Abstract
Background:
Several studies suggest that local warming therapy (LWT) may help to treat chronic wounds, such as pressure ulcers, venous ulcers, arterial ulcers, and diabetic foot ulcers. However, evidence supporting the efficacy of this treatment is still incomplete. This study aimed to assess the effects of LWT in treating chronic wounds.
Methods:
For this review, we searched the Cochrane Wounds Specialized Register (March 6, 2017); the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2017 issue 3); Ovid MEDLINE (1946 to March 6, 2017); Ovid Embase (1974 to March 6, 2017); EBSCO CINAHL (1982 to March 6, 2017); Chinese Biomedical Literature Database (1980 to March 20, 2017); China National Knowledge Infrastructure (1980 to March 20, 2017); VIP Information (1980 to March 20, 2017) (Chinese Database); and Wanfang Data (1980 to March 20, 2017). We did not apply date or language restrictions. Published or unpublished randomized controlled trials (RCTs) analyzing the effects of LWT in the treatment of chronic wounds (pressure ulcers, venous ulcers, arterial ulcers, and diabetic foot ulcers) were screened and selected. Two review authors independently conducted study selection, we planned that 2 review authors would also assess risk of bias and extract study data.
Results:
No studies (RCTs) met the inclusion criteria for this review. Thus, it was impossible to undertake a meta-analysis or a narrative description of studies.
Conclusions:
The effects of LWT for treating chronic wounds are unclear because we did not identify any studies that met the inclusion criteria for this review. Quality improvement for LWT trials is urgently needed.
Keywords: chronic wounds, effect, local warming therapy, systematic review
1. Introduction
1.1. Description of the condition
A chronic wound can be described as any interruption in the continuity of the body's surface that does not pass through an orderly and timely repair process. Common types of chronic wounds include venous leg ulcers, arterial leg ulcers, diabetic foot ulcers, and pressure ulcers.[1,2] Orderly healing follows a sequence of metabolic activities: inflammation, collagen and fibroblast deposition (scar tissue formation), angiogenesis (new blood vessel formation), wound contraction, and scar remodeling. The duration of a normal healing process depends on many factors, but is formally defined as “healing within a timeframe that could reasonably be expected with conventional treatment.”[3] Chronic wounds are more commonly encountered in the elderly and those with multiple health problems.[4,5]
1.2. Wound types
1.2.1. Pressure ulcers
Pressure ulcers, also known as bedsores or pressure sores, are regions of localized damage to the skin sometimes involving deeper tissue layers such as muscle, tendon, and bone.[6–8] They are caused by unrelieved pressure, or pressure in combination with shear, usually over bony prominences (sacrum [tailbone], back, buttocks, heels, back of the head, and elbows).[9,10] Pressure ulcer prevalence and incidence figures differ according to the method used to collect data and the classification used. A review of the international literature suggested that prevalence in the UK ranged from 4.4% in community settings to 37% in a palliative care setting.[11] The UK incidence of pressure ulcers ranged from 2.2 per 100 new admissions per year in a hospital setting to 66% over 18 months for hospitalized older patients with hip fractures.[11] In the USA and Canada, prevalence ranged from 4.7% for hospitalized patients to 33% for community-based spinal cord injured patients.[11] The USA/Canada incidence rates ranged from 0% for community settings over a 6-month period to 65.6% over 5 years for patients with spinal injuries.[11] Pressure ulcers are generally categorized according to grades numbered from 1 to 4, according to the European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel.[12]
1.2.2. Venous leg ulcers
Venous leg ulcers are thought to occur as a result of improper functioning of venous valves. Venous leg valves consist of 2 flaps that converge in order to keep the blood moving in one direction, that is, towards the heart. Damaging to valves results in venous reflux causing high venous pressure. The prevalence of venous leg ulcers has been reported between 1.5 and 3 per 1000 population, and 1% to 2% of people will suffer from a venous ulcer at least once in their life.[13,14] It has been estimated that Western healthcare systems spend around 1% to 2% of their budget on treatment, intensive nursing, and in some cases prolonged care, for people with venous ulcers.[15–18] In the United States, the cost of treatment for venous ulcers for approximately 6 million patients approaches USD 2.5 billion (GBP 1.6 billion; EUR 1.8 billion), and 2 million work days are lost annually due to venous ulcer disease.[19]
1.2.3. Arterial leg ulcers
Arterial ulcers, also referred to as ischaemic ulcers, result from an inadequate arterial blood supply. The wounds are typically very painful, especially at night. The ulcers are characterized by well-defined, even wound margins that give the wound a “punched-out” look. Risk factors for the development of arterial ulcers include age, smoking, peripheral vascular disease, diabetes mellitus, hypertension, dyslipidaemia, family history, obesity, and a sedentary lifestyle.[20] Noninvasive diagnostic options for arterial assessment include manual palpation of pulses, ankle brachial pressure index, and Doppler examination.[21,22]
Several measures are used to help distinguish between venous and arterial ulcers. Venous ulcers usually occur between the knee and the ankle, while arterial ulcers usually occur below the ankle. However, “mixed etiology” (mixed cause) ulceration can occur above the ankle. Arterial ulcers are more painful than venous ulcers when lying down. Venous leg ulcers are frequently described as “throbbing,”’ “burning,” and “itchy,” while arterial ulcer pain tends to be described as “sharp” and “hurting.” [23] An ABPI between 0.5 and 0.8 suggests that ulcers may be caused by a mixture of venous and arterial disease. An ABPI value of <0.5 indicates arterial ulcers, while an ABPI >0.8 usually indicates that ulcers are venous in etiology.[24]
1.2.4. Diabetic foot ulcers
Diabetic foot ulcers are a major health risk for people with diabetes mellitus, and can result in limb loss and mortality. Since 1996 the number of people diagnosed with diabetes has increased from 1.4 million to 2.9 million in the UK.[25] By 2025 it is estimated that 5 million people will have diabetes.[25] Global projections suggest that the worldwide prevalence of diabetes is expected to rise to 4.4% by 2030, meaning that approximately 366 million people will be affected.[26] About 25% of diabetic patients are at risk of developing a foot ulcer,[27] and 7% of them might be at risk of amputation in the next 10 years.[28] The Wagner wound classification system is well established and widely used for grading diabetic foot ulcers.[29] However, newer grading systems, such as the University of Texas Wound Classification System,[30] PEDIS system (perfusion, extent/size, depth/tissue loss, infection, and sensation),[31] and SINBAD system (site, ischemia, neuropathy, bacterial infection, and depth score) are also used.[32]
1.3. Description of the intervention
Local warming therapy (LWT) has been used for treatment of chronic wounds for about 1260 years in China, according to the China Association of Traditional Chinese Medicine,[33] and for about 30 years in the USA.[34] Different types of LWT are available for wound management. These include non-contact wound warming units, wound dressings with a removable heating element and moxibustion.
Non-contact wound warming units are designed to apply radiant heat to a wound. The objective is to raise the wound temperature in order to increase blood flow and transport of oxygen to the local area. However, practitioners need to be very careful to control the level of heat so that the patient's skin is not burned.[34]
Certain technological advances have resulted in a specifically designed wound dressing with a removable heating element capable of delivering radiant heat emitted at 38 °C to affect local wound warming at a controlled temperature.[35]
Moxibustion is a traditional Chinese therapeutic method that uses the heat generated by burning moxa sticks (usually made from herbal preparations containing Artemisia vulgaris [mugwort]) near an acupoint (location on the body used in acupuncture and other traditional Chinese therapies) to create local warming.[36–38] The purpose of moxibustion, as with most forms of traditional Chinese medicine, is to strengthen the blood, stimulate the flow of “qi” (the natural energy, improve circulation[39]), and maintain general health. In addition, it is typically an inexpensive therapy that can be self-administered at home after brief instruction.[36]
Chronic wound care has made great progress in the last few decades through the standardization seen mirrored in many medical fields. Standard wound care promotes wound healing, helps lower morbidity, improves quality of life,[40] and is often used as comparator in clinical studies.[41] The components of standard wound care include[42] application of dressings to maintain a moist wound environment, debridement of necrotic tissue, if present, cleansing of the wound initially, and at each dressing change, using a neutral, nonirritating, and nontoxic solution, evaluation of, and provision for, adequate nutritional status, and documentation of evaluation, care, and wound measurements by a licensed medical professional.
1.4. How the intervention might work
Chronic wounds under standard care are often hypoxic (i.e., have inadequate oxygen supply).[43] The usual surface temperature of ulcers is about 33 °C.[44] This temperature limits the movement and growth of tissue.[45,46] Increasing the temperature of chronic wounds to 38 °C may help to induce healing by increasing blood flow and improving the availability of oxygen.[34,47] LWT may also decrease incidence of wound infection,[48] and may eradicate established methicillin-resistant Staphylococcus aureus (MRSA) infection in pressure sores.[49]
1.5. Why it is important to do this review
Although the application of warmth to wounds is a commonly-used ancient practice, there is uncertainty about its effects.[50] The evidence for the efficacy and safety of LWT use for treating chronic wounds has not previously been summarized.
1.6. Objectives
This study aimed to assess the effects of LWT in treating chronic wounds.
2. Methods
This study is a systematic review, and does not involve individual data. Thus, it does not need approval of ethics committee. It was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines, and previous published protocol in Cochrane library.[51]
2.1. Criteria for considering studies for this review
2.1.1. Types of studies
Randomized controlled trials (RCTs) published or unpublished, in any language, would have been included.
2.1.2. Types of participants
We planned to include studies that recruited people with chronic wound(s) (pressure ulcers, venous leg ulcers, arterial ulcers, and diabetic foot ulcers). As the method of diagnosis of different types of chronic wounds may vary, we would have accepted definitions as used in the RCTs.
2.1.3. Types of interventions
Trials comparing the effects of LWT (via moxibustion, radiant heat dressing, and other local warming interventions) with standard wound care or other wound-healing interventions would have been included. Trials that compare different types of LWT would also have been considered for inclusion.
2.1.4. Types of outcome measures
Primary outcomes included time to healing assessed using appropriate survival analysis (i.e., based on censored data), proportion of people with diabetic foot ulcers undergoing amputation of the lower limb at any level, including single toes, and proportion of wounds with complete healing.
Secondary outcomes consisted of change in wound size, with change expressed as absolute change (e.g., surface area change in cm2 since baseline) or relative change (e.g., percentage change in area relative to baseline); healing rate per day, week, or other unit of time; quality of life measured by a validated scale, either generic (such as EQ-5D, SF-36, SF-12, or SF-6) or disease-specific; treatment costs (as reported by the trial author); recurrence rate (as reported by the trial author); pain from wound (measured using survey/questionnaire/data capture process or visual analogue scale); adverse events (e.g., infection).
RCTs that evaluated any of these outcomes would have been included, irrespective of the scale(s) used for assessment. If possible, outcomes would have been evaluated at 1 week, 1 month, and up to 3 months after treatment had finished.
2.2. Search methods for identification of studies
Details of the search strategy for this review are available as follows:
2.2.1. Electronic searches
We searched the following electronic databases for RCTs that evaluated the use of LWT for chronic wounds. The databases included the Cochrane Wounds Specialised Register (March 6, 2017); the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2017, issue 3) (searched March 6, 2017); Ovid MEDLINE (1946 to March 6, 2017); Ovid Embase (1974 to March 6, 2017); EBSCO CINAHL (1982 to March 6, 2017); Chinese Biomedical Literature Database (1980 to March 20, 2017) (Chinese Database); China National Knowledge Infrastructure (1980 to March 20, 2017) (Chinese Database); VIP Information (1980 to March 20, 2017) (Chinese Database); and Wanfang Data (1980 to March 20, 2017) (Chinese Database). The search strategy is described in detail in Table 1.
Table 1.
Cochrane Central Register of Controlled Trials (CENTRAL) search strategy.

We adapted this strategy to search Ovid MEDLINE, Ovid EMBASE, and EBSCO CINAHL. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity- and precision-maximising version (2008 revision). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Center. We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network. We would not restrict studies with respect to language, date of publication, or study setting.
We also searched the following clinical trials registries: ClinicalTrials.gov (http://www.clinicaltrials.gov/); WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx); and EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/).
2.2.2. Searching other resources
We searched for grey literature/unpublished work by accessing Sciencepaper online (an open-access website in China http://www.paper.edu.cn/en). This source indexes material that may be unavailable from other electronic databases. Relevant manufacturers of local warming devices were contacted to request details about any ongoing studies.
2.3. Data collection and analysis
Data collection and analysis would have been carried out according to methods outlined in the published protocol.[51]
2.4. Selection of studies
Two review authors (JHY and QHZ) independently screened the title and abstract of each potentially relevant study identified from the electronic searches. We used pre-determined eligibility criteria to identify the potentially relevant trials for which full reports should be retrieved. Disagreements among authors were resolved by discussion with a third review author (ZRS) when necessary. This process of screening was repeated for the full texts retrieved which resulted in a decision on studies eligible for inclusion in the review. As there were no studies that met the described criteria, there were no studies to include in our assessment. The PRISMA flowchart was used to select the studies[52] (Fig. 1).
Figure 1.
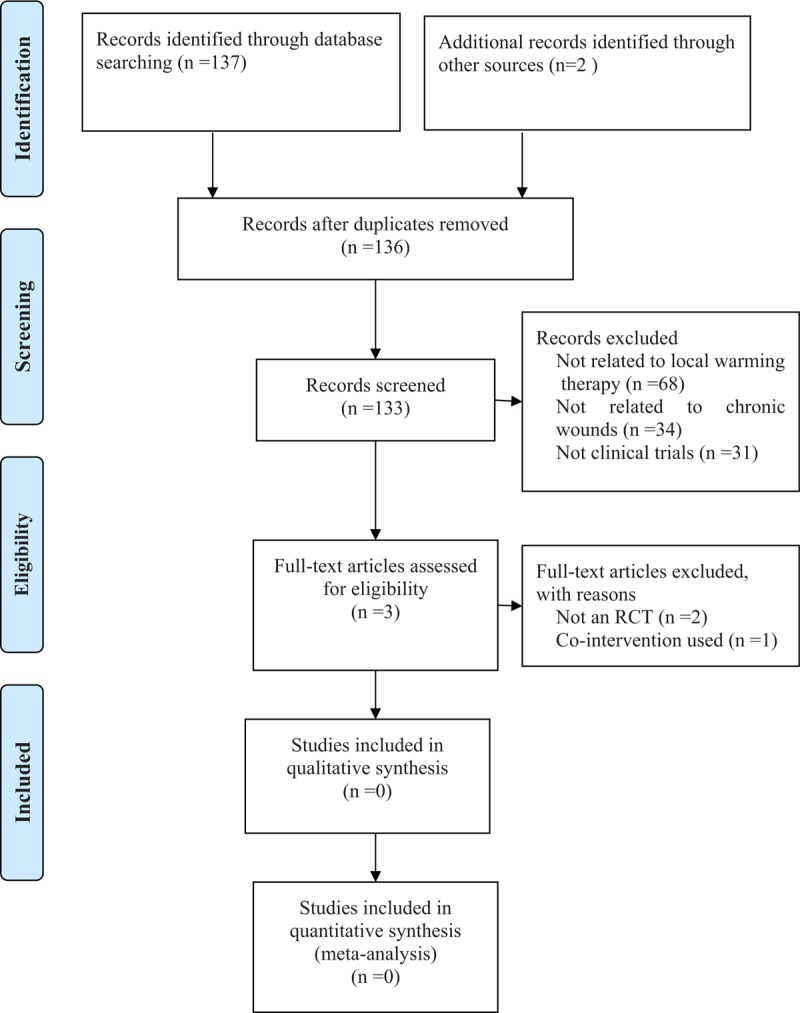
Flow diagram of the trial selection process.
2.5. Data extraction and management
Independently, two review authors (QHZ and JHY) extracted data using a data extraction sheet.
We intended to extract the following data: authors; year of publication; country of origin; trial setting; type of wound; inclusion criteria for participants; baseline characteristics of participants; number of participants randomized to each trial arm; details of the intervention (treatment and comparator); setting of treatment; duration of treatment; duration of follow-up; outcome data for primary and secondary outcomes; number of participants completing; number of withdrawals; reasons for participant withdrawal; statistical methods used in the analysis; and adverse events.
2.6. Assessment of risk of bias in included studies
For this review, two review authors would have independently assessed each included study using the Cochrane tool for assessing risk of bias.[53] This tool addresses the following domains: sequence generation, allocation concealment, blinding of participants, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias, which for this review may have included baseline imbalance. In addition, blinding was assessed separately for participants, care providers, and outcome assessors. Where possible, we intended to seek trial protocols in order to determine whether all prespecified outcomes were reported adequately. If trial protocols were unavailable, we would have made a judgment based on the inclusion or exclusion of all expected outcomes, with reference to those described in the methods sections of RCT reports. We would have completed a risk of bias table for each eligible study. We discussed any disagreement amongst all review authors to achieve a consensus.
We would have presented assessment of risk of bias using a “risk of bias summary figure,” which presents all of the judgments in a cross-tabulation of study by entry. This display of internal validity should indicate the weight a reader gave the results of each study. However, as no studies met the inclusion criteria, we could not conduct the evaluation of risk of bias.
2.7. Measures of treatment effect
2.7.1. Dichotomous data
For dichotomous data (e.g., wounds healed, amputations, ulcer recurrence, adverse events), we planned to calculate the risk ratio (RR) with 95% confidence interval (CI).
2.7.2. Continuous data
For continuous outcomes (e.g., change in wound size, quality of life), we extracted the mean difference with 95% CI.
2.7.3. Time to event data
For time to event data (time to complete wound healing), we planned to plot (and, if appropriate, pool) estimates of hazard ratios and 95% CIs as presented in the RCT reports using the generic inverse variance method in RevMan 5.3 (The Cochrane Collaboration, London, United Kingdom). We would have considered mean or median time to healing without survival analysis as a valid outcome only if reports specified that all wounds healed (i.e., if the trial authors regarded time to healing as a continuous measure with no censoring of survival metrics).
2.8. Unit of analysis issues
Healing of multiple wounds on the same patient cannot be considered as independent events. We intended to note whether RCT reported specified participants, limbs, or ulcers as the units of allocation and analysis. When multiple limbs or ulcers on the same individual were studied, we would have noted whether the analysis was appropriate (i.e., correctly taking account of highly correlated data) or inappropriate (i.e., considering outcomes for multiple ulcers on the same participant as independent). Where the number of wounds appeared to equal the number of participants, we would have assumed that the participant was the unit of analysis, unless otherwise stated. Wherever possible, measures of effect would have been based on the individual patient (as opposed to wound or limbs).
2.9. Dealing with missing data
Outcome data may be “missing at random” or “not missing at random,”[54] but in practice it is often difficult to categorize missing data in this way with any certainty. If outcome data had been missing from reports, we would have made attempts to contact the study authors to obtain missing information. If this was not successful, we would have employed the following strategy. Where RCTs reported dichotomous complete healing outcomes for only those participants completing the RCT (i.e., participants withdrawing and lost to follow up are excluded from the analysis), we would have regarded the participants not included in the analysis as if their wound did not heal (i.e., they would have been included in the denominator but not the numerator for healing outcomes). Where results were reported for participants who completed the RCT without specifying the numbers that were randomized per group initially, we would have presented only complete case data. For other outcomes, we would have presented data for all randomized participants, where reported. Otherwise we would have based estimates on complete cases only.
2.10. Assessment of heterogeneity
We would have considered both clinical and statistical heterogeneity. Consideration of clinical heterogeneity would have involved assessment of the degree of similarity between trials in terms of the clinical status of participants (e.g., wound type), intervention type, duration of intervention and type of outcome. We intended to investigate statistical heterogeneity using the Chi-squared test. We would have interpreted a Chi-squared test resulting in a P value of equal to or less than 0.10 as being indicative of significant statistical heterogeneity. We intended to measure the quantity of heterogeneity using the I-squared statistic.[55] Thresholds for the interpretation of the I-squared statistic can be misleading. A rough guide to interpretation is as follows:
0% to 30%: may represent low heterogeneity;
31% to 59%: may represent moderate heterogeneity;
60% to 100%: considerable heterogeneity.
When interpreting the I-squared statistic, we would have taken factors such as clinical and methodological heterogeneity, along with whether the heterogeneity was in the magnitude of effect or in the direction of effect, into account, particularly where confidence intervals overlapped.
Where appropriate, we would have pooled data using RevMan 5.[56] Where there were low levels or lack of heterogeneity, we intended to use a fixed effects model. Where there was moderate heterogeneity we intended to use a random effects model. Where clinical heterogeneity was evident, or where statistical heterogeneity was substantial, we would have presented the results in narrative form rather than pooling. In this review, no studies were included, thus, no substantial heterogeneity was found.
2.11. Assessment of reporting biases
In order to assess the likely presence of publication bias, funnel plots would have been constructed if at least 10 studies were available for meta-analysis of a primary outcome.
2.12. Data synthesis
We planned to combine studies using a narrative overview combined with meta-analysis of outcome data where appropriate using Review Manager Software.[56] The decision to include studies in a meta-analysis would have depended on the availability of treatment effect data and assessment of heterogeneity. Where feasible (i.e., where data were available, and where studies were similar enough) data would have been pooled. We intended to present the summary estimate as a risk ratio (RR) with 95% CI for dichotomous outcomes and difference between means with 95% CI for continuous outcomes. Where a group of RCTs had assessed the same concept with a continuous outcome but used different scales (e.g., quality of life), we would have considered using standardized mean difference (SMD) as the summary measure of effect. For time to event data, we intended to plot and pool available hazard ratio estimations using the generic inverse variance method in RevMan.[56]
2.13. ‘Summary of findings’ tables
We intended to present the main results of the review in ‘Summary of findings’ tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes.[57] The ‘Summary of findings’ tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias.[58] We would have presented the following outcomes in the ‘Summary of findings’ tables: time to healing assessed using appropriate survival analysis (i.e., based on censored data); proportion of people with diabetic foot ulcers undergoing amputation of the lower limb at any level, including single toes; and proportion of wounds with complete healing.
2.14. Subgroup analysis and investigation of heterogeneity
We would have carried out the following analyses, according to subgroup, to investigate for possible sources of heterogeneity: type of wounds; or type of interventions (e.g., moxibustion versus control intervention; wound warming unit versus control intervention).
2.15. Sensitivity analysis
If a sufficient number of trials had been found, sensitivity analyses would have been conducted to assess the robustness of the treatment effect as follows: removing studies with inadequate concealment of allocation; or removing studies in which outcome evaluation was not blinded.
3. Results
3.1. Description of studies
No RCTs met the inclusion criteria. One trial of LWT compared to an herbal powder mentioned that a random number table was used, but no other information regarding the randomization process was provided, thus the study was not included.[59]
3.2. Results of the search
The search, which included Chinese databases in addition to other International databases, resulted in 139 records that underwent further screening. Of these, 137 studies were from electronic databases, and two were from other sources. 133 studies were excluded from the title and abstract selection. Only 3 studies were considered potentially relevant after first screening and were retrieved in full text (Fig. 1). Finally, all 3 studies were excluded, and thus no trials were included. In this study, two review authors (JHY and QHZ) independently screened the title and abstract of each potentially relevant study against the inclusion criteria. Any disagreements were resolved by a third review author (ZRS).
3.3. Included studies
No studies (RCTs) were included in this review.
3.4. Excluded studies
Three studies did not meet the inclusion criteria and were excluded. One study was not an RCT.[60] Another study used a number of other cointerventions along with moxibustion, assessing only the effects of the combination therapy, but not those of moxibustion alone.[61] Although the third study mentioned the use of a random number table, we did not find any other information indicating that randomization was performed, and thus the study was excluded.[59]
3.5. Risk of bias in included studies
No studies met the inclusion criteria so we could not conduct a risk of bias assessment.
3.6. Effects of interventions
Neither a meta-analysis nor a narrative synthesis of studies was undertaken because no studies met the inclusion criteria.
4. Discussion
4.1. Summary of main results
This review highlights the lack of robust evidence for the use of LWT in the treatment of chronic wounds. We found no RCTs comparing the effects of LWT (via moxibustion, radiant heat dressing, and other local warming interventions) with standard wound care or other wound-healing interventions amongst people with chronic wound(s) (pressure ulcers, venous leg ulcers, arterial ulcers, and diabetic foot ulcers). We found 3 related trials in this review. However, all 3 studies failed to meet our inclusion criteria.[59–61] One study was not an RCT,[60] another applied cointerventions[61] and the last provided no evidence of randomization.[59]
4.2. Overall completeness and applicability of evidence
Currently there are no studies which meet the inclusion criteria for this review to assess the effects of LWT for treating chronic wounds. It is important to have information regarding this potential treatment because LWT is an intervention that is widely used for treatment of chronic wounds.[59–61] Accordingly, a valuable opportunity exists to trial LWT against standard wound care or other wound-healing interventions in a randomized control study to investigate whether LWT is effective in the treatment of chronic wounds.
4.3. Agreements and disagreements with other studies or reviews
No other reviews have presented data on LWT for treating chronic wounds. The results of our study did not find any eligible RCTs to provide analysis regarding either effectiveness or potential harms.
4.4. Implications for practice
Currently, there is no evidence that meets criteria for the use of LWT for treating chronic wounds. Thus, no definitive conclusions regarding using LWT for treating chronic wounds can be drawn from this review. As LWT therapy is commonly utilized for the management of chronic wounds in a clinical setting in many countries, the lack of adequate appropriately randomized controlled studies to document its efficacy, as well as to explore possible limitations, is concerning and must be addressed.
4.5. Implications for research
This systematic review has highlighted the need for high quality studies regarding LWT and its effects on chronic wounds. We offer the following recommendations for future studies in this area:
Strictly designed RCTs with sound methodology are needed for future research into the effects of LWT. These trials should have clear inclusion and exclusion criteria, strict methodology in randomization, allocation concealment, blinding of practitioner, participants, outcome assessors, and data analyst, adequate sample size, intention-to-treat analysis, and baseline comparability of groups. Furthermore, the results of these trials should be reported according to the guidelines set out in the CONsolidated Standards of Reporting Trials. (CONSORT) statement.
These trials should evaluate quality of life of the participants, as well as adverse events.
An economic cost analysis should also be performed to financially justify the potential benefits of LWT.
5. Conclusions
This systematic review did not include eligible studies on the local warming therapy for treating chronic wounds. More high quality trails are urgently needed to focus on this issue.
6. Author contributions
Conceptualization: J. Yue, Z. Sun.
Data curation: B. Golianu, Q. Sun, X. Wang.
Formal analysis: Q. Zhang.
Investigation: Y. Lu, S. Zhang.
Methodology: Z. Sun, B. Golianu, Y. Lu.
Resources: J. Yue, Q. Zhang.
Software: Q. Zhang.
Validation: Q. Sun.
Visualization: S. Zhang, X. Wang.
Writing – original draft: J. Yue, Q. Zhang.
Writing – review & editing: J. Yue, Q. Zhang, B. Golianu, Y. Lu.
Footnotes
Abbreviations: CENTRAL = Cochrane Central Register of Controlled Trials, CI = confidence interval, LWT = local warming therapy, MRSA = methicillin-resistant Staphylococcus aureus, PEDIS = perfusion, extent/size, depth/tissue loss, infection and sensation, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCTs = randomized controlled trials, RR = risk ratio, SINBAD = Site, Ischemia, Neuropathy, Bacterial infection, And Depth score, SMD = standardized mean difference.
JY, SZ, and QS have contributed equally to this article.
This study was partly supported by the National Natural Science Foundation of China (Grant No. 81303045), Foundation of Outstanding Innovative Talents Support Plan of Heilongjiang University of Chinese Medicine (Grant No. 2012RCQ64), Foundation of Graduate Innovative Plan of Heilongjiang Province (Grant No. YJSCX2012–357HLJ).
The authors have no conflicts of interest to disclose.
References
- [1].Doughty DB, Sparks-DeFriese B. Bryant RA, Nix DP. Wound healing physiology. Acute and Chronic Wounds: Current Management Concepts 4th ed.St Louis: Mosby-Elsevier; 2012. 63–82. [Google Scholar]
- [2].Gray M, Doughty D. Clean versus sterile technique when changing wound dressings. J Wound Ostomy Continence Nurs 2001;28:125–8. [DOI] [PubMed] [Google Scholar]
- [3].Jull AB, Cullum N, Dumville JC, et al. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2015. CD005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dealey C. The Care of Wounds: A Guide for Nurses. 4th ed.Chichester: Wiley-Blackwell; 2012. [Google Scholar]
- [5].Lauterbach S, Kostev K, Kohlmann T. Prevalence of diabetic foot syndrome and its risk factors in the UK. J Wound Care 2010;19:333–7. [DOI] [PubMed] [Google Scholar]
- [6].Reddy M, Gill SS, Kalkar SR, et al. Treatment of pressure ulcers: a systematic review. JAMA 2008;300:2647–62. [DOI] [PubMed] [Google Scholar]
- [7].Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006;14:663–79. [DOI] [PubMed] [Google Scholar]
- [8].Zhang QH, Sun ZR, Yue JH, et al. Traditional Chinese medicine for pressure ulcer: a meta-analysis. Int Wound J 2013;10:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Torpy JM, Lynm C, Glass RM. Pressure ulcers. JAMA 2003;289:254. [DOI] [PubMed] [Google Scholar]
- [10].Zeller JL, Lynm C, Glass RM. Pressure ulcers. JAMA 2006;296:1020. [DOI] [PubMed] [Google Scholar]
- [11].Kaltenthaler E, Whitfield MD, Walters SJ, et al. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare? J Wound Care 2001;10:530–5. [DOI] [PubMed] [Google Scholar]
- [12].European Pressure Advisory Panel (EPUAP), National Pressure Ulcer Advisory Panel (NPUAP), Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and treatment of pressure ulcers: quick reference guide; 2014. Available at: http://www.npuap.org/wp-content/uploads/2014/08/Updated-10-16-14-Quick-Reference-Guide-DIGITAL-NPUAP-EPUAP-PPPIA-16Oct2014.pdf. Accessed May 25, 2017. [Google Scholar]
- [13].Amsler F, Willenberg T, Blattler W. In search of optimal compression therapy for venous leg ulcers: a meta-analysis of studies comparing divers bandages with specifically designed stockings. J Vasc Surg 2009;50:668–74. [DOI] [PubMed] [Google Scholar]
- [14].Kranke P, Bennett MH, Martyn-St James M, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2015. CD004123. [DOI] [PubMed] [Google Scholar]
- [15].Gallenkemper G. Guidelines for diagnosis and therapy of venous ulcers (version 8 2008) ICD10: 183.0 (without inflammation) and 183.2 (with inflammation). Phlebologie 2008;37:308–29. [Google Scholar]
- [16].Gloviczki P, Gloviczki ML. Evidence on efficacy of treatments of venous ulcers and on prevention of ulcer recurrence. Perspect Vasc Surg Endovasc Ther 2009;21:259–68. [DOI] [PubMed] [Google Scholar]
- [17].Purwins S, Herberger K, Debus ES, et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J 2010;7:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ragnarson Tennvall G, Hjelmgren J. Annual costs of treatment for venous leg ulcers in Sweden and the United Kingdom. Wound Repair Regen 2005;13:13–8. [DOI] [PubMed] [Google Scholar]
- [19].van Gent WB, Wilschut ED, Wittens C. Management of venous ulcer disease. BMJ 2010;341:c6045. [DOI] [PubMed] [Google Scholar]
- [20].Hess CT. Arterial ulcer checklist. Adv Skin Wound Care 2010;23:432. [DOI] [PubMed] [Google Scholar]
- [21].Holloway GA., Jr Arterial ulcers: assessment and diagnosis. Ostomy Wound Manage 1996;42:46–51. [PubMed] [Google Scholar]
- [22].Perceau G. Diagnosing venous and venous/arterial ulcers. Soins 2012. 29–32. [PubMed] [Google Scholar]
- [23].Closs SJ, Nelson EA, Briggs M. Can venous and arterial leg ulcers be differentiated by the characteristics of the pain they produce? J Clin Nurs 2008;17:637–45. [DOI] [PubMed] [Google Scholar]
- [24].Vowden P, Vowden K. Doppler assessment and ABPI: interpretation in the management of leg ulceration; 2001. Available at: http://www.worldwidewounds.com/2001/march/Vowden/Doppler-assessment-and-ABPI.html. Accessed June 1, 2017. [Google Scholar]
- [25].Diabetes UK. Diabetes prevalence 2015. November 2015. Available at: https://www.diabetes.org.uk/About_us/What-we-say/Statistics/2015-as-published-2016/. Accessed May 25, 2016. [Google Scholar]
- [26].Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. [DOI] [PubMed] [Google Scholar]
- [27].Palestro CJ, Love C. Nuclear medicine and diabetic foot infections. Semin Nucl Med 2009;39:52–65. [DOI] [PubMed] [Google Scholar]
- [28].Margolis DJ, Allen-Taylor L, Hoffstad O, et al. Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen 2005;13:230–6. [DOI] [PubMed] [Google Scholar]
- [29].Wagner FW., Jr The dysvascular foot: a system of diagnosis and treatment. Foot Ankle 1981;2:64–122. [DOI] [PubMed] [Google Scholar]
- [30].Oyibo SO, Jude EB, Tarawneh I, et al. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24:84–8. [DOI] [PubMed] [Google Scholar]
- [31].Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20Suppl:S90–5. [DOI] [PubMed] [Google Scholar]
- [32].Ince P, Abbas ZG, Lutale JK, et al. Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care 2008;31:964–7. [DOI] [PubMed] [Google Scholar]
- [33].China Association of Traditional Chinese Medicine. Waitai Miyao Fang. Beijing (China): Huaxia Publishing House; 2009. [Google Scholar]
- [34].Rabkin JM, Hunt TK. Local heat increases blood flow and oxygen tension in wounds. Arch Surg 1987;122:221–5. [DOI] [PubMed] [Google Scholar]
- [35].Robinson C, Santilli SM. Warm-up active wound therapy: a novel approach to the management of chronic venous stasis ulcers. J Vasc Nurs 1998;16:38–42. [DOI] [PubMed] [Google Scholar]
- [36].Cardini F, Weixin H. Moxibustion for correction of breech presentation: a randomised controlled trial. JAMA 1998;280:1580–4. [DOI] [PubMed] [Google Scholar]
- [37].Ewies AA, Olah KS. The sharp end of medical practice: the use of acupuncture in obstetrics and gynaecology. BJOG 2002;109:1–4. [DOI] [PubMed] [Google Scholar]
- [38].World Health Organization (WHO) Regional Office for the Western Pacific. WHO international standard terminologies on traditional medicine in the western pacific region; 2007. Available at: http://www.wpro.who.int/publications/who_istrm_file.pdf?ua=1. Accessed May 25, 2017. [Google Scholar]
- [39].Deng Y, Zhu SL, Xu P, et al. Ratio of Qi with modern essential on traditional Chinese medicine Qi: Qi set, Qi element. J Mathematical Med 2003;16:346–7. [Google Scholar]
- [40].Patricia A, Slachta RN. Caring for chronic wounds: a knowledge update. Wound Care Advisor 2012;1:24–31. [Google Scholar]
- [41].Suissa D, Danino A, Nikolis A. Negative-pressure therapy versus standard wound care: a meta-analysis of randomised trials. Plast Reconstr Surg 2011;128:498e–503e. [DOI] [PubMed] [Google Scholar]
- [42].Wound Healing Society (WHS). Chronic Wound Care Guidelines. Available at: http://www.woundheal.org/whs-wound-care-guidelines. Accessed May 25, 2017. [Google Scholar]
- [43].Hopf H. Ryan TJ, Cherry GW, Harding KG. The role of warming and oxygen tension in wounds. International Congress and Symposium on Thermoregulation in Wound Care. Oxford: Royal Society of Medicine Press; 2000. 33–6. [Google Scholar]
- [44].Hellgren L, Vincent J. Degradation and liquefaction effect of streptokinase-streptodornase and stabilized trypsin on necroses, crusts of fibrinoid, purulent exudate and clotted blood from leg ulcers. J Int Med Res 1977;5:334–7. [DOI] [PubMed] [Google Scholar]
- [45].Jensen PKA, Therkelsen AJ. Cultivation at low temperature as a measure to prevent contamination with fibroblasts in epithelial cultures from human skin. J Invest Dermatol 1981;77:210–2. [DOI] [PubMed] [Google Scholar]
- [46].Yang QR, Van den Berghe D. Effect of temperature on invitro proliferative activity of human umbilical vein endothelial cells. Experimentia 1995;51:126–32. [DOI] [PubMed] [Google Scholar]
- [47].Ikeda T, Tayefeh F, Sessler DI, et al. Local radiant heating increases subcutaneous oxygen tension. Am J Surg 1998;175:33–7. [DOI] [PubMed] [Google Scholar]
- [48].Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 2001;358:876–80. [DOI] [PubMed] [Google Scholar]
- [49].Ellis SL, Finn P, Noone M, et al. Eradication of methicillin-resistant Staphylococcus aureus from pressure sores using warming therapy. Surg Infect 2003;4:53–5. [DOI] [PubMed] [Google Scholar]
- [50].Jun JY. Analysis of researches on the warming therapy for surgical patients. J Korean Acad Adult Nurs 2010;22:260–70. [Google Scholar]
- [51].Sun Z, Yue J, Zhang Q. Local warming therapy for treating chronic wounds [Protocol]. Cochrane Database Syst Rev 2015. CD011728. [Google Scholar]
- [52].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000100. [PMC free article] [PubMed] [Google Scholar]
- [53].Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available at: www.cochrane-handbook.org. Accessed October 20, 2017. [Google Scholar]
- [54].Higgins JPT, Altman DG, Sterne JAC. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available at: www.cochrane-handbook.org. Accessed October 20, 2017. [Google Scholar]
- [55].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- [57].Schünemann HJ, Oxman AD, Higgins JPT, et al. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available at: www.cochrane-handbook.org. Accessed October 21, 2017. [Google Scholar]
- [58].Schünemann HJ, Oxman AD, Higgins JPT, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. Accessed October 21, 2017. [Google Scholar]
- [59].Shang SY. Topical combination of moxibustion and its ash for treating pressure ulcers of 30 cases. Chin Med Mod Distance Educ China 2015;4:42–3. [Google Scholar]
- [60].Lin AL, Wang CR, Yu LY. The clinical observation of the combination of drugs and moxibustion for treating pressure ulcers. Nanfang J Nurs 1996;2:7–8. [Google Scholar]
- [61].Wu W, Zhou P, Huang QH. The efficacy of the topical antibiotics and. J Luzhou Med Coll 2003;6:557–8. [Google Scholar]


