Supplemental Digital Content is available in the text
Keywords: gastric cancer, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, prognostic
Abstract
The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been presented to be a prognostic indicator in several types of cancer. However, these issues have not been concluded yet. The present study was therefore performed to determine the prognostic value of NLR and PLR in gastric cancer (GC).
A total of 182 GC patients, diagnosed between January 2011 and January 2014, were enrolled in the study. The clinicopathological parameters, laboratory analyses, and outcomes were collected. The association between NLR, PLR, and clinicopathological characters was analyzed with univariate and multivariate analyses.
NLR was significantly related to age (P = .026), surgery (P = .006), node status (P = .004), and clinical stage (P = .009). The median overall survival (OS) and progression-free survival (PFS) were poor in the High-NLR group (OS: 36.0 vs 20.5 months, P < .001, PFS: 33.0 vs 12.0 months, P < .001) and High-PLR group (OS: 31.5 vs 18.5 months, P = .003, PFS: 26.0 vs 11.0 months, P = .01). Multivariate analyses indicated both surgery [for OS hazard ratio (HR) = 2.092, 95% confidence interval (95% CI): 1.345–3.253, P = .001; for PFS HR = 1.939, 95% CI: 1.259–2.988, P = .003] and NLR (for OS HR = 1.585, 95% CI: 1.011–2.485, P = .045) were independent prognostic factors.
Elevated NLR and PLR were related with poor prognosis in GC patients before treatment. The NLR was an independent prognostic factor for OS. More studies should be conducted to address the potential prognostic value of NLR and PLR in GC.
1. Introduction
Gastric cancer (GC) is one of the most common causes of cancer-related deaths even with improvements in treatment modalities.[1] The ability to predict the precise prognosis is critical for choosing the personally treatment plan and follow-up strategies for a patient. The established prognostic factors were tumor, node, tumor-node-metastasis (TNM) stage, and pathological type, etc.[2] However, it was reported that even within the same tumor stage, the clinical outcomes of patients can be heterogeneous.[3] This implies that further studies should be performed to find more prognostic factors for taking into account.
The role of the systemic inflammatory response in cancer has been highlighted in several studies.[4–6] A significant association between elevated neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) with poor prognosis in various types of cancer has been shown.[7–10] Compared with other factors, NLR and PLR are easily, routinely, and inexpensively obtained. Although several studies have valued the relationship between NLR, PLR, and the prognosis of GC, the prognostic importance of these inflammatory markers is still in controversy.
We conducted this study to evaluate the relationship between NLR, PLR, and survival of GC, and investigate the prognostic value of NLR and NLR in GC patients before treatment.
2. Materials and methods
2.1. Study population
This study included 182 histologically confirmed gastric adenocarcinoma cases, which were diagnosed in Kunshan First People's Hospital Affiliated to Jiangsu University between January 2011 and January 2014. Patients with incomplete follow-up data or active concurrent infection were excluded. At recruitment, personal data of each participant regarding clinical characters and survival information were collected from clinical record or family contact. The overall survival (OS) was defined as time from the data of diagnosis to the data of death or last visit. The progression-free survival (PFS) was calculated from the time of diagnosis to the time of progression, relapse, death, or the last follow-up. This prospective observational study was reviewed by our Institutional Review Board and written informed consent was provided by each patient.
2.2. Data collection
Clinical characteristics including age, gender, surgery, chemotherapy, tumor location, clinical TNM stage, pathologic type as well as outcomes were collected. The tumors were staged according to the TNM staging system of the American Joint Committee on Cancer (AJCC 7th ed., 2010). The blood cell counts including neutrophil, platelets, and lymphocyte before treatment were extracted from the medical records. The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. In the same way, PLR was defined as the absolute platelet count divided by the absolute lymphocyte count.
2.3. Statistical analysis
The optimal cutoff values of NLR and PLR were estimated by the receiver operating characteristics (ROC) curve. The area under the curve (AUC), sensitivity, and specificity were calculated. Continuous variables were expressed using mean ± SD. Comparisons between groups were performed using Chi-squared test. The Kaplan–Meier method and log-rank tests were used to compare survival curves. Moreover, we conducted multivariate analyses to assess the effects of multiple covariates on the survival outcome. Hazard ratios (HRs) estimated from the multivariable analysis were expressed as relative risks with 95% confidence interval (95% CI). For all the analyses, a 2-sided P value of <.05 was defined as significant. Statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL).
3. Results
3.1. Clinicopathologic characteristics
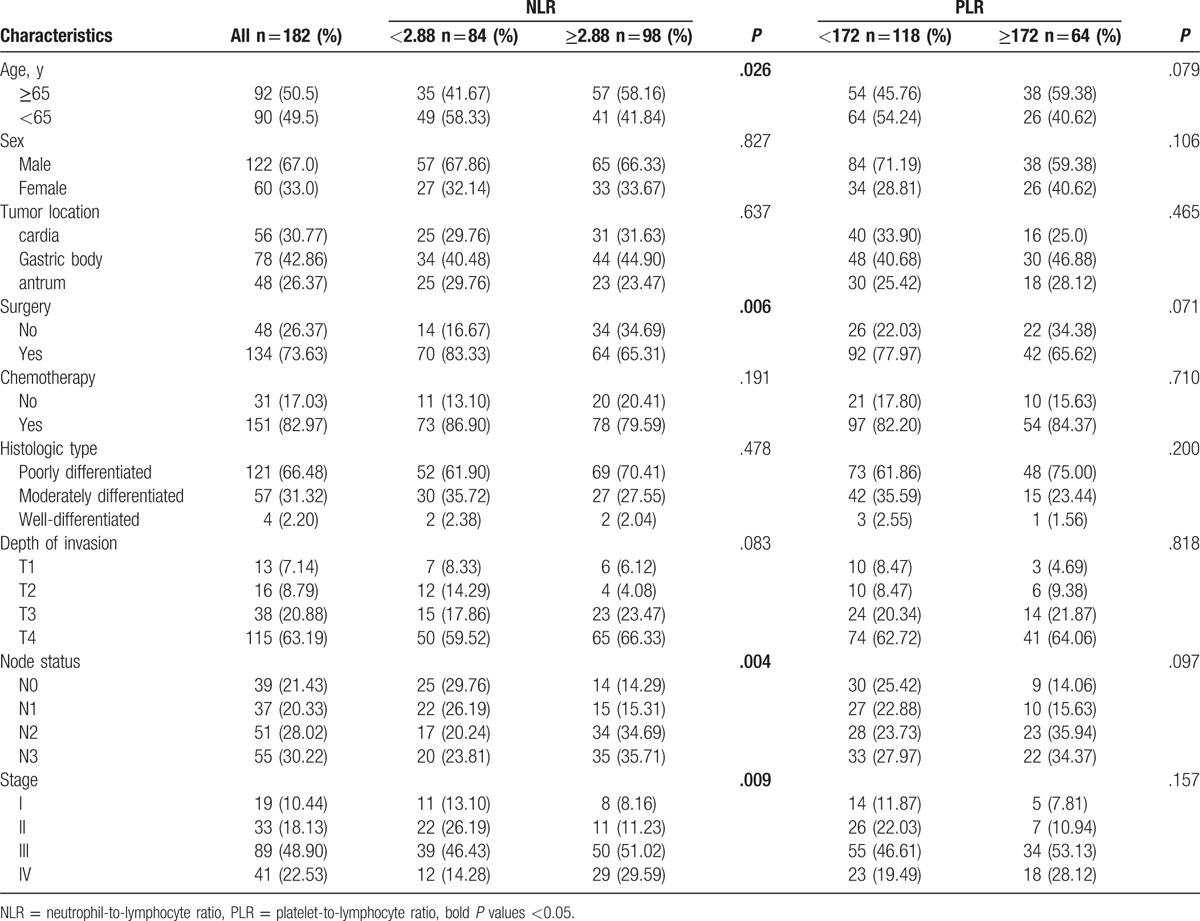
The clinical/pathological characteristics of the 182 patients are summarized in Table 1. Of 182 patients, 122 (67%) were male, and the median age was 65 years (range 29–87 years). Among all the cases, 56 (30.77%) patients were esophagogastric junction adenocarcinoma and 48 (26.37%) were diagnosed with gastric antrum cancer. Overall, 134 (73.63%) patients received surgical resection, and 151 (82.97%) patients received at least 1 cycle of chemotherapy. More than half (66.48%) of the patients were diagnosed of poorly differentiated adenocarcinoma, while the clinical stage III and IV patients were accounted for 48.90% and 22.53%, respectively.
Table 1.
Association of the patients’ characteristics with the platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios.
3.2. The association between NLR or PLR and clinicopathologic variables
The optimal cutoff level for the NLR was 2.88 for both OS and PFS when the Youden index was maximal. Similarly, the cutoff level for the PLR was 172 for both OS and PFS (supplementary Figure 1–4). The NLR level before treatment was elevated in 98 (53.85%) patients and a total of 64 (35.16%) patients were with higher PLR level. As summarized in Table 1, increased NLR level was significantly associated with age (P = .026), surgery (P = .006), node status (P = .004), and clinical stage (P = .009). However, PLR was not associated with age, sex, surgery, tumor location, histological type, or clinical stage.
3.3. Prognostic factors for OS and PFS
In this study, the median OS for all patients was 28.5 months, while PFS was 19.5 months. We further found that the median OS and PFS were poor in the High-NLR group (OS: 36.0 vs 20.5 months, P < .001, PFS: 33.0 versus 12.0 months, P < .001) and High-PLR group (OS: 31.5 vs 18.5 months, P = .003, PFS: 26.0 versus 11.0 months, P = .01), as shown in Figure (1–4).
Figure 1.
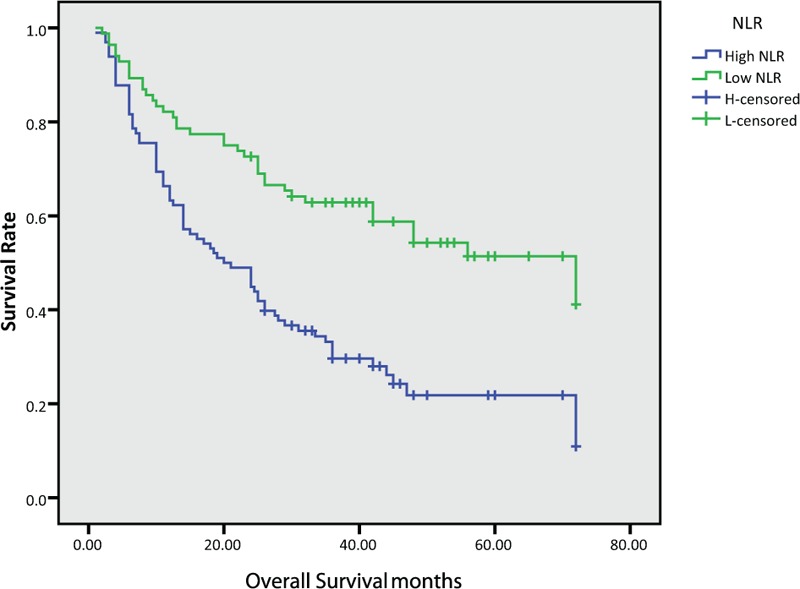
Kaplan–Meier survival curves of overall survival according to neutrophil-to-lymphocyte ratio (NLR).
Figure 4.
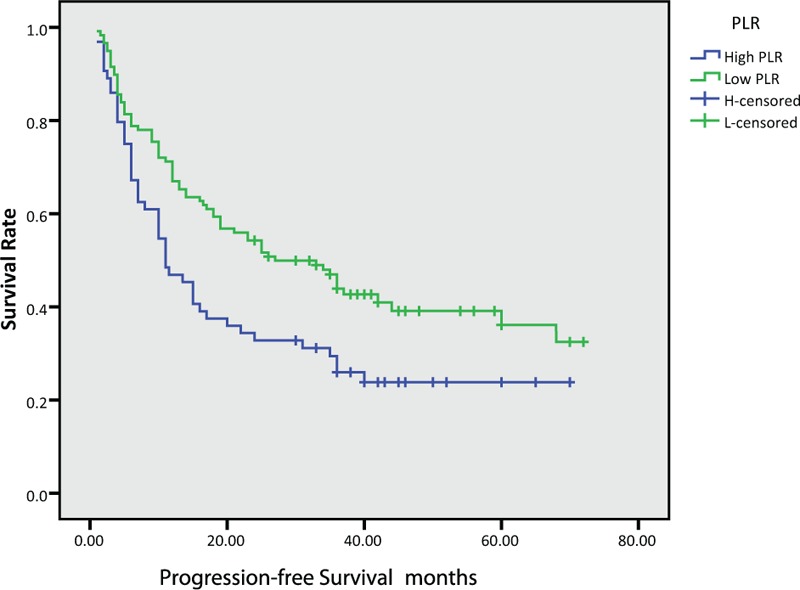
Kaplan–Meier survival curves of progression-free survival according to platelet-to-lymphocyte ratio (PLR).
Figure 2.
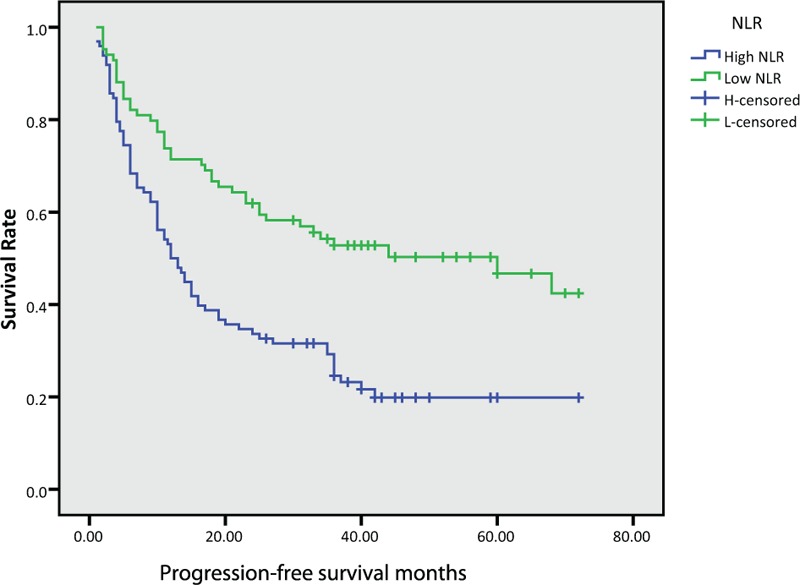
Kaplan–Meier survival curves of progression-free survival according to neutrophil-to-lymphocyte ratio (NLR).
Figure 3.
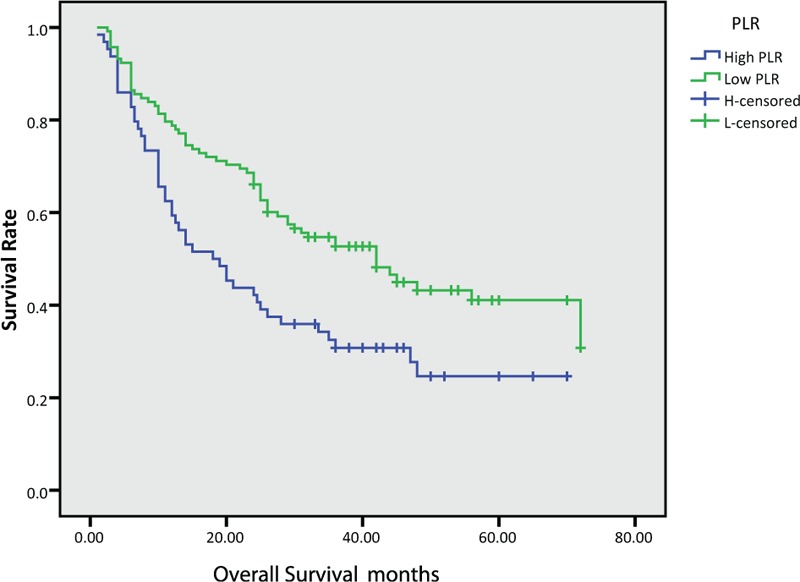
Kaplan–Meier survival curves of overall survival according to platelet-to-lymphocyte ratio (PLR).
The prognostic effect of clinicopathologic variables is summarized in Tables 2 and 3. In univariate analysis, the older age (P = .002), no surgery (P < .001), poorly differentiated histologic type (P = .048), advanced T (P < .001), N (P < .001) and clinical stage (P < .001), higher NLR (P < .001) and higher PLR (P = .004) were identified as poor prognostic factors associated with OS. Although no surgery (P < .001), advanced T (P < .001), N (P < .001) and clinical stage (P < .001), higher NLR (P < .001) and higher PLR (P = .012) were also associated with poor PFS. After multivariate analysis with these selected parameters using Cox regression model, both surgery (HR = 2.092, 95% CI: 1.345–3.253, P = .001) and NLR (HR = 1.585, 95% CI: 1.011–2.485, P = .045) were identified as independent prognostic factors associated with OS.
Table 2.
Univariate and multivariate analyses of factors for the prediction of overall survival.
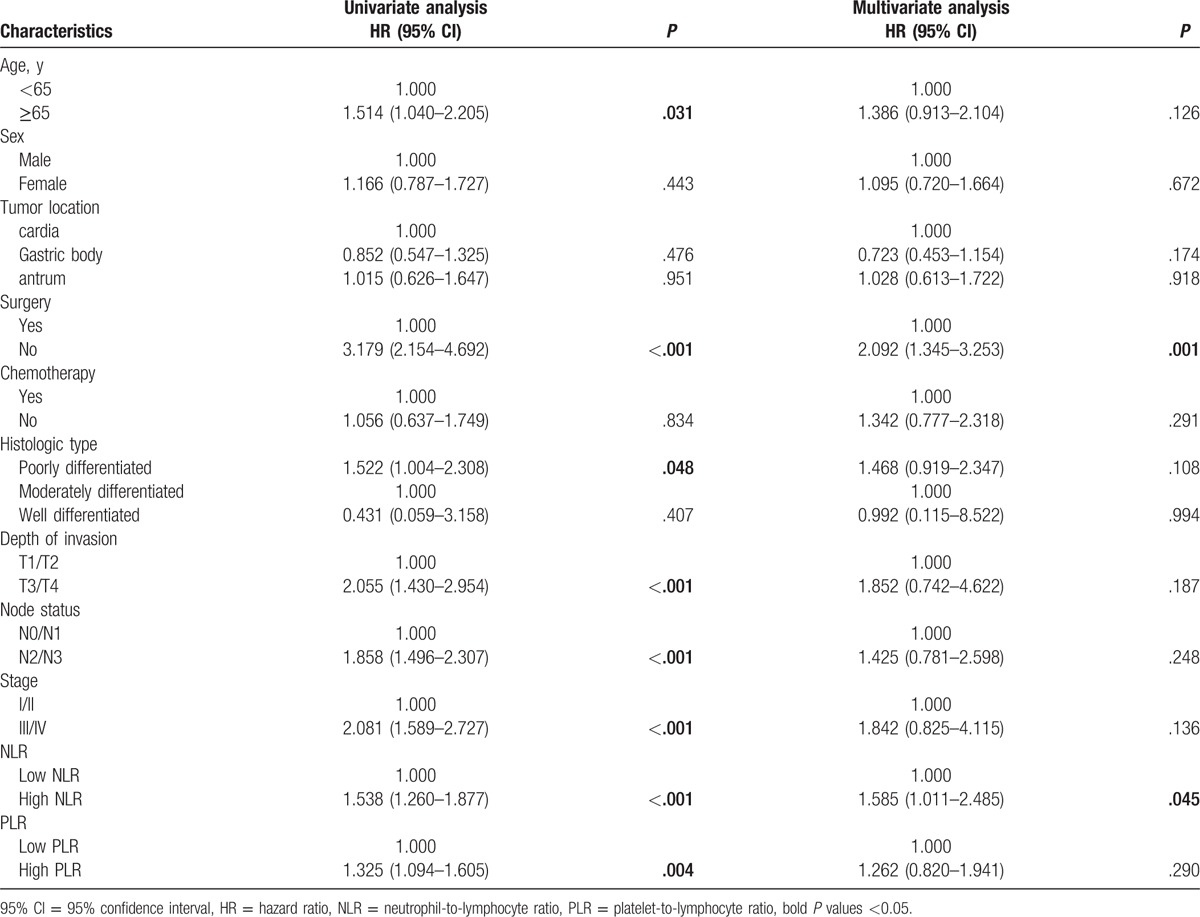
Table 3.
Univariate and multivariate analyses of factors for the prediction of progression-free survival.

4. Discussion
This study investigated the prognostic value of NLR and PLR in GC patients before treatment. We found that a high NLR was associated with age, surgery, node status, and clinical stage. Similar to previous studies, both the elevated NLR and PLR levels predicted poor OS and PFS in GC patients.[11,12] NLR was an independent risk factor for OS in Cox regression analysis.
Many types of cancer were proved to have links with infection and inflammatory reaction, such as GC. Helicobacter pylori infection is characterized by an inflammatory infiltrate, consisting mainly of neutrophils and T cells.[13] The inflammation reaction is an important factor in tumor cell microenvironment.[14,15] The inflammatory cells, chemokines, and cytokines are responsible for cell proliferation, angiogenesis, invasion, and metastasis.[16] The inflammation results are involved in lymphocytopenia, neutrophilia, and thrombocytosis.[17,18] The lymphocyte response plays an important role in immune responses, and it is also a major factor in the suppression of cancer progression.[19] The mechanisms of neutrophilia in proliferation and metastasis include release of reactive oxygen species or nitric oxide and remodeling of the extracellular matrix.[20] Platelets might participate in the inflammatory reaction by increasing angiogenesis or releasing growth factors.[21,22]
The concept of inflammation-based scores, such as the NLR and PLR, has been revealed as negative prognostic factors in various types of solid tumors, such as breast cancer,[7] colorectal cancer,[23,24] esophageal squamous cell carcinoma,[25] liver cancer,[26] and combined small cell lung cancer.[27] Several studies also focused on the association between NLR, PLR, and GC.[28–30] A meta-analysis including 10 studies with a total of 2952 cases indicated that elevated NLR predicated poor survival in GC.[31] Dogan et al[32] found that patients with high PLR and/or NLR have shorter PFS and OS in metastatic GC receiving first-line modified docetaxel, cisplatin, and 5-fluorouracil (mDCF). Deng et al[30] reported that preoperative NLR may serve as potential prognostic biomarkers in patients with GC who underwent surgical resection; however, they did not find that NLR was significantly associated with OS (P = .648) in multivariate analysis. Due to the differences in assays measuring neutrophil and lymphocyte, different population and different survival end-point, we optimized the algorithm results and employed different cut-off values. Fortunately, our results have shown that NLR was the independent prognostic factor for OS (P = .045) in multivariate analysis.
Some researchers also compared the prognostic value of NLR and PLR. Kim et al[12] reported that although both the NLR and PLR can reflect the prognosis, the NLR is more predictive of OS than the PLR. In Our study, we also found that higher NLR and higher PLR were associated with poor OS and PFS. After multivariate analysis, only NLR was identified as an independent prognostic factor for OS. We supposed that NLR has more predictive value than PLR. As NLR is the ratio of neutrophils to lymphocytes, high NLR means a relatively higher neutrophils and lower lymphocyte levels. A high NLR indicated an imbalance in the immune response, which impaired the normal anti-tumor functions.[20] Thus, tumor recurrence and invasion may occur. Given that some potential limitations exist in the present study, bias is inevitable. This retrospective study was conducted in 1 single institution, and some potential cofactors related to systematic inflammation and immunity have not been considered in all analyses. Our results still need more evidences to support.
In conclusion, high level of NLR and PLR was associated with poor OS and PFS in GC patients. NLR was identified as an independent prognostic factor for GC patients. Larger, prospective, and randomized studies are needed to confirm these findings and to elucidate the potential mechanism of systemic inflammatory response against tumor cells.
5. Author contributions
Data curation: Y-P. Du, C-X. Feng.
Formal analysis: C-X. Feng.
Funding acquisition: M. Chen.
Methodology: L-Q. Wang, M. Chen.
Software: J.J. Lu, Y-P. Du.
Supervision: L-Q. Wang.
Writing – original draft: Y. Zhang.
Writing – review & editing: M. Chen.
Supplementary Material
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, GC = gastric cancer, HR = hazard ratio, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PFS = progression-free survival, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristics, TNM = tumor-node-metastasis.
Funding/support: This work is supported by the National Natural Science Foundation (Grant numbers: 81472786); Suzhou Municipal Health Bureau projects (Grant number: LCZX201318, LCZX201619); The Foundation of tumor clinical and basic research team (KYC005).
The authors have declared that no competing interests exist.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Zhu YL, Yang L, Sui ZQ, et al. Clinicopathological features and prognosis of Borrmann type IV gastric cancer. J BUON 2016;21:1471–5. [PubMed] [Google Scholar]
- [3].Shah MA, Ajani JA. Gastric cancer: an enigmatic and heterogeneous disease. JAMA 2010;303:1753–4. [DOI] [PubMed] [Google Scholar]
- [4].Coffelt SB, de Visser KE. Cancer: inflammation lights the way to metastasis. Nature 2014;507:48–9. [DOI] [PubMed] [Google Scholar]
- [5].Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest 2015;125:3347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol 2016;46:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Orditura M, Galizia G, Diana A, et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open 2016;1:e000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miyazaki T, Sakai M, Sohda M, et al. Prognostic significance of inflammatory and nutritional parameters in patients with esophageal cancer. Anticancer Res 2016;36:6557–62. [DOI] [PubMed] [Google Scholar]
- [9].Moon H, Roh JL, Lee SW, et al. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother Oncol 2016;118:330–4. [DOI] [PubMed] [Google Scholar]
- [10].Zhang H, Zhang L, Zhu K, et al. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One 2015;10:e0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li S, Xu X, Liang D, et al. [Prognostic value of blood neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in patients with gastric cancer]. Zhonghua Zhong Liu Za Zhi 2014;36:910–5. [PubMed] [Google Scholar]
- [12].Kim EY, Lee JW, Yoo HM, et al. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol 2015;22:4363–70. [DOI] [PubMed] [Google Scholar]
- [13].Amedei A, Munari F, Bella CD, et al. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med 2014;9:303–9. [DOI] [PubMed] [Google Scholar]
- [14].Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One 2014;9:e106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Semenza GL, Ruvolo PP. Introduction to tumor microenvironment regulation of cancer cell survival, metastasis, inflammation, and immune surveillance. Biochim Biophys Acta 2016;1863:379–81. [DOI] [PubMed] [Google Scholar]
- [16].Rivas-Fuentes S, Salgado-Aguayo A, Pertuz Belloso S, et al. Role of chemokines in non-small cell lung cancer: angiogenesis and inflammation. J Cancer 2015;6:938–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shoda K, Komatsu S, Ichikawa D, et al. [Thrombocytosis associated with poor prognosis in patients with gastric cancer]. Gan To Kagaku Ryoho 2015;42:1980–2. [PubMed] [Google Scholar]
- [18].Chen Y, Zhang L, Liu WX, et al. Prognostic significance of preoperative anemia, leukocytosis and thrombocytosis in Chinese women with epithelial ovarian cancer. Asian Pac J Cancer Prev 2015;16:933–9. [DOI] [PubMed] [Google Scholar]
- [19].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [20].Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res 2015;2015:983698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mezouar S, Frere C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res 2016;139:65–76. [DOI] [PubMed] [Google Scholar]
- [22].Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int J Cancer 2016;138:2078–87. [DOI] [PubMed] [Google Scholar]
- [23].Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol 2014;31:305. [DOI] [PubMed] [Google Scholar]
- [24].Gu X, Gao XS, Qin S, et al. Elevated platelet to lymphocyte ratio is associated with poor survival outcomes in patients with colorectal cancer. PLoS One 2016;11:e0163523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xu XL, Yu HQ, Hu W, et al. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One 2015;10:e0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun XD, Shi XJ, Chen YG, et al. Elevated preoperative neutrophil-lymphocyte ratio is associated with poor prognosis in hepatocellular carcinoma patients treated with liver transplantation: a meta-analysis. Gastroenterol Res Pract 2016;2016:4743808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shao N, Cai Q. High pretreatment neutrophil-lymphocyte ratio predicts recurrence and poor prognosis for combined small cell lung cancer. Clin Transl Oncol 2015;17:772–8. [DOI] [PubMed] [Google Scholar]
- [28].Jiang N, Deng JY, Liu Y, et al. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers 2014;19:444–51. [DOI] [PubMed] [Google Scholar]
- [29].Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Deng Q, He B, Liu X, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med 2015;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang X, Zhang W, Feng LJ. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PLoS One 2014;9:e111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dogan M, Eren T, Ozdemir N, et al. The relationship between platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and survival in metastatic gastric cancer on firstline modified docetaxel and cisplatinum plus 5 Fluorourasil regimen: a single institute experience. Saudi J Gastroenterol 2015;21:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


