Abstract
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease. To date, the specific mechanisms that drive RA disease remain unknown and provide the impetus for genetic investigations into the development of RA. Researchers hope to identify gene polymorphisms that could serve as treatment targets in patients with RA. We have previously suggested that the gene encoding the pro-inflammatory adipokine resistin (RETN) may correlate with RA development. In this report, we sought to determine whether selected RETN single nucleotide polymorphisms (SNPs) are associated with RA susceptibility and clinicopathological characteristics. Four RETN SNPs (rs3745367, rs7408174, rs1862513, and rs3219175) were assessed using TaqMan genotyping in Chinese Han patients with RA and healthy controls. We found that carriers with the C allele of the RETN SNP rs7408174 as well as those with the AG allele or who had at least one A allele of the SNP rs3219175 are at greater risk of developing RA disease compared with wild-type carriers. Moreover, RA patients with the AG allele of the RETN SNP rs3219175 had higher serum C-reactive protein expression compared with controls, and these patients had a high likelihood of being on tumor necrosis factor (TNF) inhibitor therapy. This study is the first to discuss risk factors associated with RETN SNPs in RA progression in a Chinese Han population.
Keywords: resistin, rheumatoid arthritis, single nucleotide polymorphism
1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammation and joint deformation, affecting an estimated 0.5% to 1.6% of the population in developed countries and resulting in severe disability and premature mortality.[1] The etiology of RA is unclear, although considerable clinical data suggest that genetic components and environmental factors contribute to the development of this disease.[2] Indeed, genetic factors may be responsible for up to 60% of susceptibility to RA.[2] We therefore sought to define genetic aberrations in RA, as these may lead to more effective therapeutic strategies and a better understanding of risk prediction.
Human resistin is a 12.5 kDa cysteine-rich protein that is constitutively secreted by adipose tissue.[3] Evidence shows that the pro-inflammatory nature of resistin plays a critical regulatory role in inflammation and immune responses.[4] The gene encoding resistin, RETN, is localized on chromosome 9. Several single nucleotide polymorphisms (SNPs) have been identified in the RETN promoter, intron, and 3′-untranslated regions.[5] Genetic variances in RETN are associated with a greater risk of various diseases, including metabolic syndrome and colon cancer.[6,7] Notably, a functional RETN gene polymorphism, rs3219175, has demonstrated higher susceptibility to inflammatory and autoimmune diseases[8] and several RETN SNPs are known to be associated with type 2 diabetes mellitus or breast cancer.[9,10] Importantly, investigations have described differential resistin gene expression in human breast cancer tissues.[11] However, despite increasing evidence revealing the role played by resistin in various diseases, the relationship between RETN gene polymorphisms and RA prognosis remains unclear. We therefore performed this case–control study to investigate the association of four RETN SNPs with RA risk in Chinese Han patients.
2. Materials and methods
2.1. Patients and blood samples
We collected blood specimens from 266 patients (cases) who had been diagnosed with RA at Dongyang People's Hospital between 2014 and 2016. The control group randomly comprised 451 healthy volunteers without a history of RA in 2016. Written (signed) informed consent was obtained from all patients and participants. The study protocol was approved by the Dongyang People's Hospital Ethics Committee and Institutional Review Board (2015-YB002). Clinicopathological characteristics in all patients were determined based on medical records. We used a standardized questionnaire and searched the patients’ electronic medical records to obtain detailed clinical data on age, sex, and disease duration, as well as concurrent treatment with methotrexate, prednisolone, and tumor necrosis factor (TNF) inhibitors. At baseline, serum samples were collected from all RA patients and analyzed for the presence of anticitrullinated protein antibodies (ACPAs), rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Samples were ACPA-positive if anti-CCP2 titers were ≥17 IU/mL and RF-positive if IgM RF titers were ≥30 IU/mL. Whole blood samples (3 mL) were collected from all study participants and stored at −80°C for subsequent DNA extraction.
2.2. Selection of RETN polymorphisms
Three RETN SNPs were selected from a 2-kb region upstream of RETN (rs7408174, rs1862513, and rs3219175), and one (rs3745367) was selected from the intron of RETN; all SNPs had minor allele frequencies of greater than 5%. Most RETN SNPs were known to be associated with type II diabetes mellitus or breast cancer.[9,10]
2.3. Genomic DNA extraction
Genomic DNA was extracted from peripheral blood leukocytes using a QIAamp DNA blood kit (Qiagen, CA) according to the manufacturer's instructions. Extracted DNA was stored at −20°C and prepared for genotyping by polymerase chain reaction (PCR).
2.4. Genotyping by real-time PCR
Four RETN SNP probes were purchased from Thermo Fisher Scientific Inc., and assessment of allelic discrimination for RETN SNPs was conducted using a Roche LightCycler 480 Instrument II (Roche, Germany). Data were further analyzed with LightCycler 480 Software, Version 1.5 (Roche). PCR was carried out in a total volume of 10 μL, containing 20 to 70 ng genomic DNA, 1 U Taqman Genotyping Master Mix (Applied Biosystems, Foster City, CA), and 0.25 μL probes. The protocol included an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
2.5. Statistical analysis
Differences between the 2 groups were considered significant if P values were less than 0.05. Bonferonni's correction was used for multiple comparisons. Hardy–Weinberg equilibrium (HWE) was assessed using chi-square goodness-of-fit tests for biallelic markers. Since the data were independent and normal distribution, Fisher's exact test was used to compare differences in demographic characteristics between healthy controls and patients with RA. The odds ratios (ORs), adjusted odds ratios (AORs), and 95% confidence intervals (CIs) for associations between genotype frequencies and the risk of RA or clinicopathological characteristics were estimated by multiple logistic regression models, after controlling for other covariates. All data were analyzed using Statistical Analytic System software (v. 9.1, 2005; SAS Institute, Cary, NC).
3. Results
In this study, we compared clinical and demographic characteristics of 266 patients with RA and 451 controls. All study participants were of Chinese Han ethnicity (Table 1). Between-group differences were significant for age and gender (both P < .001). The majority of RA patients were female (83.8%), with RA duration of 35.96 ± 42.76 months, were naïve to anti-TNF drugs (43.6%), were being treated concurrently with methotrexate (56%) or prednisolone (60.5%), and were positive for RF status (85%) and ACPA antibodies (77.1%).
Table 1.
Comparison of demographic characteristics and clinical parameters of 451 healthy controls and 266 patients with RA.
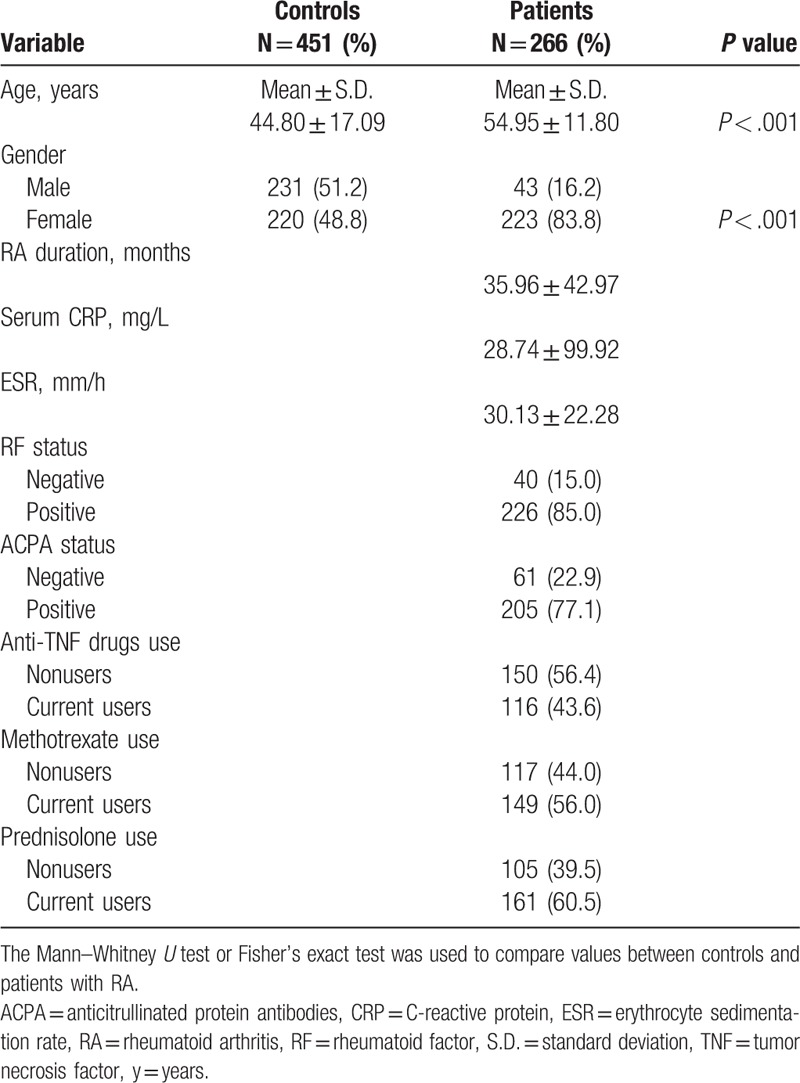
First, we genotyped controls and patients to determine any associations between the RETN SNPs (rs7408174, rs1862513, rs3219175, and rs3745367) and risk of RA. Genotyping distributions and associations between RA and RETN gene polymorphisms are presented in Table 2. In both patients with RA and controls, the alleles with the highest distribution frequency for RETN rs3745367, rs7408174, rs1862513, and rs3219175 were, respectively, heterozygous to A/G, homozygous to T/T, heterozygous to C/G, and homozygous to G/G (Table 2). Individuals carrying the C allele at rs7408174 and AG at rs3219175 had a 1.717-fold (95% CI: 1.177–2.506, P < .05) and a 1.638-fold (95% CI: 1.192–2.250, P < .05) higher risk of RA, respectively, compared with individuals carrying the T allele and wild-type GG polymorphic allele. To reduce the possible interference of confounding variables, AORs with 95% CIs were estimated by multiple logistic regression models controlling for age and gender in each comparison. In the adjusted analyses, subjects with C/C homozygotes of the RETN rs7408174 polymorphism and those with A/G heterozygotes of the RETN rs3219175 polymorphism had a 2.278-fold (95% CI: 1.430–5.203, P < .05) and 1.522-fold (95% CI: 1.070–2.165, P < .05) significantly higher risk of developing RA, respectively, compared to those with T/T and G/G homozygotes. The rates of RA patients with the rs3745367 and rs1862513 polymorphisms did not differ from those of controls with the same polymorphisms.
Table 2.
Comparison of the genotype and allele frequencies of the RETN polymorphism in 451 controls and 266 patients with RA.
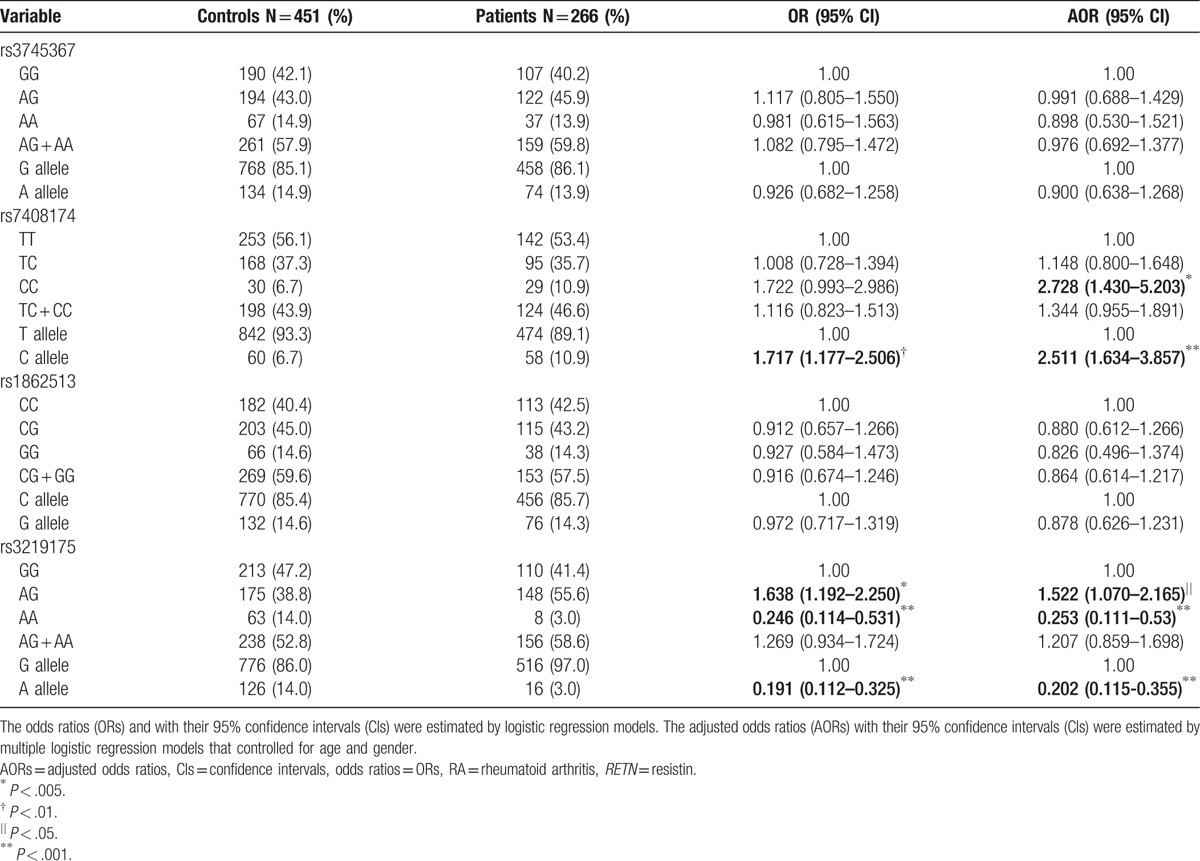
Next, we performed RETN genotyping in patients with RA to clarify the role of RETN polymorphisms regarding the clinical status of RF and ACPA and drug usage of anti-TNF agents, methotrexate, and prednisolone (Table 3). RA patients who were GG or AA carriers of the rs3219175 variant were significantly more likely to be receiving anti-TNF agents (OR: 1.870, 95% CI: 1.127–3.104, P < .05); this likelihood persisted in multivariate analysis adjusting for confounders (AOR: 1.886, 95% CI: 1.132–3.114, P < .05) (Table 3). In contrast, no such significant findings were observed between the RETN rs7408174 polymorphism and clinicopathological status (data not shown).
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) of the clinical status and genotype frequencies of the RETN rs3219175 polymorphism in 258 patients with RA.
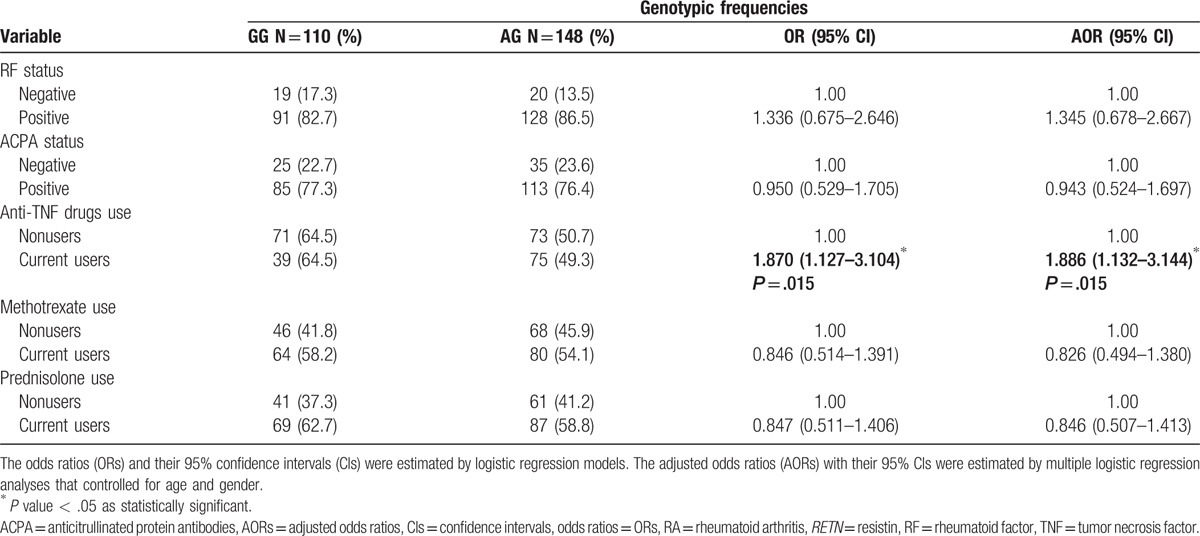
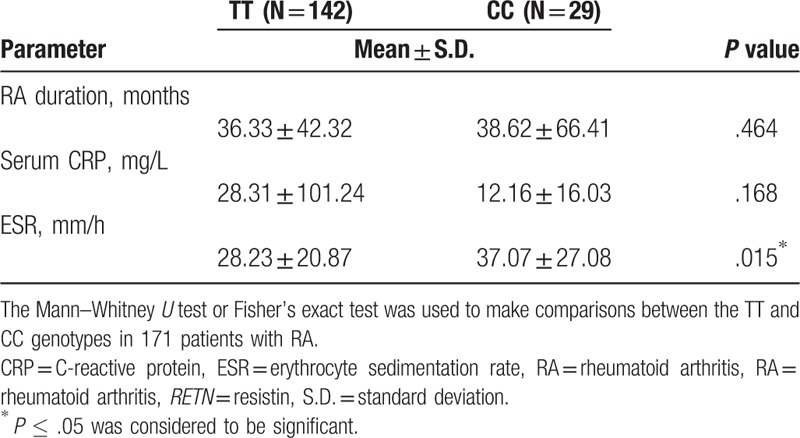
We then sought to determine potential associations between RETN gene polymorphisms and clinical serum markers of RA. As regard the rs3219175 polymorphism, in 258 patients with RA, serum CRP was significantly higher and ESR values were significantly lower among GG carriers compared with AG carriers (Table 4). Similarly, among 171 patients with RA with the rs7408174 polymorphism, serum ESR was significantly lower in those with the TT genotype than in those with the CC genotype (Table 5). No such significant associations were found between other RETN SNP genotypes and RA clinical markers (data not shown).
Table 4.
Comparison of the clinical parameters and genotype frequencies of the RETN rs3219175 polymorphism in 258 patients with RA.
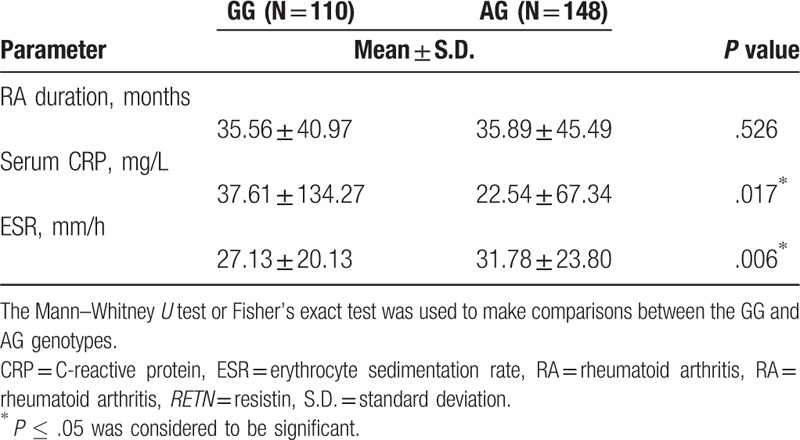
Table 5.
Comparison of the clinical parameters and genotype frequencies of the RETN rs7408174 polymorphism in 171 patients with RA.
4. Discussion
RA is a complex immune-related disease, with many genetic components and environmental factors implicated in its development. An increasing number of newer therapies that have become available in the last few years have enabled around 30% of patients with RA to achieve remission, reducing signs and symptoms of their disease.[12] However, a substantial proportion of patients remain treatment refractory and continue to suffer the active form of RA that leads to disability. These patients have substantial therapeutic needs. These limitations of current RA therapies emphasize the importance of continuing to investigate the pathogenesis of RA disease and identifying new therapeutic targets. This treatment goal informed our study, in which we sought to determine whether genetic polymorphisms from adipokines contribute to a higher susceptibility to RA. In our study population, the majority of RA patients were female and aged over 50 years. We were unable to show any correlation between RA disease and smoking or alcohol consumption, owing to a lack of data on these health behaviors.
Polymorphisms in the RETN gene have been reported in various cancers.[7,13] In the present study, we describe involvement of the RETN polymorphism in RA disease. Specifically, we identified two RETN polymorphisms are associated with a significantly higher risk of developing RA disease; the C allele at rs7408174 and the AG allele at rs3219175. In contrast, RETN polymorphisms at rs1862513 and rs3745367 did not significantly increase the risk of RA compared with controls. In a previous study that investigated the effect of resistin on the response to interferon therapy in hepatitis C virus (HCV) infection, patients with the rs3219175 SNP were far less likely to achieve a sustained virological response.[14] In agreement with the findings of that research, we encourage the development of resistin-targeted therapy for RA.
A previous epigenetic study has demonstrated that the promoter region of RETN could have dual genetic and epigenetic effects on plasma resistin.[15] The expression of the RETN polymorphism at rs3219175 is located in the promoter region, and we have examined the correlation of this SNP with the clinicopathological status of patients with RA. We found that patients with the RETN re3219175 SNP were significantly more likely to be using TNF inhibitors. In addition, rs7408174 and rs3219175 SNPs correlated with ESR status.
Although our present results indicate that patients with the RETN rs3219175 SNP are at greater risk of developing RA disease, the reconstructed linkage disequilibrium plot of the 4 RETN SNPs showed that rs3219175 had low linkage disequilibrium with rs1862513 (data not shown). Detailed functional analysis of rs3219175 is required. Moreover, it remains unclear as to how these SNPs affect resistin gene expression in RA cells. Additionally, some patient survival data were unavailable because patients had just recently enrolled in the study. On the other hand, since the missing information of body mass index and waist circumference which was associated with resistin and metabolic disorders, we could not adjusted for those factors in the logistic regression. Further studies are needed using larger populations of patients to confirm the role of RETN polymorphisms in RA progression.
Taken together, our results demonstrate an association between RETN gene variants and risk of RA disease. We found that RETN SNPs were significantly associated with clinical therapies and CRP marker of RA in the Chinese Han population. This study is the first to report a correlation between RETN polymorphisms and high risk for RA disease. The evidence indicates that RETN could serve as a genetic prognostic marker for RA therapy.
5. Author contributions
Conceptualization: C-M. Su.
Data curation: C-C. Huang, C-H. Tang, C-M. Su, G. Xu, L. Wang, T. Lu, Y. Sun.
Methodology: C-M. Su, S-F. Yang.
Project administration: C-M. Su.
Resources: L. Wang.
Software: C-M. Su.
Supervision: C-M. Su.
Validation: C-M. Su, S-F. Yang, C-M. Su, S-F. Yang.
Writing – original draft: C-M. Su.
Writing – review & editing: C-H. Tang, C-M. Su, S-F. Yang.
Footnotes
Abbreviations: ACPAs = anticitrullinated protein antibodies, AOR = adjusted odds ratios, CI = confidence interval, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, GTEx = genotype-tissue expression, HWE = Hardy-Weinberg equilibrium, OR = odds ratio, PCR = polymerase chain reaction, RA = rheumatoid arthritis, RETN = resistin, RF = rheumatoid factor, SNP = single nucleotide polymorphisms, TNF = tumor necrosis factor.
LW and C-HT contributed equally to this work.
This work was supported by grants from the Dongyang People's Hospital (2015-YB002).
The authors have no conflicts of interest to disclose.
References
- [1].Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- [2].Bowes J, Barton A. Recent advances in the genetics of RA susceptibility. Rheumatology 2008;47:399–402. [DOI] [PubMed] [Google Scholar]
- [3].Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature 2001;409:307–12. [DOI] [PubMed] [Google Scholar]
- [4].Reilly MP, Lehrke M, Wolfe ML, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005;111:932–9. [DOI] [PubMed] [Google Scholar]
- [5].Steppan CM, Brown EJ, Wright CM, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA 2001;98:502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kumar S, Gupta V, Srivastava N, et al. Resistin 420C/G gene polymorphism on circulating resistin, metabolic risk factors and insulin resistance in adult women. Immunol Lett 2014;162:287–91. [DOI] [PubMed] [Google Scholar]
- [7].Alharithy RN. Polymorphisms in RETN gene and susceptibility to colon cancer in Saudi patients. Ann Saudi Med 2014;34:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sasayama D, Hori H, Nakamura S, et al. Increased protein and mRNA expression of resistin after dexamethasone administration. Horm Metab Res 2015;47:433–8. [DOI] [PubMed] [Google Scholar]
- [9].Chung CM, Lin TH, Chen JW, et al. Common quantitative trait locus downstream of RETN gene identified by genome-wide association study is associated with risk of type 2 diabetes mellitus in Han Chinese: a Mendelian randomization effect. Diabetes Metab Res Reviews 2014;30:232–40. [DOI] [PubMed] [Google Scholar]
- [10].Vallega KA, Liu N, Myers JS, et al. Elevated resistin gene expression in African American estrogen and progesterone receptor negative breast cancer. PLoS One 2016;11:e0157741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rhodes B, Merriman ME, Harrison A, et al. A genetic association study of serum acute-phase C-reactive protein levels in rheumatoid arthritis: implications for clinical interpretation. PLoS Med 2010;7:e1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aletaha D, Bingham CO, Tanaka Y, et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet 2017;389:1206–17. [DOI] [PubMed] [Google Scholar]
- [13].Hu WW, Tang CH, Sun Y, et al. Correlation between resistin gene polymorphism and clinical aspects of lung cancer. Medicine 2017;96:e9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chang ML, Liang KH, Ku CL, et al. Resistin reinforces interferon lambda-3 to eliminate hepatitis C virus with fine-tuning from RETN single-nucleotide polymorphisms. Sci Rep 2016;6:30799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Onuma H, Tabara Y, Kawamura R, et al. A at single nucleotide polymorphism-358 is required for G at -420 to confer the highest plasma resistin in the general Japanese population. PLoS One 2010;5:e9718. [DOI] [PMC free article] [PubMed] [Google Scholar]


