage of Children 0–18. State Health Facts. http://kff.org/other/state-indicator/children-0-18/?currentTimeframe=0.
- Katz R. NDA 18-936/S-064. Rockville, MD: 2003. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2003/18936se5-064ltr.pdf. [Google Scholar]
- Keeler EB, Manning WG, Wells KB. The demand for episodes of mental health services. J Health Econ. 1988;7(4):369–392. doi: 10.1016/0167-6296(88)90021-5. [DOI] [PubMed] [Google Scholar]
- Libby AM, Brent DA, Morrato EH, Orton HD, Allen R, Valuck RJ. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry. 2007;164(6):884–891. doi: 10.1176/ajp.2007.164.6.884. [DOI] [PubMed] [Google Scholar]
- Lu CY, Soumerai SB, Ross-Degnan D, Zhang F, Adams AS. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar illness. Med Care. 2010;48(1):4–9. doi: 10.1097/MLR.0b013e3181bd4c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Zhang F, Lakoma MD, Madden JM, Rusinak D, Penfold RB, Simon G, Ahmedani BK, Clarke G, Hunkeler EM, Waitzfelder B, Owen-Smith A, Raebel MA, Rossom R, Coleman KJ, Copeland LA, Soumerai SB. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi: 10.1136/bmj.g3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Foundation for Medical Education and Research. Depression Medication Choice. Mayo Clinic Shared Decision Making National Resource Center; 2011. [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Druss BG. Effects of Food and Drug Administration warnings on antidepressant use in a national sample. Arch Gen Psychiatry. 2008;65(1):94–101. doi: 10.1001/archgenpsychiatry.2007.5. [DOI] [PubMed] [Google Scholar]
- Olson AL, Kelleher KJ, Kemper KJ, Zuckerman BS, Hammond CS, Dietrich AJ. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1(2):91–98. doi: 10.1367/1539-4409(2001)001<0091:pcprap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38–44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care. 2003;19(4):613–623. doi: 10.1017/s0266462303000576. [DOI] [PubMed] [Google Scholar]
- Rector TS, Adabag S, Cunningham F, Nelson D, Dieperink E. Outcomes of Citalopram Dosage Risk Mitigation in a Veteran Population. Am J Psychiatry. 2016;173(9):896–902. doi: 10.1176/appi.ajp.2016.15111444. [DOI] [PubMed] [Google Scholar]
- Saloner B, Carson N, Cook BL. Episodes of Mental Health Treatment Among a Nationally Representative Sample of Children and Adolescents. Medical Care Research and Review. 2014;71(3):261–279. doi: 10.1177/1077558713518347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ND, V, Montori M, Krumholz HM, Tu K, Alexander GC, Jackevicius CA. Responding to an FDA warning–geographic variation in the use of rosiglitazone. N Engl J Med. 2010;363(22):2081–2084. doi: 10.1056/NEJMp1011042. [DOI] [PubMed] [Google Scholar]
- Stone MB. The FDA warning on antidepressants and suicidality–why the controversy? N Engl J Med. 2014;371(18):1668–1671. doi: 10.1056/NEJMp1411138. [DOI] [PubMed] [Google Scholar]
- Tansella M, Micciolo R, Biggeri A, Bisoffi G, Balestrieri M. Episodes of care for first-ever psychiatric patients. A long-term case-register evaluation in a mainly urban area. Br J Psychiatry. 1995;167(2):220–227. doi: 10.1192/bjp.167.2.220. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. National Drug Code Directory. Retrieved May 29, 2014, from http://www.fda.gov/drugs/informationondrugs/ucm142438.htm.
- Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363(9418):1341–1345. doi: 10.1016/S0140-6736(04)16043-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 3. Rates of Incident Fluoxetine Prescription Fills Over Time


