Abstract
Minimal information is available concerning platelet-albumin–bilirubin (PALBI) grades in patients with hepatocellular carcinoma (HCC) following liver resection. This study aimed to investigate the predictive ability of PALBI grades in patients with a Child–Pugh class A score and hepatitis B virus-related (HBV-related) HCC after liver resection.
The data of patients with HBV-related HCC who underwent liver resection from 2010 to 2017 at our center were reviewed (n = 785). Cox regression was used to determine factors independently associated with postoperative recurrence and mortality. The area under the receiver operating characteristic curve (AUC) was used to estimate the predictive accuracy of different tools.
During the follow-up period, 505 (64.3%) patients experienced recurrence, and 374 (47.6%) patients died. Multivariate analysis revealed that the tumor-node-metastasis (TNM) stage (HR = 1.591, 95%CI = 1.414–1.789, P < .001), PALBI grade (HR = 1.326, 95%CI = 1.139–1.544, P < .001), a high AFP level (HR = 1.382, 95%CI = 1.158–1.649, P < .001) and transfusion (HR = 1.364, 95%CI = 1.087–1.712, P = 0.007) were independently associated with recurrence. Additionally, microvascular invasion (HR = 1.674, 95%CI = 1.292–2.169, P < .001), beyond the Milan criteria (HR = 0.477, 95%CI = 0.346–0.657, P < .001), PALBI grade (HR = 1.356, 95%CI = 1.151–1.598, P < .001), a high AFP level (HR = 1.542, 95%CI = 1.252–1.900, P < .001), and transfusion (HR = 1.548, 95%CI = 1.199–1.999, P = 0.001) adversely impacted the overall survival. The AUCs of the PALBI grades for postoperative recurrence and mortality were significantly higher than the albumin–bilirubin grade and Child–Pugh score. The prognostic significance of the PALBI grade for postoperative recurrence and mortality was maintained when stratified by the TNM stage.
The preoperative PALBI grade is a surrogate marker for the postoperative prognosis in patients with HBV-related HCC after liver resection.
Keywords: hepatocellular carcinoma, liver resection, platelet-albumin–bilirubin grades
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and third most frequent cancer-related cause of death worldwide.[1] Every year, over 700,000 new HCC cases are diagnosed, with a similar number of deaths due to its highly aggressive behavior.[1] Unfortunately, more than 50% of new cases and deaths occur in China because of the high prevalence of hepatitis B virus (HBV) infection.[1] Liver resection is a widely used treatment for patients with HCC and well-preserved liver function. However, the long-term outcomes of HCC patients after liver resection remain unsatisfactory because of a high incidence of postoperative recurrence. Previous investigations reported a postoperative recurrence rate of 70% at 5 years, even in patients with early stage HCC.[2]
Liver function plays a key role in liver resection for HCC. Several investigations have reported that liver function not only contributed to surgical safety but was also associated with postoperative recurrence and long-term survival following liver resection.[3,4] Currently, the Child–Pugh score is the most commonly used system to evaluate liver function in patients. However, the Child–Pugh score includes some subjective factors, including encephalopathy and ascites. Moreover, the interrelationship between ascites and albumin can confound the Child–Pugh score. In 2015, the albumin–bilirubin (ALBI) grade was proposed to assess a patient's liver function, and it only includes 2 objective factors (albumin and total bilirubin).[5] Since it was introduced, a number of investigations have confirmed that the ALBI grade was better than the Child–Pugh score in predicting a patient's liver function as well as in predicting a patient's prognosis following liver resection and radioembolization.[3,6,7] However, the ALBI grade does not incorporate portal hypertension, which is considered to be an unfavorable factor for long-term survival of HCC patients.[8,9] Recently, based on the ALBI grade, the platelet-albumin–bilirubin (PALBI) grade was developed to assess a patient's liver function. This grade incorporates blood platelet count as a surrogate maker of portal hypertension.[10] Both ALBI and PALBI include only objective factors. Despite some external validations of the ALBI and PALBI grades, whether the PALBI score could predict a HCC patient's outcome after liver resection remains unclear.[10–12] In this study, we attempted to clarify this issue.
2. Patients and methods
The data of patients with HBV-related HCC who underwent liver resection at West China Hospital between 2010 and 2017 were reviewed. Patients who met the following criteria were excluded: re-resection, ruptured HCC, no history of HBV infection, coinfection with hepatitis C virus, received preoperative antitumor treatment, a positive surgical margin, and the presence of other types of tumors. HCC was confirmed by postoperative pathological examination. The hepatitis B surface antigen was detected in all patients. This study was approved by the ethics committee of West China Hospital.
2.1. Follow-up
All preoperative blood tests were performed 2 days before the operation. After liver resection, patients were regularly followed up at one month post-op, every 3 months during the first postoperative 3-year period, and every 6 months thereafter. Before and after the operation, antiviral drugs (entecavir or lamivudine) were conventionally administered to patients who were positive for hepatitis B virus-DNA (HBV-DNA). During follow-up, blood cell tests, liver function tests, serum alpha-fetoprotein (AFP) measurement, HBV-DNA tests, visceral ultrasonography, computed tomography or magnetic resonance imaging, and chest radiography were performed for all patients. Bone scintigraphy was performed whenever HCC recurrence was suspected. Postoperative recurrence was defined as positive imaging findings compared with preoperative examination values or confirmation by biopsy or resection.[13]
2.2. Definitions
The ABLI grade was calculated based on the following formula: ALBI = (log10 bilirubin (μmol/L) × 0.66) + (albumin (g/L) × −0.085).[5] ALBI values were divided into 3 grades as follows: grade 1 (less than −2.60), grade 2 (between −2.60 and −1.39) and grade 3 (above −1.39).[5] The PALBI score was calculated using the following equation: 2.02 × log10 bilirubin–0.37 × (log10 bilirubin)2–0.04 × albumin–3.48 × log10 platelets + 1.01 × (log10 platelets).[2,11] Serum bilirubin was expressed in μmol/L; serum albumin level was expressed in g/L; and blood platelet count was expressed in 1000/μL.[11] The PALBI score was divided into 3 grades as follows: grade 1 (≤−2.53), grade 2 (>−2.53 and ≤−2.09), grade 3 (>−2.09).[11] The neutrophil-to-lymphocyte ratio (NLR) was defined as the absolute neutrophil count divided by the lymphocyte count.[13] The platelet-to-lymphocyte ratio (PLR) was defined as the absolute platelet count divided by the lymphocyte count.[13] A high preoperative AFP was defined as an AFP level greater than 400 ng/mL.[13]
2.3. Statistical analysis
All statistical analyses were performed using SPSS 21.0 (SPSS Company, Chicago, IL) for Windows. All continuous variables were shown as the means ± standard deviation and compared using one-way analysis of variance. Categorical variables were analyzed using the χ2 test or Fisher's exact test. Recurrence-free survival (RFS) and overall survival (OS) were determined using the Kaplan–Meier method, and comparisons were determined using the log-rank test. Independent risk factors for RFS and OS were identified using Cox regression analysis. Potential risk factors with a P-value < .05 in the univariate analysis were included in the multivariate analysis. The area under the receiver operating characteristic curve (AUC) was used to estimate the predictive accuracy of the PALBI grade, ALBI grade and Child–Pugh score. A P-value of < .05 was considered statistically significant.
3. Results
A total of 785 patients were included in the current study; 681 (86.8%) males and 104 (13.2%) females. The mean age was 50.8 ± 11.8 years. The mean tumor size was 7.6 ± 4.3 cm, and 327 (41.7%) patients had multiple tumors. High preoperative AFP levels were observed in 341 (43.4%) patients. Positive HBV-DNA was detected in 324 (41.3%) patients. Microvascular invasion (MVI) was observed in 429 (54.6%) patients, and a portal vein tumor thrombus (PVTT) was observed in 125 (15.9%) patients. A total of 232 (29.6%) patients were staged as TNM stage I, 268 (34.1%) patients as TNM stage II, and 285 (36.3%) patients as TNM stage III. A total of 125 (15.9%) patients had transfusions. A total of 535 (68.2%) patients were designated as ALBI grade 1, 250 (31.8%) patients as ALBI grade 2, and no patients as ALBI grade 3. A total of 544 (69.3%) patients were designated as PALBI grade 1, 211 (26.9%) patients as PALBI grade 2, and 30 (3.8%) patients as PALBI grade 3. A total of 750 (95.5%) patients received a Child–Pugh score of 5 points, and 35 (4.5%) patients received a Child–Pugh score of 6 points.
During the follow-up period (33.9 ± 18.1 months), 505 (64.3%) patients experienced recurrence, and 374 (47.6%) patients died. The 1-, 3-, and 5-year RFS rates were 77.5%, 41.0% and 26.4%, respectively. The 1-, 3-, and 5-year OS rates were 91.8%, 56.9% and 35.4%, respectively.
3.1. Prognostic factors for RFS
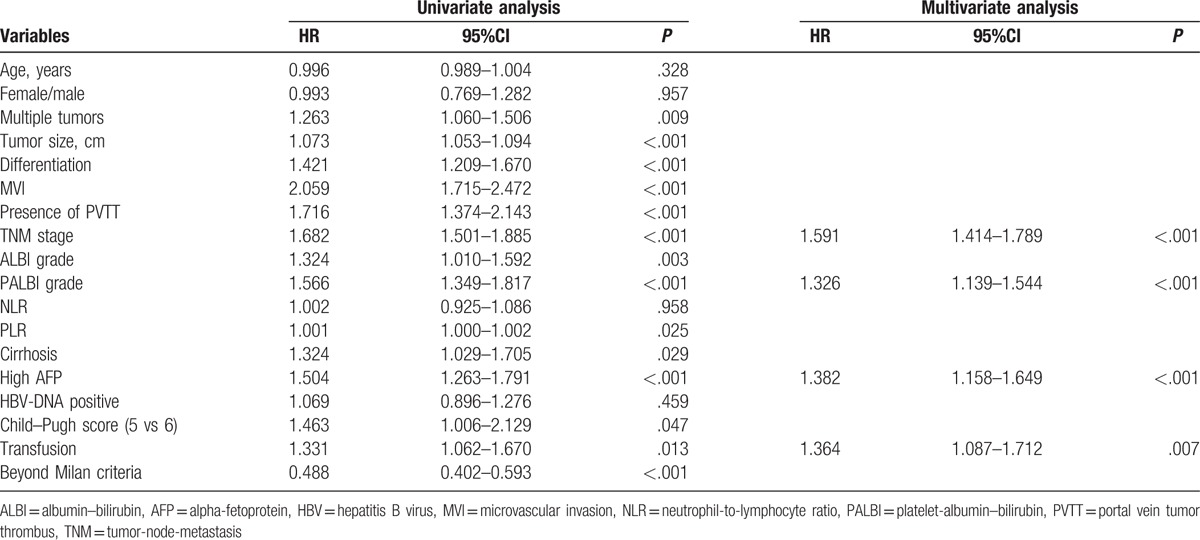
Using univariate analysis, the following factors contributed to recurrence after liver resection: tumor size, tumor differentiation, presence of PVTT, presence of MVI, TNM stage, ALBI grade, PALBI grade, multiple tumors, PLR, transfusion, high AFP level, beyond Milan criteria, and cirrhosis (Table 1). As shown in Table 1, multivariate analysis revealed that the following parameters were independently associated with recurrence: TNM stage (HR = 1.591, 95%CI = 1.414–1.789, P < .001), PALBI grade (HR = 1.326, 95%CI = 1.139–1.544, P < .001), high AFP level (HR = 1.382, 95%CI = 1.158–1.649, P < .001), and transfusion (HR = 1.364, 95%CI = 1.087–1.712, P = 0.007).
Table 1.
Univariate and multivariate analyses of factors associated with recurrence-free survival.
3.2. Prognostic factors for OS
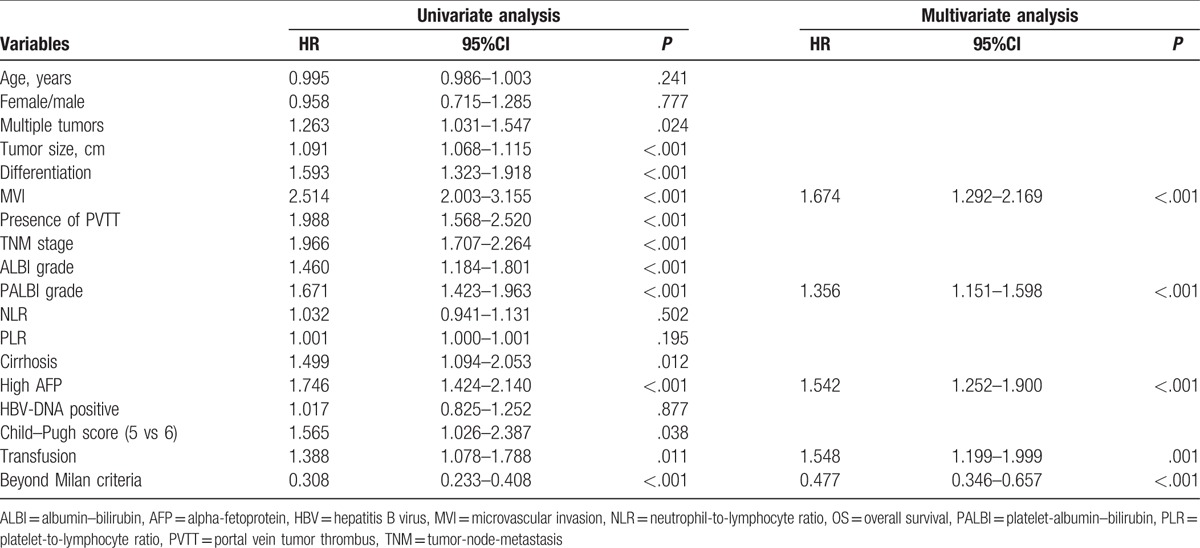
As listed in Table 2, univariate analysis demonstrated that tumor size, tumor differentiation, presence of PVTT, presence of MVI, TNM stage, ALBI grade, PALBI grade, multiple tumors, transfusion, high AFP level, beyond Milan criteria, and cirrhosis were significant predictive factors for OS. Multivariate analysis revealed that the independent risk factors for OS were the following: presence of MVI (HR = 1.674, 95%CI = 1.292–2.169, P < .001), beyond Milan criteria (HR = 0.477, 95%CI = 0.346–0.657, P < .001), PALBI grade (HR = 1.356, 95%CI = 1.151–1.598, P < .001), high AFP level (HR = 1.542, 95%CI = 1.252–1.900, P < .001), and transfusion (HR = 1.548, 95%CI = 1.199–1.999, P = 0.001).
Table 2.
Univariate and multivariate analyses of factors associated with overall survival.
3.3. Comparison of patients with different PALBI grades
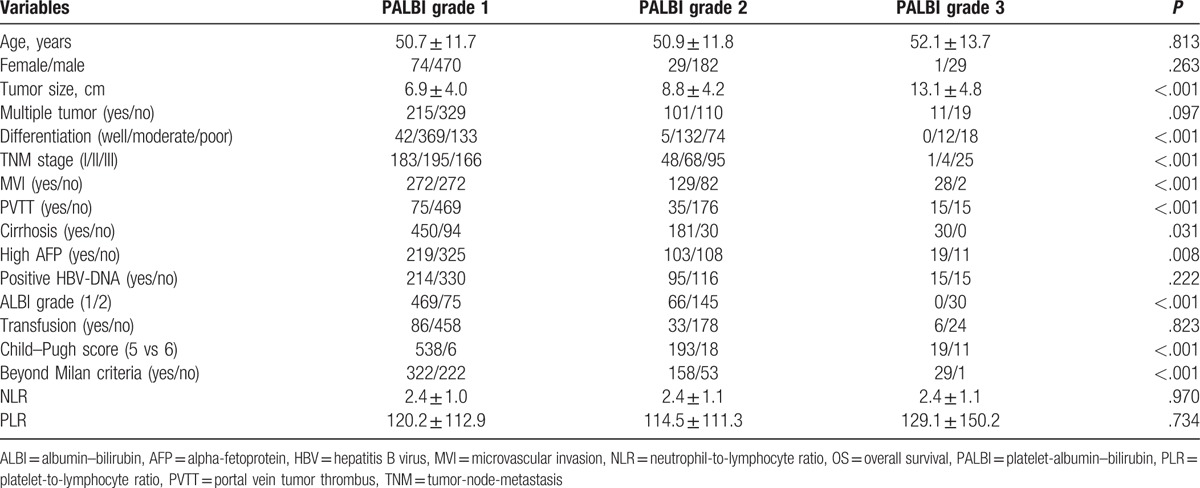
As shown in Figure 1, the 1-, 3-, and 5-year RFS rates were 80.5%, 46.6% and 31.8%, respectively, for patients with PALBI grade 1; 71.6%, 31.5% and 16.3%, respectively, for patients with PALBI grade 2 and 63.3%, 8.9% and 3.4%, respectively, for patients with PALBI grade 3. A significant difference was observed in RFS across the PABLI grades. The 1-, 3-, and 5-year OS rates of patients with PALBI grade 1 were 92.8%, 62.0% and 44.5%, respectively, and these values were significantly better than those with PALBI grade 2 or 3 (90.0%, 48.8% and 22.1%, respectively, for PALBI grade 2; 93.3%, 30.2% and 3.8%, respectively, for PALBI grade 3). We also compared the clinicopathological characteristics of patients with different PALBI grades (Table 3). Larger tumors, more MVI, PVTT, poor differentiation, cirrhosis, high preoperative AFP levels, beyond Milan criteria, high ALBI grade and advanced TNM stage were observed in patients with high PALBI grades (Table 3).
Figure 1.
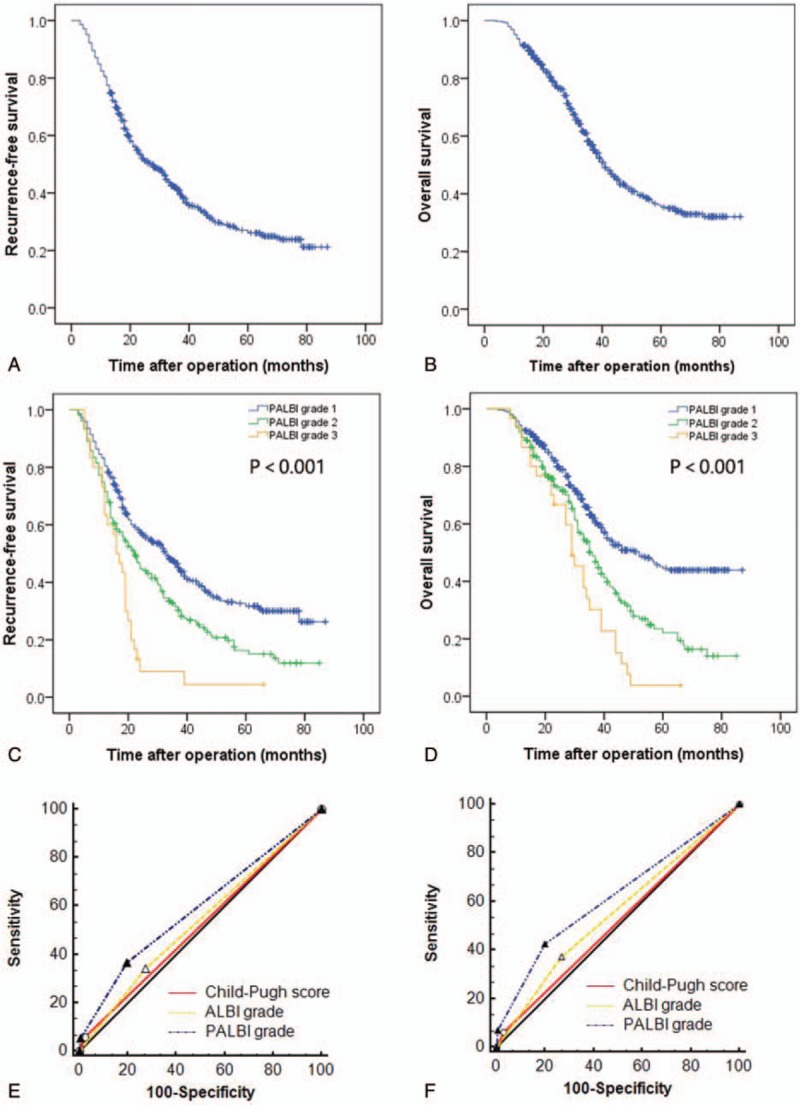
The RFS (A) and OS (B) curves for the whole cohort; comparison of RFS (C) and OS (D) in patients with different PALBI grades; comparison of the AUC for the PALBI grade, ALBI grade and Child–Pugh score in predicting RFS (E) and OS (F). AUC = area under the receiver operating characteristic curve, OS = overall survival, PALBI = platelet-albumin–bilirubin, RFS = recurrence-free survival.
Table 3.
Comparison of clinicopathological parameters of patients with different PALBI grades.
3.4. Comparison of the predictive ability of PALBI grade, ALBI grade and Child–Pugh score
The discriminatory capabilities of the PALBI grade, ALBI grade and Child–Pugh score were tested by the AUC methods. When we compared the AUC for the PALBI grade, ALBI grade and Child–Pugh score in predicting postoperative recurrence, the PABLI grade had the highest AUC (AUC = 0.590), followed by the ALBI grade (AUC = 0.534) and Child–Pugh score (0.518). Significant differences were observed (PPALBIvs. ALBI < 0.001, PPALBI vs. Child–Pugh score < 0.001). When we compared the AUC for the PALBI grade, ALBI grade and Child–Pugh score in predicting postoperative survival, the PABLI grade had the highest AUC (AUC = 0.618), followed by the ALBI grade (AUC = 0.553) and Child–Pugh score (0.516). Significant differences were observed (PPALBI vs. ALBI < 0.001, PPALBI vs. Child–Pugh score < 0.001).
3.5. The prognostic significance of PALBI stratified by TNM stage
We analyzed the prognostic significance of PALBI when stratified by TNM stage. As shown in Figure 2, when we divided patients into 3 groups according to TNM stage, the prognostic significance of the PALBI grade for RFS and OS was maintained when stratified by TNM stage. Patients with a high PALBI grade had a higher incidence of recurrence and lower long-term survival than those with a low PALBI grade.
Figure 2.
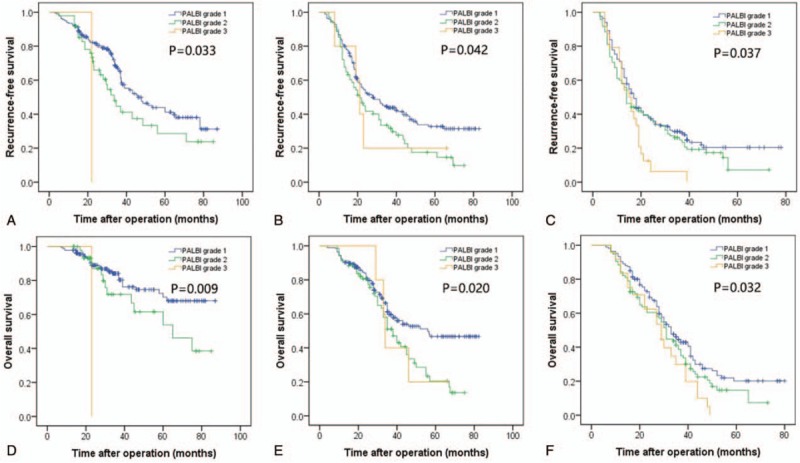
Comparison of RFS of patients with different PALBI grades in TNM stage I (A), II (B) and III (C); comparison of OS in patients with different PALBI grades in TNM stage I (D), II (E) and III (F). OS = overall survival, PALBI = platelet-albumin–bilirubin, RFS = recurrence-free survival, TNM = tumor-node-metastasis.
4. Discussion
Liver function plays an important role in predicting the prognosis in HCC patients. Traditionally, we have used the Child–Pugh score to assess a patient's liver function. However, the prognostic power of the Child–Pugh score was weakened when some subjective factors were included. Previous investigations confirmed that the ALBI grade was a simple, evidence-based and objective tool to evaluate a patient's liver function.[3,12] The ALBI grade did not involve factors related to portal hypertension. A number of studies have revealed that portal hypertension was associated with a high incidence of postoperative complications, recurrence and reduced long-term survival in HCC patients following liver resection.[14,15] The PALBI grade not only includes factors (total bilirubin and albumin) associated with liver function but also includes factors (platelet) reflecting portal hypertension. This may be an explanation for why the PALBI grade showed better predictive ability than other tools in the current study. We acknowledge that platelet count cannot exactly mirror portal hypertension. However, previous studies suggested a low platelet count negatively influences a patient's prognosis. For example, Shehta et al[16] confirmed that a preoperative low platelet count was related to a high HCC recurrence rate after liver resection. Maithel et al[17] revealed that a low preoperative platelet count was independently associated with increased postoperative major complications and mortality in liver resection in HCC patients. Recently, a meta-analysis that was performed by Zhang et al[18] also confirmed that a low platelet count was associated with poor RFS and OS in HCC patients after liver resection.
There were some investigations concerning the PALBI score in predicting the prognosis of HCC. Liu et al[11] suggested that both ALBI and PALBI were good tools to assess liver dysfunction. In their study, Liu et al included patients with Child–Pugh class A, B, and C liver function who underwent liver resection, radiofrequency ablation and transarterial chemoembolization (TACE).[11] Recently, Hansmann et al[10] confirmed that PALBI was accurate in the survival metrics in high-risk HCC patients who received TACE. In their study, Hansmann et al involved patients with Child–Pugh class A, B, and C liver function.[10] In our study, we only included patients with Child–Pugh class A liver function who had liver resection, which was different from the abovementioned studies.[10,11] Well-preserved liver function is very important for liver resection. Moreover, our study further identified the prognostic performance of TNM stage stratified by the PALBI grade. Our study confirmed that the PALBI grade can predict not only the OS but also the RFS for patients with HCC after liver resection.
Consistent with previous studies, in our study, a high AFP level, TNM stage, MVI, beyond Milan criteria and transfusion were suggested to be negative factors for prognosis.[19,20] Our study further suggested that the PALBI grade was an independent risk factor for both RFS and OS following liver resection. For patients in Child–Pugh class A, the Child–Pugh score has only 2 points (5 or 6). The ALBI grade also classifies these patients into 2 grades. However, the PALBI grade divides these patients into 3 grades. In our study, 223 patients with the Child–Pugh score 5 were classified into PALBI grade 2 or 3. In other words, the PALBI grade may be more useful in subdividing patients in the Child–Pugh class A than the Child–Pugh score. The subdivision of the Child–Pugh class A is useful for predicting a patient's prognosis. For instance, Okajima et al[21] confirmed that both the RFS and OS of patients with a Child–Pugh score 5 was better than that of patients with a Child–Pugh score 6, although all patients were designated as Child–Pugh class A. This finding may offer another potential explanation for why the PALBI grade showed good prognostic power in our study. Additionally, the PALBI grade only contains total bilirubin, albumin and platelets. These 3 paraments are objective and can be easily obtained from routine blood tests. Thus, the PALBI grade is an objective tool to assess liver function, and therefore is better than the Child–Pugh score.
There are some limitations in this study. This study is a single-center retrospective study. In the current study, we only included patients with HBV-related HCC. The predictive ability of the PALBI grade for other causes of HCC needs further investigation.
In conclusion, our study confirmed that the PALBI grade, an objective and evidence-based tool, is a surrogate marker for the prognosis in patients with HCC following liver resection.
5. Author contributions
LHM and CLP proposed this study. LHM and ZSZ collected the data. LHM, LC, and ZSZ analyzed the data.
Footnotes
Abbreviations: AF P = alpha-fetoprotein, ALBI = albumin–bilirubin, AUC = area under the receiver operating characteristic curve, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, MVI = microvascular invasion, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PALBI = platelet-albumin–bilirubin, PLR = platelet-to-lymphocyte ratio, PVTT = portal vein tumor thrombus, RFS = recurrence-free survival, TNM = tumor-node-metastasis.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].de Haas RJ, Lim C, Bhangui P, et al. Curative salvage liver transplantation in cirrhotic patients with hepatocellular carcinoma: an intention-to-treat analysis. Hepatology 2017;67:204–15. [DOI] [PubMed] [Google Scholar]
- [3].Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child–Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725–34. [DOI] [PubMed] [Google Scholar]
- [4].Kokudo T, Hasegawa K, Amikura K, et al. Assessment of preoperative liver function in patients with hepatocellular carcinoma—The Albumin-Indocyanine Green Evaluation (ALICE) Grade. PLoS One 2016;11:e0159530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Toyoda H, Lai PB, O’Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gui B, Weiner AA, Nosher J, et al. Assessment of the albumin–bilirubin (ALBI) grade as a prognostic indicator for hepatocellular carcinoma patients treated with radioembolization. Am J Clin Oncol 2017;doi: 10.1097/COC.0000000000000384. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Faitot F, Allard MA, Pittau G, et al. Impact of clinically evident portal hypertension on the course of hepatocellular carcinoma in patients listed for liver transplantation. Hepatology 2015;62:179–87. [DOI] [PubMed] [Google Scholar]
- [9].Choi SB, Kim HJ, Song TJ, et al. Influence of clinically significant portal hypertension on surgical outcomes and survival following hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci 2014;21:639–47. [DOI] [PubMed] [Google Scholar]
- [10].Hansmann J, Evers MJ, Bui JT, et al. Albumin-bilirubin and platelet-albumin-bilirubin grades accurately predict overall survival in high-risk patients undergoing conventional transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2017;28:1224–31. e1222. [DOI] [PubMed] [Google Scholar]
- [11].Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol 2017;32:879–86. [DOI] [PubMed] [Google Scholar]
- [12].Chan AW, Kumada T, Toyoda H, et al. Integration of albumin–bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1300–6. [DOI] [PubMed] [Google Scholar]
- [13].Li C, Wen TF, Yan LN, et al. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res 2015;198:73–9. [DOI] [PubMed] [Google Scholar]
- [14].Berzigotti A, Reig M, Abraldes JG, et al. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 2015;61:526–36. [DOI] [PubMed] [Google Scholar]
- [15].Harada N, Shirabe K, Maeda T, et al. Comparison of the outcomes of patients with hepatocellular carcinoma and portal hypertension after liver resection versus radiofrequency ablation. World J Surg 2016;40:1709–19. [DOI] [PubMed] [Google Scholar]
- [16].Shehta A, Han HS, Ahn S, et al. Post-resection recurrence of hepatocellular carcinoma in cirrhotic patients: Is thrombocytopenia a risk factor for recurrence? Surg Oncol 2016;25:364–9. [DOI] [PubMed] [Google Scholar]
- [17].Maithel SK, Kneuertz PJ, Kooby DA, et al. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg 2011;212:638–48. discussion 648–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Z, Zhang Y, Wang W, et al. Thrombocytopenia and the outcomes of hepatectomy for hepatocellular carcinoma: a meta-analysis. J Surg Res 2017;210:99–107. [DOI] [PubMed] [Google Scholar]
- [19].Zhang N, Gu J, Yin L, et al. Incorporation of alpha-fetoprotein (AFP) into subclassification of BCLC C stage hepatocellular carcinoma according to a 5-year survival analysis based on the SEER database. Oncotarget 2016;7:81389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhao H, Chen C, Fu X, et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget 2017;8:5474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Okajima C, Arii S, Tanaka S, et al. Prognostic role of Child–Pugh score 5 and 6 in hepatocellular carcinoma patients who underwent curative hepatic resection. Am J Surg 2015;209:199–205. [DOI] [PubMed] [Google Scholar]


