Abstract
Low concentrations of sugars altered the sensitivity of seed germination to inhibition by exogenous abscisic acid (ABA). Germination of wild-type and ABA-insensitive (abi) Arabidopsis seeds was assayed on media containing ABA and a variety of sugars and sugar alcohols. The inhibitory effects of ABA were strongly repressed in the presence of 15 to 90 mm glucose (Glc), sucrose, or fructose, but not by comparable concentrations of sorbitol or mannitol. Several features of the response to Glc are inconsistent with a purely nutritional effect: The optimal sugar concentration is low and differs between the wild type and the abi mutants. Furthermore, Glc suppression of ABA inhibition is light dependent and limited to the process of radicle emergence.
Seed germination can be divided into three phases, imbibition, increased metabolic activity, and initiation of growth, that loosely parallel the triphasic water uptake of mature dry seeds (Bewley, 1997). Imbibition is a physical phenomenon driven by the extremely low matric potential of dry seeds and is not subject to physiological control (Bewley and Black, 1985). Morphologically, initiation of growth corresponds to radicle emergence; subsequent growth is generally defined as seedling growth. Progression through the phases of increased metabolic activity and initiation of growth is tightly regulated by environmental and hormonal signals because seedlings become committed to growth upon entry into the final phase of germination, when they lose desiccation tolerance. For example, light promotes seed germination in many species and its effects are thought to be mediated, at least in part, by a combination of increased synthesis and perception of gibberellins and decreased abscisic acid (ABA) levels (Koornneef and Karssen, 1994; Toyomasu et al., 1994, 1998; Yang et al., 1995). In contrast, germination is strongly inhibited by ABA and high solute concentrations or limited water availability (Bewley and Black, 1985). By using molecular markers, such as enzymes required for mobilization of food reserves in cereal grains, it has been established that GA and ABA exert their antagonistic effects through multiple regulatory mechanisms, including transcriptional control and synthesis of specific inhibitors of enzyme activities (for review, see Jacobsen and Chandler, 1987; Jacobsen, 1995).
Recently, many examples of carbohydrate-regulated gene expression in plants have been described. Most of these gene products are involved in carbon metabolism, transport, or uptake affecting source-sink relationships within the plant body (for review, see Koch, 1996; Jang and Sheen, 1997; Smeekens and Rook, 1997). Studies of the mechanisms of carbohydrate regulation in plants have shown that the sugars themselves are often the signal molecules. These results are highly reminiscent of microbial and animal catabolite repression responses. However, although plant hexokinase genes can complement the catalytic functions that are lacking in yeast hexokinase mutants, overexpression of a yeast hexokinase in plants reduces sugar sensitivity, indicating that the regulatory mechanisms are not interchangeable (Jang et al., 1997).
Interactions between sugar and hormonal signaling have been demonstrated for ethylene (Zhou et al., 1998), auxin (Dewald et al., 1994) and cytokinins (Jang et al., 1997). In the present study, we provide evidence that sugar availability also affects the regulation of germination by ABA. By comparing germination of wild-type and ABA-insensitive (abi) Arabidopsis seeds on media containing ABA and a variety of sugars, we found that the inhibitory effects of ABA are dramatically suppressed in the presence of relatively low concentrations of Glc, Suc, or Fru. Similar results were reported by Garciarrubio et al. (1997), but were interpreted as evidence that sugars or amino acids overcome ABA inhibition of germination by supplying energy and nutrients, thereby relieving a metabolic block. The relationship we observe between the Glc dose and suppression of ABA inhibition of germination, as well as the lack of continued seedling growth in the presence of added nutrients, is not consistent with a solely nutritional effect.
MATERIALS AND METHODS
Plant Material
The Arabidopsis ecotype Landsberg erecta (Ler) (the wild type) and abi1-1 mutant (Koornneef et al., 1984) were used. Seeds of both genotypes were collected from plants grown at 22°C in continuous light, then stored dry at room temperature for several months before being used in germination assays.
Germination Assays
For germination assays, Arabidopsis seeds were surface-sterilized in 5% (v/v) hypochlorite and 0.02% (v/v) Triton X-100, then rinsed three to four times with sterile water before plating on minimal medium (Haughn and Somerville, 1986) containing 0.7% (w/v) agar and ABA (mixed isomers, Sigma, St. Louis), sugars, analogs, or inhibitors at the indicated concentrations in 15- × 100-mm Petri dishes. At least 40 seeds were used for viability controls in each experiment, and 100 to 200 seeds per treatment were used for experimental conditions. The dishes were incubated for 3 d at 4°C to break any residual dormancy, then transferred to 22°C in continuous light (50–70 μE m−2 s−1).
Isocitrate Lyase (ICL) Activity Assays
Brassica rapa seeds (obtained from University of Wisconsin-Madison Crucifer Genetics Coop.) were surface-sterilized and cultured as described for Arabidopsis. At the indicated times, seedlings were harvested, weighed, and flash-frozen in liquid nitrogen. Frozen samples were ground in 10 to 20 mL/g fresh weight of buffer (100 mm KPO4, pH 6.9, 6 mm MgCl2, and 3 mm dithiothreitol [DTT]) using a sintered glass homogenizer, then microfuged to remove insoluble material. ICL activity of extracts was assayed spectrophotometrically, as described in Cooper and Beevers (1969).
Histochemical Staining for Invertase Activity
Arabidopsis seedlings were fixed in 4% (v/v) formalin for 30 min, then rinsed extensively in water to remove endogenous sugars. Invertase was assayed histochemically as described in Duke et al. (1991).
RNA Analysis
RNA was isolated by hot phenol extraction as described previously (Finkelstein et al., 1985). Total RNA from plantlets (10 μg per lane) was size fractionated on 1% (w/v) agarose 3-(N-morpholino)-propanesulfonic acid (MOPS)-formaldehyde gels (Sambrook et al., 1989), then transferred to Nytran membranes using 20× SSPE as blotting buffer. RNA was bound to the filters by UV cross-linking (120 mJ/cm2 at 254 nm). Uniformity of loading and transfer was assayed qualitatively by methylene blue staining of the filters (Herrin and Schmidt, 1988). The cor6.6 and RAB18 mRNAs were detected by hybridization to cDNA clones (Hajela et al., 1990; Parcy et al., 1994) and labeled by random priming to a specific activity of 108 cpm/μg (Hodgson and Fisk, 1987). Hybridization conditions were (50% [v/v] formamide, 5× SSPE, 5× Denhardts, 0.1% [w/v] SDS, and 200 μg/mL DNA) at 43°C in a rotisserie oven (Hyb-Aid, Middlesex, UK). Filters were washed twice at 60°C in 2× SSC, 0.1% (w/v) SDS and once at 60°C in 0.2× SSC, 0.1% (w/v) SDS for 30 to 60 min. All hybridization reagents were prepared as described in Sambrook et al. (1989).
RESULTS
Metabolizable Sugars Reduce Sensitivity of Germination to Inhibition by ABA
In experiments testing for improved growth of a near-lethal ABA-insensitive (abi) Arabidopsis mutant, we noticed that survival on Glc-containing medium was significantly enhanced relative to that on medium with Suc or no carbon source (R. Finkelstein, unpublished observations). When Glc was included in the selection medium, wild-type control seeds were much less sensitive to ABA than on sugar-free medium. To determine whether this was a metabolic or an osmotic effect, we compared the effects of a variety of mono- and di-saccharides and sugar alcohols on ABA sensitivity. Germination (i.e. radicle emergence) of wild-type and abi1 seeds was tested in the presence and absence of 3 μm ABA and 60 mm sugars (Fig. 1). In addition, we tested 30 mm Suc because it can be metabolized into Glc and Fru at 30 mm each, for a total of 60 mm monosaccharides.
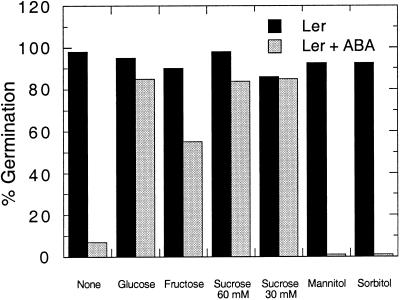
Figure 1.
Germination of wild-type (Ler) seeds after 7 d of incubation in the presence and absence of 3 μm ABA and the indicated sugars (60 mm unless noted otherwise). Percentages represent assays of 40 to 200 seeds per treatment for each genotype.
When added to minimal medium, 3 μm ABA was sufficient to limit germination of wild-type Ler seeds to less than 10% (Fig. 1). Abi1 mutant seeds were resistant to this concentration of ABA, and all culture conditions permitted essentially complete germination (data not shown). The addition of 60 mm Glc or Suc to medium containing 3 μm ABA permits 90% germination of wild-type seeds, while Fru is a slightly less-effective suppressor of ABA inhibition, allowing 60% germination of wild-type seeds. In contrast, germination of wild-type seeds in the presence of ABA did not occur when 60 mm mannitol or sorbitol was added. Similar results were obtained for wild-type seeds of the Columbia (Col) and Wassilewskija (WS) ecotypes, although both are intrinsically somewhat less sensitive to ABA than Ler. After 1 week on minimal medium supplemented with 3 μm ABA, the germination percentage of Ws and Col seeds was 2- or 7-fold higher, respectively, than that of Ler seeds. Although these data indicate that the inclusion of Glc obscures the differential ABA sensitivity of wild-type versus the abi mutant, the germination percentages reflect only radicle emergence from the seed coat. Subsequent growth of the wild-type seedlings was still ABA sensitive, resulting in unexpanded whitish-purple plants, while the abi1 seedlings were green and fully expanded when exposed to 3 μm ABA (data not shown).
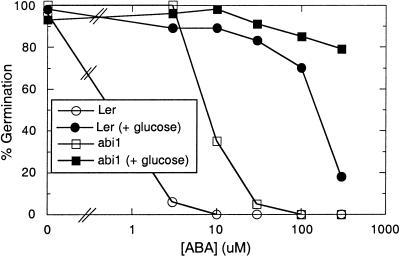
When germination was tested in the presence and absence of 60 mm Glc, addition of Glc significantly reduced the sensitivity to ABA in both wild-type and abi1 seeds (Fig. 2). Radicle emergence was observed in wild-type seeds exposed to 100-fold more ABA than was tolerated on Glc-free medium, while abi1 seeds tolerated nearly 40-fold more ABA. At ABA concentrations above 30 μm in the presence of Glc, the wild-type and abi1 seeds looked similar: both genotypes were stunted and whitish-purple, presumably due to stress-induced anthocyanin accumulation. However, the genotypes were still distinguishable because the wild-type seeds germinated several days later than the abi1 seeds.
Figure 2.
ABA dose response of wild-type (Ler) or abi1 seed germination in the absence (white symbols) or presence (black symbols) of 60 mm Glc. Germination was scored after 7 d of incubation on the indicated [ABA]. Percentages represent assays of 60 to 200 seeds per treatment for each genotype.
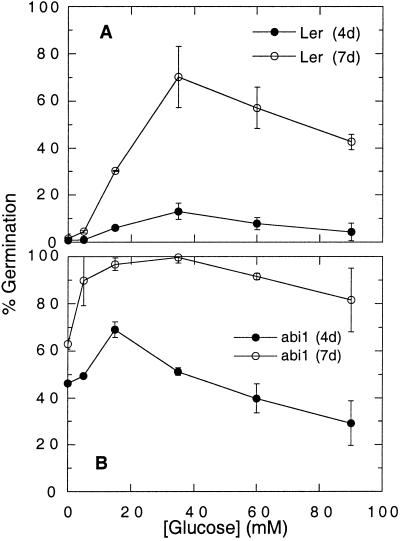
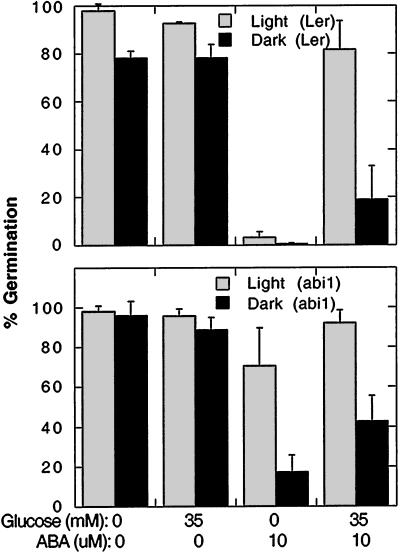
To determine whether the Glc effect was nutritional, we assayed the effects of a series of Glc concentrations on germination in the presence of 10 μm ABA (Fig. 3). Over a range of Glc concentrations from 0 to 90 mm, none of which is high enough to inhibit germination by osmotic effects (Fig. 1 and data not shown), the strongest suppression of ABA inhibition of germination occurred at 35 mm for wild-type seeds and at 15 mm Glc for abi1 seeds (Fig. 3). The fact that the higher Glc concentrations (60–90 mm) were less effective in relieving the ABA inhibition than the optimal Glc concentrations, in addition to the lack of continued seedling growth described above, suggests that the Glc effect is not purely nutritional. Moderate concentrations (0.04%) of reduced nitrogen in the form of either Gln or casamino acids had no effect on ABA inhibition of germination (data not shown). Garciarrubio et al. (1997) reported that 0.3% (w/v) peptone could partially relieve ABA inhibition of germination and acted synergistically with 29 mm Glc to permit nearly complete germination after 7 d on 10 μm ABA. However, even with 0.3% (w/v) peptone added, wild-type growth was arrested and the seedlings remained stunted and whitish-purple for at least 10 d, starting to green only after 2 to 3 weeks (data not shown).
Figure 3.
Dose response of Glc suppression of ABA inhibition of germination. Germination of wild-type Ler (A) and abi1 (B) seeds was scored after 4 d (black symbols) and 7 d (white symbols) incubation on 10 μm ABA supplemented with the indicated concentrations of Glc. Data points represent averages ± sd of duplicate assays of at least 100 seeds per treatment for each genotype.
Germination can be inhibited by a variety of factors, including high osmoticum and ABA. To determine whether Glc produces a generalized increase in germination potential under otherwise inhibitory conditions, we tested whether Glc ameliorated the germination inhibition (approximately 50%) due to a high concentration of sorbitol (500 mm). Glc had no effect on osmotic inhibition of wild-type seed germination (data not shown), indicating that it probably does not increase germination potential by reducing seedling water potential to enhance water uptake and subsequent cell expansion.
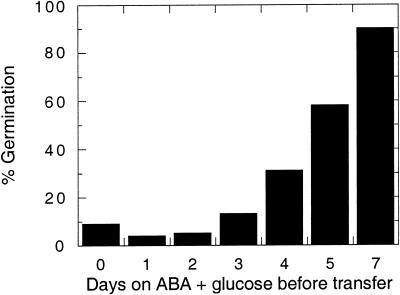
To determine whether the Glc suppression of ABA sensitivity depended on continuous or transient exposure to Glc, we performed a medium-shift experiment. Seeds were plated initially on ABA plus Glc, then transferred at daily intervals to medium containing ABA alone. Wild-type germination was first observed after 4 d on ABA/Glc, but did not occur to any significant extent in seeds transferred to ABA alone prior to 4 d (Fig. 4). This indicated that exposure to Glc was required during the 3 to 7 d post-imbibition period to suppress ABA inhibition of germination.
Figure 4.
Dependence on continuous exposure to Glc. Wild-type (Ler) seeds were incubated on 10 μm ABA and 35 mm Glc for the indicated number of days before transfer to 10 μm ABA. Percent germination was scored after 7 d (total incubation time). Percentages represent assays of 50 to 170 seeds per treatment.
Light Enhances the Glc Effect on ABA Sensitivity
Many Arabidopsis ecotypes, including Ler, require light and/or chilling to break dormancy (Koornneef and Karssen, 1994). To determine whether the sugar repression of ABA inhibition of germination was light dependent, we tested dark germination of wild-type and abi1 seeds in the presence and absence of Glc and ABA following a dormancy-breaking chilling treatment. Glc only partially suppressed the ABA inhibition of germination when seeds were incubated in the dark (Fig. 5). While 10 μm ABA almost completely blocked germination of wild-type seeds in either light or dark, supplementing with 35 mm Glc permitted approximately 20% or 80% germination of wild-type seeds after 1 week in the dark or light, respectively. However, even the abi1 mutant did not germinate well on ABA in the dark. The abi1 mutant, although generally described as non-dormant, has some residual photodormancy (Koornneef et al., 1984) that can usually be overcome by a chilling treatment.
Figure 5.
Glc suppression of ABA-inhibited seed germination is partially light dependent. Germination was scored after 7 d of incubation in light or dark on the indicated media. Data points represent averages ± sd of duplicate assays of 50 to 140 seeds per treatment for each genotype.
Glc Effects on Metabolic Aspects of Germination
The germination assays with Arabidopsis seeds indicated that Glc could partially overcome ABA inhibition of emergence from the seed coat. We were interested in determining whether this morphological change was accompanied by the standard metabolic changes such as increased glyoxylate cycle enzyme activities. To easily obtain enough tissue for measuring enzyme activities, we used a larger-seeded relative, the rapid-cycling B. rapa. After establishing that Glc suppressed ABA inhibition of radicle and cotyledon emergence in this species as well (Fig. 6A), we compared the time course of changes in ICL activity in the presence and absence of 10 μm ABA and 35 mm Glc.
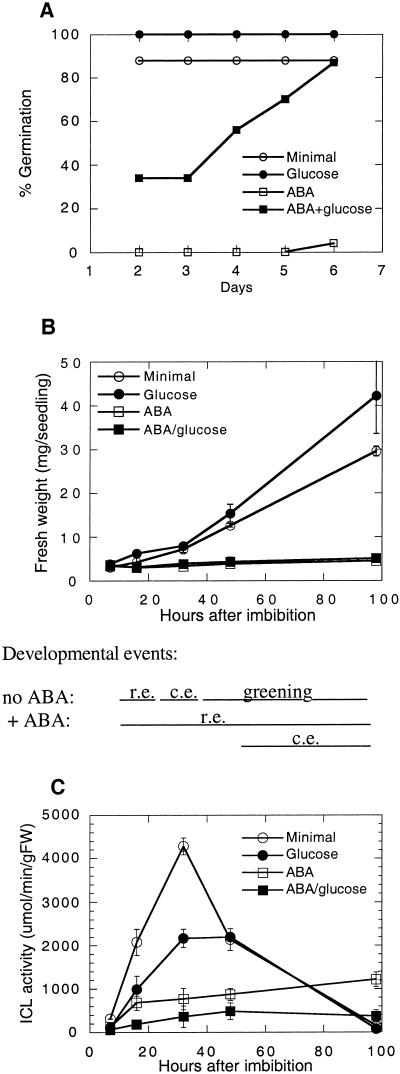
Figure 6.
Time course of B. rapa germination. A, Radicle emergence on media with or without 35 mm Glc and/or 10 μm ABA. Percentages represent assays of 23 to 25 seeds per treatment. B, Changes in fresh weight and developmental events (r.e., radicle emergence; c.e., cotyledon emergence) on media with or without 35 mm Glc and/or 10 μm ABA. C, Effects of Glc and/or ABA on ICL activity in B. rapa seedlings. Data points represent means ± se of duplicate samples, each comprised of three to 10 seeds/seedlings.
Seeds and seedlings were harvested after 7, 16, 32, 48, and 98 h of incubation in continuous light post imbibition. These time points were chosen because they bracket the period from radicle emergence to cotyledon expansion and greening (Fig. 6B), by which time these enzyme activities usually decline in seeds incubated without ABA. For seeds incubated with ABA, the entire process takes longer, so the asynchronies of the populations are more apparent. In the presence of ABA, radicle emergence is first observed at 8 h, but is not completed by all seeds until after 98 h.
Emergence of cotyledons from the seed coat of ABA-treated seeds occured almost exclusively on the Glc-supplemented medium (starting after 48 h), and full expansion and greening of the cotyledons was never observed on any ABA-containing medium. Thus, the 7-h no-ABA samples were morphologically similar to all of the plus-ABA samples. As anticipated for the no-ABA controls, induction of ICL activity was delayed and depressed in Glc-treated seedlings, which is consistent with control by catabolite repression (Fig. 6C) (Graham et al., 1994). As has been described previously for other species (Marriott and Northcote, 1977), the ABA treatments also repressed ICL activity, even for seedlings of a comparable developmental stage, e.g. 16-h no-ABA and 98-h 10 μm ABA (Fig. 6C). ICL activity was even lower in seeds treated with Glc and ABA than in those treated with ABA alone, indicating that the morphological aspects of germination, i.e. radicle emergence, were not correlated with the standard metabolic changes, e.g. storage reserve mobilization, during Glc/ABA treatment.
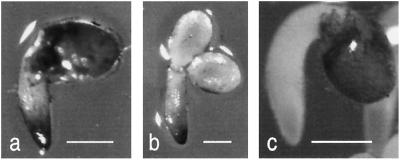
Glc signaling has been shown to induce apoplastic invertase activity in suspension-culture cells (Roitsch et al., 1995). In intact plants, the corresponding gene is expressed only in roots. Recently, antisense expression of a cell wall invertase gene was shown to severely limit root growth, and antisense expression of a vacuolar invertase gene resulted in a moderate reduction of root growth (Tang et al., 1999). These data are consistent with the hypothesis that sugars regulate carbohydrate partitioning by increasing extracellular invertase activity, thereby increasing the phloem sap-cell wall Suc gradient driving phloem unloading in sink tissues. The Glc effect on reducing ABA sensitivity is limited to the earliest visible phases of germination: emergence of radicles and cotyledons from the seed coat. The radicle must become an effective sink to sustain the substantial growth of the emerging root. Therefore, to determine whether Glc stimulates ABA-resistant germination via increased invertase activity, we histochemically stained emerging wild-type Arabidopsis roots from the various media treatments. Radicle emergence, which was accompanied by invertase staining in emerging root tips, occurred after 2 d on medium containing Glc (Fig. 7a) or 4 d on ABA and Glc (Fig. 7b). On hormone-free medium, root growth and invertase activity were sustained, while seeds on ABA and Glc stopped growing and invertase activity had disappeared by 10 d (data not shown). Thus, continued growth was correlated with sustained invertase activity. Because histochemical staining does not distinguish among the various isoforms of invertase, it is not clear whether the observed invertase activity is apoplastic, enhancing sink strength, or intracellular, promoting cell enlargement and/or metabolizing Suc.
Figure 7.
Histochemical localization of invertase activity in germinating wild-type seeds incubated for 2 d on medium containing Glc (a and c) or 4 d on medium containing ABA and Glc (b). The formation of blue formazon reaction product in root tips (a and b) was dependent on Suc in the reaction medium (missing in c). Bars = 0.5 mm.
Glc Does Not Alter ABA-Induced Gene Expression in Plantlets
Having observed a dramatic interaction between sugar and ABA effects on germination, we wanted to determine whether this was just one example of a more general antagonism between sugar and ABA responses. Many ABA-inducible genes have been identified through studies of a variety of stress responses, and we tested the accumulation of two such transcripts, cor 6.6 (Hajela et al., 1990) and Rab18 (Parcy et al., 1994), in 13-d-old wild-type plants that had been cultured for 48 h on ABA-containing or control medium, with or without a 35 mm Glc supplement. These genes were equally ABA responsive whether or not Glc was present (data not shown).
DISCUSSION
While exogenously supplied sugars relieve ABA inhibition of some aspects of germination, this effect is limited to emergence from the seed coat and does not extend to greening and mobilization of lipid reserves. These results are similar to those of Garciarrubio et al. (1997), who reported that sugars and peptone cooperatively promoted germination, i.e. radicle emergence, of wild-type Arabidopsis even in the presence of up to 30 μm ABA, but did not alter ABA-dependent protein synthesis or promote storage protein degradation. In determining the optimal concentration for promoting germination in the presence of ABA, we tested Glc concentrations ranging from 5 to 90 mm, while Garciarrubio et al. (1997) tested 0.5% to 20% (29 mm--1.16 m). Although we both found the 29 to 58 mm range to be optimal for wild-type seeds, our interpretations are different. Because they did not test any intermediate Glc concentrations between 58 and 290 mm, they ascribed the decrease in effectiveness at higher concentrations to the onset of an osmotic inhibition, and interpreted the effect of low concentrations as overcoming a nutritional deficiency. The observation that inclusion of a reduced nitrogen source permitted even more extensive growth was consistent with this interpretation. In contrast, because we observe a decrease in effectiveness at Glc concentrations well below an osmotically inhibitory level, we conclude that the Glc effect is unlikely to be solely nutritional. Furthermore, the differing optima for wild-type versus abi1 mutant seeds, the light dependence of the Glc effect, and the failure of added nutrients to promote continued seedling growth are not easily reconciled with a simple nutrient deficiency mechanism.
Many recent studies have demonstrated that sugars have important regulatory and nutritional effects on plants (for review, see Koch, 1996; Jang and Sheen, 1997; Smeekens and Rook, 1997). There have been documented interactions between sugar signaling and signaling by phytohormones including ethylene (Zhou et al., 1998), auxin (Dewald et al., 1994), and cytokinins (Jang et al., 1997). Our data suggest an interaction between sugar and ABA signaling. Consistent with this, many mutants with altered sugar sensitivity have been identified recently by screening for growth, lack of anthocyanin synthesis, or aberrant expression of sugar-regulated genes on high concentrations (300–360 mm) of sugars (Gibson et al., 1996; Zhou et al., 1996; Boxall et al., 1997; Mita et al., 1997), and several of these have been found to be allelic to some of the previously identified ABI loci, e.g. ABI4 (Gibson et al., 1999; Huijser et al., 1999). Since most of these mutant screens were based on processes other than germination, interactions between ABA and sugar signaling may be a more general phenomenon than is described in this report.
The observation that other processes regulated by ABA (e.g. expression of specific genes) show equivalent sensitivity to ABA regardless of whether exogenous sugar is present suggests that the suppression of ABA-inhibited germination is a specific effect, not merely a consequence of altered ABA uptake or metabolism in the presence of sugars. This interpretation is supported by the observation that Glc does not significantly alter endogenous ABA concentrations in seeds (Garciarrubio et al., 1997). However, because we do not understand the molecular mechanism(s) of germination, we cannot say whether the relevant events depend on the presence of ABA in the same compartments (cellular or tissue level) for effects on radicle emergence as for effects on gene expression. There is substantial evidence for multiple sites and mechanisms of ABA perception leading into multiple signaling pathways (for review, see Leung and Giraudat, 1998), so it is possible that sugars alter ABA availability to one or a few classes of receptors and their dependent responses.
We have also found evidence for light regulation of the sugar effect on ABA sensitivity of germination. Light, GA, and ABA are well-documented regulators of germination, with light and GA generally having promotive effects and ABA having inhibitory effects. We found that the germination-promoting effects of Glc were largely dependent on light. Although light has previously been suggested to promote germination by altering the osmotic potential of the radicle (Nabors and Lang, 1971), exogenous Glc does not alter the wild-type response to osmotic inhibition of germination and therefore does not appear to work via a similar mechanism. Analysis of Arabidopsis mutants defective in ABA or GA synthesis or response has led to the view that GA is required for germination only if dormancy has already been induced by ABA produced during embryogenesis (Karssen and Lacka, 1985).
Exposure to light appears to shift seeds toward greater GA synthesis and sensitivity and, therefore, improved germination. Consistent with this, recent studies have shown that red light dramatically induces expression of a gene encoding the GA biosynthetic enzyme GA 3β-hydroxylase in photoblastic lettuce seeds (Toyomasu et al., 1998). Furthermore, endogenous ABA levels are lower in light- or GA-exposed seeds (Toyomasu et al., 1994), and the light effect could be due to increased ABA degradation, as described in Nicotiana plumbaginifolia seeds (Kraepiel et al., 1994). If phytochrome control of ABA levels also operates in germinating Arabidopsis seeds, this would shift the balance toward germination in the light. Conversely, exogenous ABA can override the germination-promoting effects of light or GA, even for mutants with reduced ABA sensitivity and dormancy (e.g. abi1). The abi1 mutant does not respond to one of the negative regulators of germination (ABA), and consequently requires less of the positive regulators (e.g. GA or Glc) than the wild type does to promote germination. The fact that the presence of exogenous ABA in the dark shifts abi1 seeds back toward non-germination suggests that the non-dormancy of the abi1 mutant is still partially dependent on positive regulators of germination. Consistent with this, Steber et al. (1998) isolated mutants defective in GA biosynthesis and signaling as suppressors of the ABA-resistant germination of abi1-1. However, while exposure to light may alter GA and ABA levels and sensitivity such that the need for additional positive regulators of germination is reduced, substantial ABA-resistant germination of wild-type seeds still depends on added sugars.
The dosage dependence of the Glc effect on ABA sensitivity is suggestive of a signaling rather than a nutritional role for Glc. There is now substantial evidence that Glc acts as a signal of the metabolic state in plants, similar to its role in bacteria, fungi, and animals (for review, see Koch, 1996; Jang and Sheen, 1997; Smeekens and Rook, 1997), although the specific mechanisms of sugar sensing are a matter of debate (Halford et al., 1999; Moore and Sheen, 1999). At least three distinct mechanisms of Glc signaling have been described in microbes and animals: (a) intracellular hexose-activated signaling involving hexokinase, (b) membrane-transport-associated signaling that may or may not involve a hexokinase function, and (c) derepression of Glc-repressed genes by the Suc nonfermenting-1 (SNF1) protein-Ser/Thr kinase complex.
Plant homologs of both hexokinase and SNF1 have been identified, and both classes appear to be involved in mediating some sugar sensing. Hexokinase signaling can be induced by Glc analogs such as 2-deoxyglucose and Man and inhibited by mannoheptulose. Evidence for Glc sensing during transport across the plasma membrane can be provided by the effectiveness of other Glc analogs, e.g. 3-O-methyl-Glc, that are actively taken up by plant cells, but neither metabolized or phosphorylated. However, even low concentrations of either of these classes of analogs (Man at or above 3 mm, 3-O-methyl-Glc at 35–60 mm) inhibit germination in the absence of ABA, and this effect is enhanced in the presence of ABA (Pego et al., 1999, and data not shown). The basis of the Man inhibition has recently been shown to be a hexokinase-mediated step, possibly limiting germination by depletion of energy and carbon sources (Pego et al., 1999). Consistent with this, Glc treatment represses the onset of glyoxylate cycle enzyme activities. Consequently, we were not able to use these analogs to test for possible sugar signaling mechanisms modifying ABA responsiveness of germination. Interestingly, a mutant displaying Man-resistant germination has been found to have a defect in one of the ABI genes (Huijser et al., 1999), ABI4, strengthening the link between sugar and ABA signaling in germination.
It seems initially paradoxical that a single mutation can confer resistance to sugar inhibition of seedling growth and to both Man and ABA inhibition of germination, while sugar also antagonizes ABA inhibition of germination. However, these opposite effects on growth occur at different sugar concentrations and different developmental stages. Metabolizable sugars inhibit seedling growth when present at concentrations greater than 300 mm, and sugar concentrations above 580 mm inhibit germination; both of these inhibitions may be largely osmotic effects that could be mediated by ABA. Consistent with this, several of the abi mutants can germinate in the presence of non-metabolizable sugar alcohols (e.g. sorbitol) or salt at concentrations that inhibit wild-type germination (Werner and Finkelstein, 1995, and data not shown). In contrast, sugars promote germination when present at 10-fold-lower concentrations (29–60 mm) and may act through a different signaling pathway, as well as by augmenting the energy supplies of seeds that are prevented from mobilizing their stored reserves. Although this permits radicle emergence, subsequent growth is arrested. This arrest may reflect the seedling's inability to mobilize sufficient non-carbohydrate reserves to maintain their growth or the loss of vacuolar invertase activity and the concomitant loss of osmotic potential required for root expansion. However, even a combination of sugar and amino acid supplements is not sufficient to sustain seedling growth in the presence of ABA, presumably due to ABA's additional growth inhibitory effects.
Germination of any individual seed is an all-or-nothing event, subject to control by the combined effects of an assortment of positive and negative regulatory signals. In addition to the well-documented hormonal and environmental signals (e.g. ABA, GA, light, and chilling), metabolizable sugars appear to function as both nutritional and signaling stimulants of germination. These results also illustrate the importance of eliminating or minimizing germination-promoting substances such as the low concentrations of sugars commonly included in basal medium (Valvekens et al., 1988) when quantitatively assessing the ABA sensitivity of particular genotypes.
ACKNOWLEDGMENTS
We thank Drs. D. Bush, J. Cooper, and M. Chrispeels for stimulating discussions during the course of this project, and an anonymous reviewer for constructive suggestions regarding this manuscript.
Footnotes
T.J.L. was supported by the U.S. Department of Agriculture (grant no. 95–37304–2217).
LITERATURE CITED
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: Physiology of Development and Germination. New York: Plenum Press; 1985. p. 73. , 80, 115–120, 231–232, 246. [Google Scholar]
- Boxall SF, Martin TR, Graham IA. Arabidopsis thaliana mutants that are carbohydrate insensitive (abstract no. 1266) Plant Physiol. 1997;114:S-247. [Google Scholar]
- Cooper TG, Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. J Biol Chem. 1969;244:3507–3513. [PubMed] [Google Scholar]
- Dewald DB, Sadka A, Mullet JE. Sucrose modulation of soybean Vsp gene expression is inhibited by auxin. Plant Physiol. 1994;104:439–444. doi: 10.1104/pp.104.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke ER, McCarty DR, Koch KE. Organ-specific invertase deficiency in the primary root of an inbred maize line. Plant Physiol. 1991;97:523–527. doi: 10.1104/pp.97.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Tenbarge K, Shumway J, Crouch M. Role of abscisic acid in maturation of rapeseed embryos. Plant Physiol. 1985;78:630–636. doi: 10.1104/pp.78.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarrubio A, Legaria JP, Covarrubias AA. Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta. 1997;203:182–187. doi: 10.1007/s004250050180. [DOI] [PubMed] [Google Scholar]
- Gibson S, Kincaid MS, Kuo M-Y, Ciau N. Sugar insensitive (SIS) mutants of Arabidopsis thaliana (abstract no. 800) Plant Physiol. 1996;111:S-169. [Google Scholar]
- Gibson SI, Laby RJ, Kim D (1999) Sugar-insensitive mutants of Arabidopsis with defects in phytohormone metabolism and/or response. Presented at the 10th International Conference on Arabidopsis Research, July 4–8, 1999, Melbourne, Australia
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajela R, Horvath D, Gilmour S, Thomashow M. Molecular cloning and expression of cor (cold-regulated) genes in Arabidopsis thaliana. Plant Physiol. 1990;93:1246–1252. doi: 10.1104/pp.93.3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford NG, Purcell PC, Hardie DG. Is hexokinase really a sugar sensor in plants? Trends Plant Sci. 1999;4:117–120. doi: 10.1016/s1360-1385(99)01377-1. [DOI] [PubMed] [Google Scholar]
- Haughn G, Somerville C. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet. 1986;204:430–434. [Google Scholar]
- Herrin D, Schmidt G. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988;6:196–200. [PubMed] [Google Scholar]
- Hodgson C, Fisk R. Hybridization probe size control: optimized “oligolabelling.”. Nucleic Acids Res. 1987;14:6295. doi: 10.1093/nar/15.15.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser JC, Pego J, Kortstee A, Smeekens S (1999) The sugar sensing mutant sun6 is insensitive to abscisic acid: involvement of abscisic acid and the ABI genes in sugar sensing. Presented at the 10th International Conference on Arabidopsis Research, July 4–8, 1999, Melbourne, Australia
- Jacobsen JV. Gibberellin action in germinated cereal grains. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry, and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 246–271. [Google Scholar]
- Jacobsen JV, Chandler PM. Gibberellin and abscisic acid in germinating cereals. In: Davies PJ, editor. Plant Hormones and Their Role in Growth and Development. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1987. pp. 164–193. [Google Scholar]
- Jang J, Sheen J. Sugar sensing in higher plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Jang J-C, Leon P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssen C, Lacka E. A revision of the hormone balance theory of seed dormancy: studies on gibberellin and/or abscisic acid-deficient mutants of Arabidopsis thaliana. In: Bopp M, editor. Plant Growth Substances 1985. Heidelberg: Springer-Verlag; 1985. pp. 315–323. [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. In: Jones RL, editor. Annual Review of Plant Physiology and Plant Molecular Biology. Vol. 47. Palo Alto, CA: Annual Reviews; 1996. pp. 509–540. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Karssen CM. Seed Dormancy and Germination. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 313–334. [Google Scholar]
- Koornneef M, Reuling G, Karssen C. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. [Google Scholar]
- Kraepiel Y, Rousselin P, Sotta B, Kerhoas L, Einhorn J, Caboche M, Miginiac E. Analysis of phytochrome- and ABA-deficient mutants suggests that ABA degradation is controlled by light in Nicotiana plumbaginifolia. Plant J. 1994;6:665–672. [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Marriott KM, Northcote DH. The influence of abscisic acid, adenosine 3′,5′ cyclic phosphate, and gibberellic acid on the induction of isocitrate lyase activity in the endosperm of germinating castor bean seeds. J Exp Bot. 1977;28:219–224. [Google Scholar]
- Mita S, Murano N, Akaike M, Nakamura K. Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for beta-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 1997;11:841–851. doi: 10.1046/j.1365-313x.1997.11040841.x. [DOI] [PubMed] [Google Scholar]
- Moore BD, Sheen J. Plant sugar sensing and signaling: a complex reality. Trends Plant Sci. 1999;4:250. doi: 10.1016/s1360-1385(99)01433-8. [DOI] [PubMed] [Google Scholar]
- Nabors MW, Lang A. The growth physics and water relations of red-light-induced germination in lettuce seeds: I. Embryos germinating in osmoticum. Planta. 1971;101:1–25. doi: 10.1007/BF00387687. [DOI] [PubMed] [Google Scholar]
- Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. 1994;6:1567–1582. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JV, Weisbeek PJ, Smeekens SCM. Mannose inhibits Arabidopsis germination via a hexokinase-mediated step. Plant Physiol. 1999;119:1017–1023. doi: 10.1104/pp.119.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Bittner M, Godt DE. Induction of apoplastic invertase of Chenopodium rubrum by D-glucose and a glucose analog and tissue-specific expression suggest a role in sink-source regulation. Plant Physiol. 1995;108:285–294. doi: 10.1104/pp.108.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 7.43–7.52. , B.11–B.15. [Google Scholar]
- Smeekens S, Rook F. Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber CM, Cooney SE, McCourt P. Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics. 1998;149:509–521. doi: 10.1093/genetics/149.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G-Q, Luscher M, Sturm A. Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell. 1999;11:177–189. doi: 10.1105/tpc.11.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T, Kawaide H, Mitsuhashi W, Inoue Y, Kamiya Y. Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiol. 1998;118:1517–1523. doi: 10.1104/pp.118.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T, Yamane H, Murofushi N, Inoue Y. Effects of exogenously applied gibberellin and red light on the endogenous levels of abscisic acid in photoblastic lettuce seeds. Plant Cell Physiol. 1994;35:127–129. [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J, Finkelstein R. Arabidopsis mutants with reduced response to NaCl and osmotic stress. Physiol Plant. 1995;93:659–666. [Google Scholar]
- Yang Y-Y, Nagatani A, Zhao Y-J, Kang B-J, Kendrick RE, Kamiya Y. Effects of gibberellins on seed germination of phytochrome-deficient mutants of Arabidopsis thaliana. Plant Cell Physiol. 1995;36:1205–1211. [PubMed] [Google Scholar]
- Zhou L, Jang J, Sheen J (1996) Glucose insensitive (gin) mutants define downstream pathways for sugar signaling in Arabidopsis development. Seventh International Conference on Arabidopsis, June, 1996, Norwich, UK
- Zhou L, Jang J-C, Jones TL, Sheen J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA. 1998;95:10294–10299. doi: 10.1073/pnas.95.17.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]