Abstract
Background:
There were many reports suggesting that different kinds of tumors can express B7-H4; however, the prognostic value in cancer was still unclearly. Therefore, we conducted a meta-analysis to investigate the relationship between overexpression of B7-H4 with the prognostic value in pancreatic cancer patients.
Materials and Methods:
The Pubmed, Embase, Cochrane Library, Ovid, Web of Science, and Chinese research database (including CBM, CNKI, and WAN FANG) were searched for related literature published until October 12, 2017. The pooled odds ratios (ORs) and/or pooled hazard ratios (HRs) for clinical pathological factors and overall survival (OS) were calculated and analyzed using Stata software. To assess whether an individual study had an impact on the result, sensitivity analysis was performed for all included individual studies using the fixed-effects model. Publication bias was evaluated using Egger's and Begg's tests.
Results:
Data from 6 observational studies including 442 patients were summarized in this meta-analysis, and each study was eligible for inclusion based on included and exclude criteria. The pooled results indicated that the B7-H4 overexpression could predict the presentation of lymph node metastasis (OR = 3.94, 95% CI: 1.22–12.66, P = .022), advanced TNM stage (T = the extent of the primary tumor, N = regional lymph nodes, M = distant metastases) (III+IV vs I+II; OR = 7.63, 95% CI: 2.46–23.66, P < .001), and the poor OS (HR = 3.00, 95%CI = 2.20–4.10, P < .001) in PC patients.
Conclusions:
This study reveals that high expression of B7-H4 is an unfavorable prognostic factor for patients with pancreatic cancer. These results may guide the clinical management of this patient population.
Keywords: B7-H4, meta-analysis, pancreatic cancer, prognosis, survival
1. Introduction
Pancreatic cancer ranks fourth in cancer-related mortality in the United States in 2017,[1] and it is predicted to become the second leading causes of death due to cancer by 2030.[2] Pancreatic cancer is known to have a poor prognosis, mostly due to the fact that early stage of pancreatic cancer is often asymptomatic and poor early detection rates as well as the clinical presentation (such as liver metastases) usually precludes surgical resection in the most patients at advanced stage. Consequently, the average 5-year survival rate among patients with pancreatic cancer <3% .[3,4] Therefore, it is crucial to seek more reliable prognostic indictors in future scientific research. The costimulatory molecule B7-H4 may be a new prognostic marker for pancreatic cancer.
B7-H4, a member of the B7 family of immunoregulatory proteins, is generally expressed on the surface of antigen-presenting cells (APCs) and interacts with ligands bound to unknown receptors presented by activated T cells. This proteins can inhibits T cell proliferation and cytokine production in tumor microenvironment.[5] Unlike in normal tissues, high levels of B7-H4 protein expression have been reported in a variety of human tumors, including ovarian carcinoma, breast cancer, bladder cancer, glioma, prostate cancer, pancreatic cancer, and so on.[6] Besides, there is a study showed that shRNA-mediated disruption of B7-H4 markedly inhibited tumor cell proliferation, invasion, and migration, increased cell apoptosis and arrested cell cycle at G0/G1.[7] So, we assume that B7-H4 may serve as a potential target for cancer immunotherapy.
Several clinical studies have suggested that high B7-H4 expression is associated with shorter overall survival (OS) in solid cancer.[8–10] Nevertheless, the reliability and degree of the prognostic impact of B7-H4 in pancreatic carcinoma have not yet been methodically analyzed. Therefore, we conducted a meta-analysis to assess the prognostic effect of elevated B7- H4 in patients with PC.
2. Materials and methods
2.1. Search strategy and study selection
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We performed a comprehensive literature search of the following databases: Pubmed, Embase, Cochrane Library, Ovid, Web of Science, and Chinese research database (including CBM, CNKI, WAN FANG). The last search was updated on October 12, 2017. The published languages were limited to English and Chinese. The searching items in English were used: “B7-H4,” “B7H4,” “B7S1,” “pancreat∗ ,” “pancreatic cancer,” “pancreatic carcinoma,” “pancreatic neoplasm,” “pancreatic tumor,” “PC,” and “PADC”. The references in the relevant studies were also searched.
Studies were eligible for inclusion based on the following criteria: diagnosis of PC was proven by histopathological methods; B7-H4 was detected by any method; studies reported the correlation of B7-H4 with clinicopathological factors or overall survival (OS); when there were multiple studies on the same patients population, the largest or the most recent one was selected; HR with 95% CI was available for data extraction; articles were published in English or Chinese. The exclusion criteria were as follows: meeting abstracts, reviews, letters, or case reports; duplicated studies and repeated analyses; studies which lack necessary information.
2.2. Data extraction and qualitative assessment
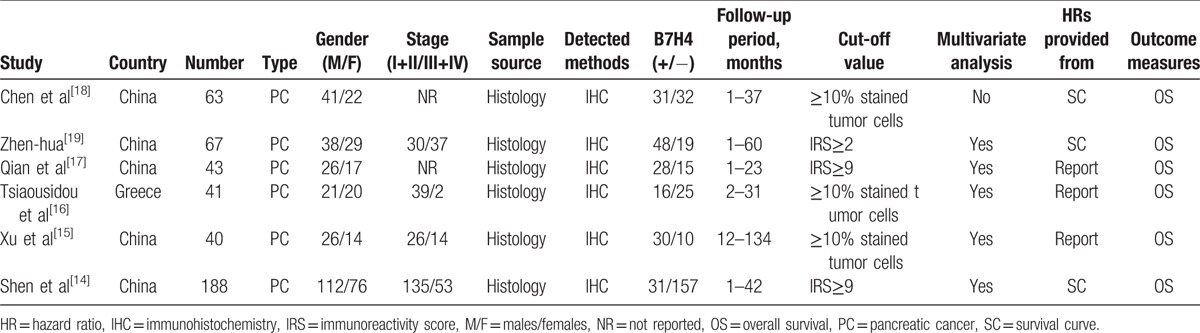
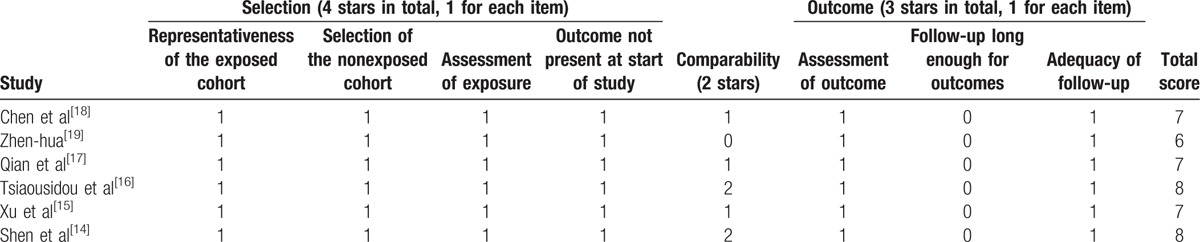
The following information of each eligible study was extracted independently by 2 investigators (CX and TL): first author; publication year; country; number of participants; cancer type; gender; tumor stage; sample source (histology/cytology); detected methods; follow-up period; cut-off value; outcome measures; HR for survival outcome and corresponding 95% CI (Table 1). If a study provided univariate and multivariate results both, considered the precision of data, the multivariate analysis would be selected. Some HRs and their 95% CI were not directly reported in the articles, we used the graphical data to extract information. Any discrepancies were resolved by discussion to reach a consensus. The quality assessment of eligible studies was independently performed by 2 reviewers (CX and TL) according to the NOS,[11] which included criteria of patient selection (0–4 points), comparability of groups (0–2 points), and outcome assessment (0–3 points). If NOS scores were ≥6, this study was defined as a high-quality study.
Table 1.
Summary of the studies included in the meta-analysis.
2.3. Statistical analysis
The B7-H4 cut-off values classified cancer patients into high and low expression group. HRs and their 95% CIs were combined to measure the effective value of elevated B7-H4 expression on prognosis. Also the associations between B7-H4 and clinicopathologic features were expressed as odds ratios (ORs) and their 95% CIs. If the statistical variables were described in the article, we used them directly. Otherwise, we read the Kaplan–Meier survival curves through Engauge Digitizer version 10.4 to extract the data according to the method described by Tierney et al.[12] This process was conducted by 2 independent researchers (CX and TL) to avoid the reading variability. Of course, we also contacted the authors of the literature to request additional information and original data. An observed HR>1 indicated a worse prognosis in patients with B7-H4 overexpression and HR<1 suggested a better prognosis. Statistical heterogeneity across the eligible studies was assessed by visual inspection of forest plots, processing the Chi-square test (assessing the P value) and calculating the I2 statistic. If the I2 was greater than 50% and P-value was <.05, indicating a significant heterogeneity, a random-effects model (the DerSimonian–Laird method) was used. Otherwise the fixed-effects (Mante–Haenszel method) was applied to analyze the pooled HRs. To assess whether an individual study had an impact on the result, sensitivity analysis was performed for all included individual studies using the fixed-effects or random-effects model. Finally, if more than 10 studies were included, publication bias was estimated by visually assessing the asymmetry of an inverted funnel plot; otherwise Egger's and Begg's tests were used for analysis only. If publication bias was observed, we adjusted for the effect using the Duval and Tweedie trim-and-fill method.[13] For all analyses, STATA version 14.0 (Stata Corporation, College Station, TX) was performed, and a P value <.05 in the statistical tests was considered as statistically significant.
3. Results
3.1. Studies selection and characteristics
Around 381 publications, of which 86 were duplicated studies, were found from the database search by using the described searching strategy (Fig. 1). After screening the titles and abstracts, a total of 16 studies were assessed for eligibility by full-text screening. Then, 10 studies that met the exclusion criteria were removed after full text review. Finally, 6 eligible studies[14–19] were enrolled for meta-analysis. Table 1 provided the basic and summarized characteristics of the enrolled studies. All studies were scored in accordance with the NOS and the quality score ranged from 6 to 8 (Table 2).
Figure 1.
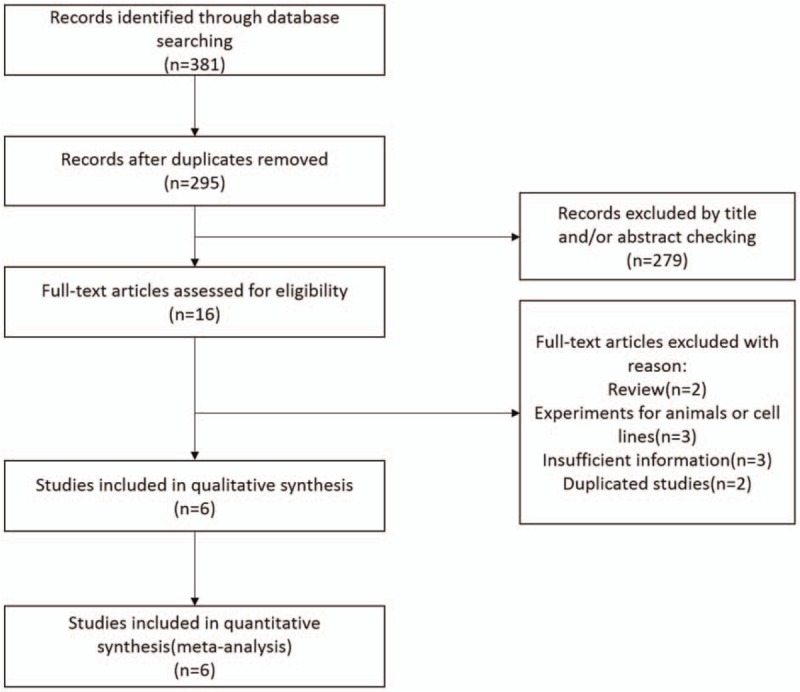
Flow diagram of the inclusion and exclusion of studies.
Table 2.
Quality assessment of the included studies according to the Newcastle–Ottawa scale.
3.2. Association between B7-H4 and clinical pathological factors in PC
To investigate the association between increased expression of B7-H4 and characteristics of PC patients, we identified 6 clinicopathological factors that included gender, age, lymph node metastasis, TNM stage (T = the extent of the primary tumor, N = regional lymph nodes, M = distant metastases) (III+IV vs I+II), tumor differentiation (poorly vs moderately/well) and treatment (chemotherapy vs none), and the results were summarized in Table 3 and Figure 2. The aggregated analysis showed that overexpression of B7-H4 was significantly connected with lymph node metastasis (yes vs no; OR = 3.94, 95% CI: 1.22–12.66, P = .022) and TNM stage (III+IV vs I+II; OR = 7.63, 95% CI: 2.46–23.66, P<.001). However, the pooled data suggested that there were no significant association between the level of B7-H4 and gender (male vs female; OR = 1.08, 95% CI: 0.69–1.68, P = .735), age (≥65 vs < 65 years; OR = 0.90, 95% CI: 0.51–1.57, P = .706), tumor differentiation (poorly vs moderately/well; OR = 2.12, 95% CI: 0.76–5.92, P = .150) or treatment (chemotherapy vs none; OR = 0.93, 95% CI: 0.52–1.68, P = .811) in PC.
Table 3.
Association between B7-H4 and clinical pathological factors in PC.
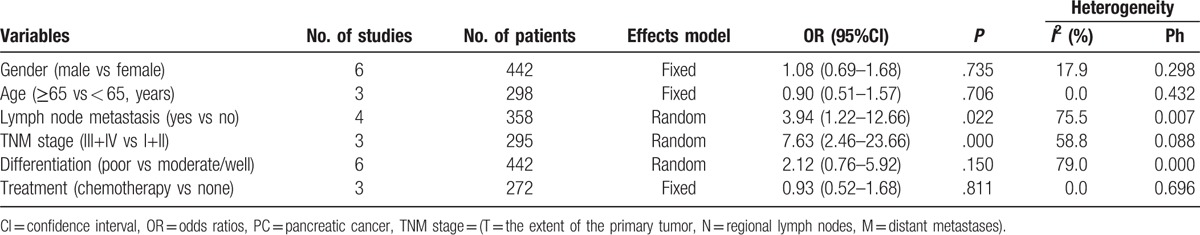
Figure 2.
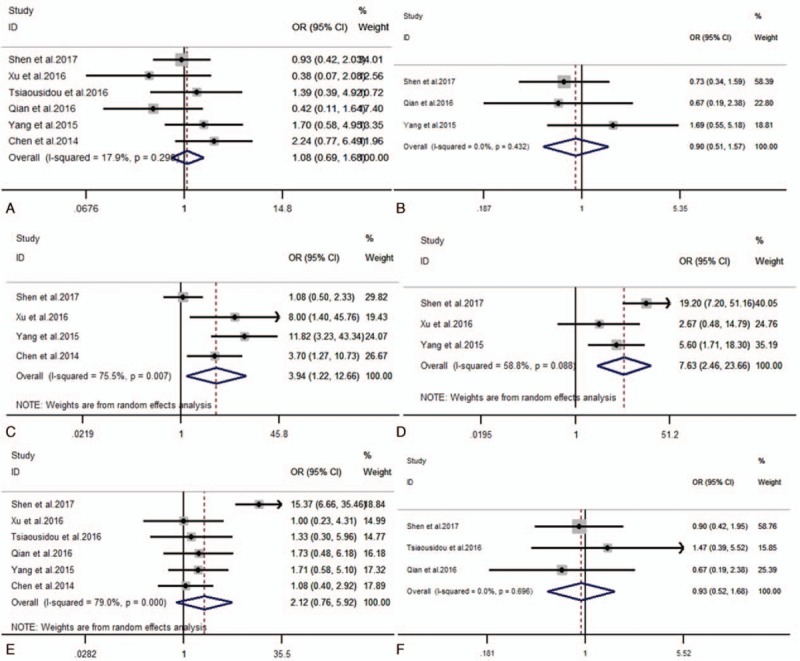
Forest plots of OR for the relation between B7-H4 expression and (A) gender; (B) age; (C) lymph node metastasis; (D) TNM stage; (E) differentiation and (F) treatment. OR = odds ratios, TNM stage = (T = the extent of the primary tumor, N = regional lymph nodes, M = distant metastases).
3.3. The prognostic value of B7-H4 in OS
The HRs for OS were available in 5 studies[14–18] involving 375 patients. As the heterogeneity among studies was not significant (I2 = 3.05; Ph = 0.550), the fixed-effects model was utilized (Fig. 3). The pooled HR showed a significant correlation of B7-H4 overexpression with shorter OS (HR = 3.00, 95%CI = 2.20–4.10, P < .001; Fig. 3). Subgroup analyses based on different cut-off values were not carried out because the scale of included studies is too small. To assess whether an individual study had an impact on the result, sensitivity analysis was performed to analysis using the fixed-effects model. The result pattern was not obviously impacted by any single study (Fig. 4).
Figure 3.
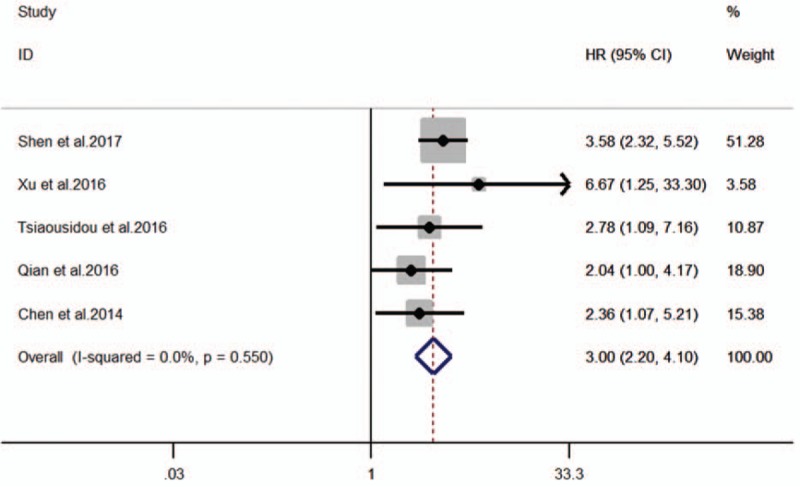
Forest plot for the association of B7-H4 expression with overall survival.
Figure 4.
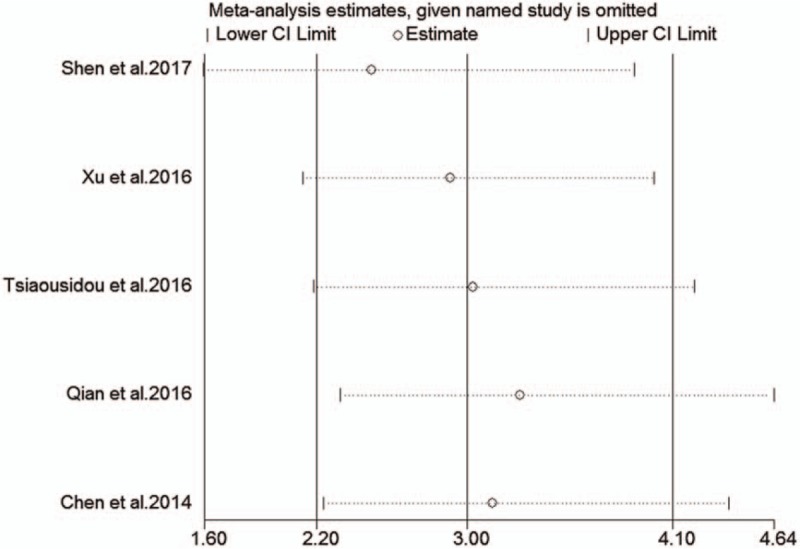
Sensitivity analysis on the relationships between B7-H4 expression and overall survival in pancreatic cancer patients.
3.4. Publication bias
We measured the publication bias of articles through Egger's and Begg's tests, considering that 6 studies were included. Egger's and Begg's tests showed, respectively, the P values of .903 and .806, and the result showed no evidence of publication bias.
4. Discussion
For now, at least, our meta-analysis is the first time to investigate the relationship between high B7-H4 expression and clinical prognosis value in patients with pancreatic cancer. This study shows that an elevated B7-H4 is a strong predictor of worse OS in patients with PC, and we also find that the association between B7-H4 overexpression with some clinical pathological features in PC patients (the express levels of B7-H4 were positively correlated with lymph node metastasis and higher grade tumor staging, such as III/IV staging). In a word, our research finding indicates that B7-H4 could serve as a potential prognostic biomarker for the patients with PC, and it may be a new target for future cancer treatments.
B7-H4, which is a novel B7 ligand that possibly acts as a negative regulatory factor in the T cell-mediated immune response,[20] is aberrantly expressed in some tumor tissues (such as breast,[8,21] urothelial bladder ,[22] ovarian,[23,24] cervical,[25] lung,[26] pancreatic,[27] renal cell,[28] and gastric cancers[9,29]). So, it suggests that these molecules might be new tumor diagnostic and prognostic molecular biomarkers. Some studies have investigated the role of high B7-H4 expression for cancer cell in the tumor immune escape, it is achieved mainly through the following ways: the B7-H4 molecular can directly arrest the proliferation of cytotoxic T lymphocytes in G0/G1 phase, and it also indirectly suppress tumor-associated antigen-specific T-cell immunity (including inhibiting the proliferation and cytokine production of T cells).[7,30–33] Therefore, the high expression of B7-H4 in cancer tissue may play an important role in promoting tumor progression and metastasis.
In our analysis, the pooled HR of B7-H4 on OS for PC patients is 3.00 (95%CI = 2.20–4.10, P < .001) which indicates B7-H4 was related to a worse outcome for patients. By reviewing the literature, we also find that some authors use meta-analysis to investigate the correlation between B7-H4 and prognosis of cancer patients.[34–36] Their research also suggests that the high expression of B7-H4 is associated with worse OS in different cancer. However, different studies suggest that the level of B7-H4 expression is associated with different clinical pathological features in various cancers. For example, Tan and Shen[34] reported that the correlation between B7-H4 and lymph node metastasis, advanced TNM stage and poor differentiation in patients with nonsmall cell lung cancer. But our study reveals that it correlates with lymph node metastasis and higher TNM tumor staging in PC. Although the included studies use the same detected methods (immunohistochemistry/IHC) to detect the level of B7-H4 expression in our study, but the assessment methods of B7-H4 staining vary, resulting in inconsistent cut-offs in some studies. This problem may have some potential impacts on this meta-analysis. Therefore, further studies with the same assessment way are needed.
There are several limitations to the present study. First, most included studies are conducted in China and several studies could not include because of its negative results which could not be published. Therefore, patient selection bias and publication bias maybe exist. Second, the data of several studies are extracted from survival curves; the statistical error that could affect the precision of the results is existed. Third, some studies[19] that their SC images are fuzzy are not included in analyzing the relationship between B7-H4 and OS. Fourth, only 5 studies were included for the OS analysis and the total sample was small, we need more large scale studies for meta-analysis to make compelling conclusions.
5. Conclusion
In summary, despite there are some limitations in this study, but this meta-analysis suggests that overexpression B7-H4 is significantly associated with presence of lymph node metastasis, poor tumor differentiation, and shorter OS in PC. High B7-H4 expression is an unfavorable prognostic factor for patients with PC.
6. Author contributions
Dr XD and CX conceived and designed this study; CX and TL performed the analysis and interpretation of data; CX, TL, YC, and XD wrote the manuscript. All authors read and approved the final manuscript
Acknowledgments
This study was supported by a grant from the Capital Characteristic Clinical Application Research and Achievement Promotion Project (No. Z171100001017121) and the National Natural Science Foundation of China (No. 81672862).
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, IHC = immunohistochemistry, IRS = immunoreactivity score, M/F = males/females, NR = not reported, OR = odds ratios, OS = overall survival, PC = pancreatic cancer, PRISMA = the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SC = survival curve.
Institutional review board approval: Given these date did not include personal identifying information and it was based on a publicly available database, informed consent was not required in this study.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- [3].Gong Z, Holly EA, Bracci PM. Survival in population-based pancreatic cancer patients: San Francisco Bay area, 1995–1999. Am J Epidemiol 2011;174:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sohal DP, Mangu PB, Laheru D. Metastatic pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract 2017;13:261–4. [DOI] [PubMed] [Google Scholar]
- [5].Podojil JR, Miller SD. Potential targeting of B7-H4 for the treatment of cancer. Immunol Rev 2017;276:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].MacGregor HL, Ohashi PS. Molecular pathways: evaluating the potential for B7-H4 as an immunoregulatory target. Clin Cancer Res 2017;23:2934–41. [DOI] [PubMed] [Google Scholar]
- [7].Zhang X, Cai L, Zhang G, et al. B7-H4 promotes tumor growth and metastatic progression in lung cancer by impacting cell proliferation and survival. Oncotarget 2017;8:18861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang H, Li C, Ren G. Clinical significance of the B7-H4 as a novel prognostic marker in breast cancer. Gene 2017;623:24–8. [DOI] [PubMed] [Google Scholar]
- [9].Arigami T, Uenosono Y, Ishigami S, et al. Clinical significance of the B7-H4 coregulatory molecule as a novel prognostic marker in gastric cancer. World J Surg 2011;35:2051–7. [DOI] [PubMed] [Google Scholar]
- [10].Xu CH, Wang W, Wang YC, et al. Diagnosis value of serum soluble B7-H4 expression in non-small cell lung cancer. Clin Respir J 2016;12:1334–9. [DOI] [PubMed] [Google Scholar]
- [11].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [12].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. PubMed PMID: 10877304. [DOI] [PubMed] [Google Scholar]
- [14].Shen L, Qian Y, Wu W, et al. B7-H4 is a prognostic biomarker for poor survival in patients with pancreatic cancer. Hum Pathol 2017;66:79–85. [DOI] [PubMed] [Google Scholar]
- [15].Xu H, Chen X, Tao M, et al. B7-H3 and B7-H4 are independent predictors of a poor prognosis in patients with pancreatic cancer. Oncol Lett 2016;11:1841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tsiaousidou A, Tsaroucha AK, Lambropoulou M, et al. Increased B7H4 tissue expression correlates with high CA19.9 serum levels and a worse prognosis of pancreatic adenocarcinoma. Clin Exp Med 2016;16:351–6. [DOI] [PubMed] [Google Scholar]
- [17].Qian Y, Sang Y, Wang FX, et al. Prognostic significance of B7-H4 expression in matched primary pancreatic cancer and liver metastases. Oncotarget 2016;7:72242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen Y, Sun J, Zhao H, et al. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco Targets Ther 2014;7:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [19].Zhen-hua Y. Affect of B7-H4 and IRF-2 level on the prognosis of patients with pancreatic cancer. Mod Digestion Intervent 2015;20:212–5. [Google Scholar]
- [20].He C, Qiao H, Jiang H, et al. The inhibitory role of b7-h4 in antitumor immunity: association with cancer progression and survival. Clin Dev Immunol 2011;2011:695834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ozgoz A, Samli H, Ozturk KH, et al. An investigation of the effects of FGFR2 and B7-H4 polymorphisms in breast cancer. J Cancer Res Ther 2013;9:370–5. [DOI] [PubMed] [Google Scholar]
- [22].Ozgoz A, Samli M, Dincel D, et al. Association of B7-H4 gene polymorphisms in urothelial bladder cancer. Turk J Med Sci 2017;47:443–6. [DOI] [PubMed] [Google Scholar]
- [23].Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res 2007;67:8900–5. [DOI] [PubMed] [Google Scholar]
- [24].Leandersson P, Kalapotharakos G, Henic E, et al. A biomarker panel increases the diagnostic performance for epithelial ovarian cancer type I and II in young women. Anticancer Res 2016;36:957–65. [PubMed] [Google Scholar]
- [25].Huang C, Zhou L, Chang X, et al. B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: Associations with patient outcome and clinical significance. Oncol Rep 2016;35:2183–90. [DOI] [PubMed] [Google Scholar]
- [26].Li ZY, Zhang XH, Chen Y, et al. Clinical significance of B7-H4 expression in matched non-small cell lung cancer brain metastases and primary tumors. Onco Targets Ther 2013;6:869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Awadallah NS, Shroyer KR, Langer DA, et al. Detection of B7-H4 and p53 in pancreatic cancer: potential role as a cytological diagnostic adjunct. Pancreas 2008;36:200–6. [DOI] [PubMed] [Google Scholar]
- [28].Safaei HR, Rostamzadeh A, Rahmani O, et al. Prognostic investigations of B7-H1 and B7-H4 expression levels as independent predictor markers of renal cell carcinoma. Tumour Biol 2016;37:7583–7. [DOI] [PubMed] [Google Scholar]
- [29].Geng Y, Wang H, Lu C, et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 2015;20:273–81. [DOI] [PubMed] [Google Scholar]
- [30].Simon I, Zhuo S, Corral L, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res 2006;66:1570–5. [DOI] [PubMed] [Google Scholar]
- [31].Kristensen NN, Schmidt EG, Rasmussen S, et al. B7-H4-Ig treatment of normal mice changes lymphocyte homeostasis and increases the potential of regulatory T cells. Immunopharmacol Immunotoxicol 2013;35:505–13. [DOI] [PubMed] [Google Scholar]
- [32].Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 2006;203:871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sica GL, Choi IH, Zhu G, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003;18:849–61. [DOI] [PubMed] [Google Scholar]
- [34].Tan Z, Shen W. Prognostic role of B7-H4 in patients with non-small cell lung cancer: A meta-analysis. Oncotarget 2017;8:27137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meng Z, Wang F, Zhang Y, et al. B7-H4 as an independent prognostic indicator of cancer patients: a meta-analysis. Oncotarget 2017;8:68825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Song X, Shao Y, Gu W, et al. Prognostic role of high B7-H4 expression in patients with solid tumors: a meta-analysis. Oncotarget 2016;7:76523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


