Abstract
The aim of this study was to compare the clinical features of patients with avian influenza A (H7N9) and influenza A (H1N1) complicated by acute respiratory distress syndrome (ARDS).
The clinical data of 18 cases of H7N9 and 26 cases of H1N1 with ARDS were collected and compared in the respiratory intensive care unit (RICU) of Fuzhou Pulmonary Hospital of Fujian from March 2014 to December 2016.
Patients with H7N9 had a higher acute physiology and chronic health evaluation-II score (P < .05) and lung injury score (P < .05). The rates of coexisting diabetes mellitus, hyperpyrexia, and bloody sputum production were significantly higher in the H7N9 group than in the H1N1 group (P < .05). The H7N9 group had a longer duration of viral shedding from the onset of illness (P < .05) and from the initiation of antiviral therapy (P < .05) to a negative viral test result than the H1N1 group. Patients with H7N9 had higher rates of invasive mechanical ventilation; serious complications, including alimentary tract hemorrhage, pneumothorax or septum emphysema, hospital-acquired pneumonia (HAP) and multiple organ dysfunction syndrome (MODS); and hospital mortality (P < .05). At the 6th month of follow-up, the rates of bronchiectasia, reticular opacities, fibrous stripes, and patchy opacities on chest computed tomography (CT) were significantly higher in the H7N9 group than in the H1N1 group (P < .05). Based on multiple logistic regression analysis, H7N9 influenza viral infection was associated with a higher risk of the presence of severe ARDS than H1N1 influenza viral infection (odds ratio 8.29, 95% confidence interval [CI] 1.53–44.94; P < .05).
Compared to patients with H1N1, patients with H7N9 complicated by ARDS had much more severe disease. During long-term follow-up, more changes in pulmonary fibrosis were observed in patients with H7N9 than in patients with H1N1 during the convalescent stage.
Keywords: acute respiratory distress syndrome, avian influenza A (H7N9), influenza A (H1N1), tomography, x-ray computed
1. Introduction
Humans infected with either avian influenza A (H7N9)[1] or influenza A (H1N1)[2–4] can exhibit severe respiratory disease. Acute respiratory distress syndrome (ARDS) has a high incidence in severe cases and is the main cause of avian influenza-related death.[5–7] To date, data regarding comparisons between patients with H7N9 and those with H1N1 have been reported,[8–11] but specific studies comparing the 2 diseases in terms of ARDS are rare. Herein, the clinical features and prognoses of patients with H7N9 avian influenza and those with H1N1 influenza were retrospectively and comparatively analyzed to further improve the understanding of ARDS caused by influenza viruses.
2. Methods
2.1. Patients
This study was established in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Fuzhou Pulmonary Hospital of Fujian, Educational Hospital of Fujian Medical University. The Ethics Committee waived the requirement for informed consent for this study, but all patient data were analyzed anonymously. The clinical data, including epidemiology, clinical manifestations, imaging examinations, and laboratory examinations, were collected from 18 patients with avian influenza A (H7N9) and 26 patients with influenza A (H1N1) complicated by ARDS in the respiratory intensive care unit (RICU) of the Fuzhou Pulmonary Hospital of Fujian from March 2014 to December 2016. All patients with H7N9 or H1N1 were diagnosed according to the diagnosis and treatment programmes for influenza issued by the Chinese Ministry of Health (National Health and Family Planning Commission).[12,13] The respiratory specimens (nasopharyngeal swab, sputum, extracted tracheal aspirate, or bronchoalveolar lavage fluid) in all clinically suspected cases of influenza viral infection were positive for avian influenza A (H7N9) virus or influenza A (H1N1) virus by reverse transcription polymerase chain reaction (RT-PCR), which was performed as a qualitative assay. After admission, serial sampling of all respiratory secretions mentioned above (nasopharyngeal swab or lower respiration sample) was performed daily for viral monitoring until the clinical specimens were consistently negative twice during a time interval of 24 hours. Patients with ARDS were diagnosed according to the diagnostic criteria of the Berlin definition[14] as follows: the timing was within 1 week of a known clinical insult or new/worsening respiratory symptoms; chest imaging (chest x-ray or computed tomography [CT] scan) showed bilateral opacities that were not fully explained by effusions, lobar/lung collapse, or nodules; hypoxemia (positive end expiratory pressure [PEEP] or continuous positive airway pressure [CPAP] ≥5 cm H2O) was classified as mild (the ratio of arterial partial pressure of oxygen to inspiratory oxygen fraction [PaO2/FiO2] 201–300 mmHg), moderate (PaO2/FiO2 101–200 mmHg), or severe (PaO2/FiO2 ≤100 mm Hg); and the origin of edema was assessed as respiratory failure that was not fully explained by cardiac failure or fluid overload, and an objective assessment (e.g., echocardiography) was necessary to exclude hydrostatic edema if no risk factor was present.
2.2. Statistical analysis
Data were described as the median (interquartile range, IQR) or number (%). The comparisons of the features between the different subtypes of influenza (H7N9 and H1N1) were performed with the Wilcoxon signed-rank test to compare the medians of continuous variables and Fisher exact test or the chi-squared test to compare proportions. The association between the different subtypes of influenza viral infection (H7N9 and H1N1) and the risk of severe ARDS presentation and death was assessed using multivariable logistic regression analysis, adjusted for age, sex, and coexisting diabetes. All analyses were performed with SPSS 19.0 statistical analysis software (IBM, Armonk, NY). A P-value <.05 was considered statistically significant.
3. Results
3.1. Epidemiological characteristics
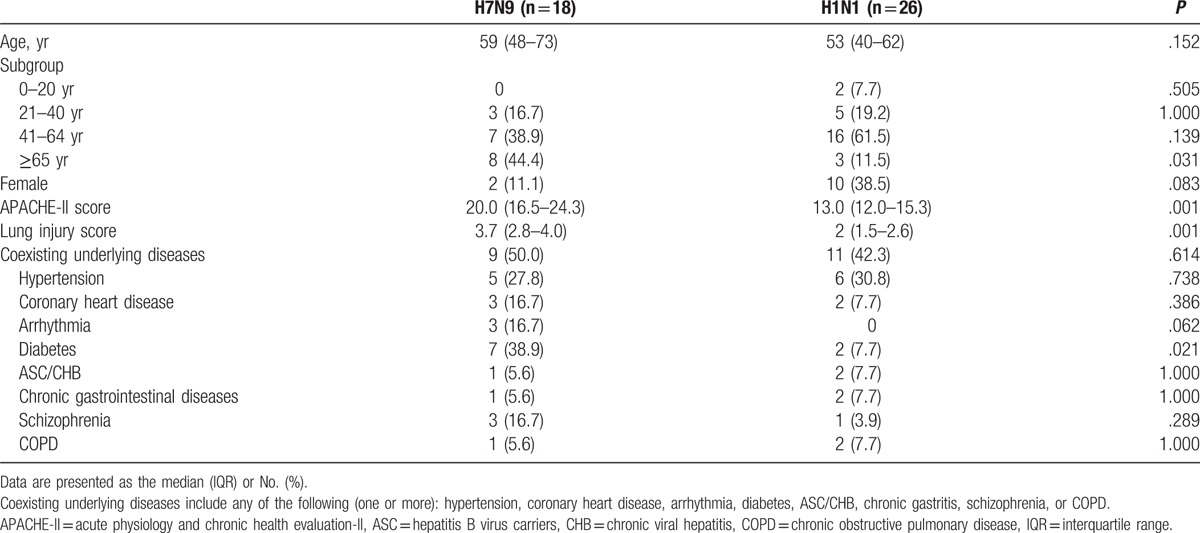
The epidemiological characteristics of the 2 groups are shown in Table 1. The number of patients aged ≥65 years, the acute physiology and chronic health evaluation-II (APACHE-II) score and the lung injury score were higher in the H7N9 group than in the H1N1 group (P < .05) on admission. The rate of diabetes mellitus was statistically higher in the H7N9 group than in H1N1 group (P < .05).
Table 1.
Epidemiological features in the H7N9 and H1N1 groups.
3.2. Clinical manifestations, laboratory results, and imaging findings
The clinical characteristics of the patients in the 2 groups are shown in Table 2. The 2 groups were mainly characterized by fever, shortness of breath, cough, hemoptysis, poor appetite, and fatigue, and hyperpyrexia (>39.0 °C) and bloody sputum were more common in the H7N9 group than in the H1N1 group (P < .05).
Table 2.
Clinical symptoms, laboratory results, and imaging features of the H7N9 and H1N1 groups.
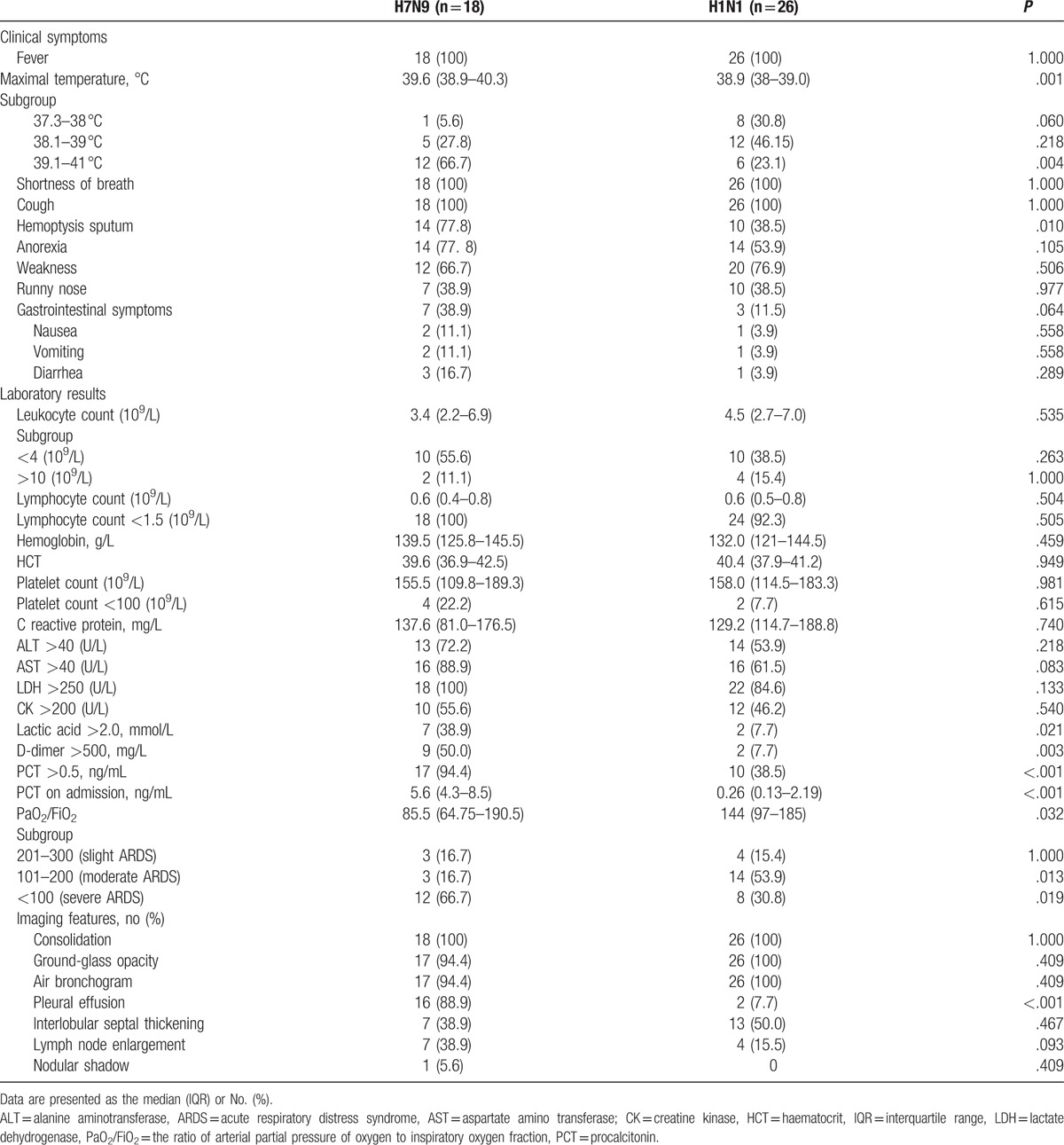
The procalcitonin (PCT) level, elevated lactic acid, and D-dimer rates on admission and PCT >0.5 ng/mL rate after onset were significantly higher in the H7N9 group than in the H1N1 group (P < .05). The severity of ARDS was more serious in the H7N9 group than in the H1N1 group (P < .05).
The typical chest CT characteristics of the 2 groups were similar. A nodular shadow was extremely rare, and bronchiectasis was not observed. The pleural effusion rate was higher in the H7N9 group than in the H1N1 group (P < .05).
3.3. Treatment and prognosis
All patients were treated with antiviral drugs and other treatment measures (Table 3). The rate of invasive mechanical ventilation was significantly higher in the H7N9 group than in the H1N1 group (P < .05). The H7N9 group had longer durations of viral shedding from the onset of illness and from the initiation of antiviral therapy to a negative viral test result (P < .05) than the H1N1 group.
Table 3.
Treatment and prognosis of the H7N9 and H1N1 groups.
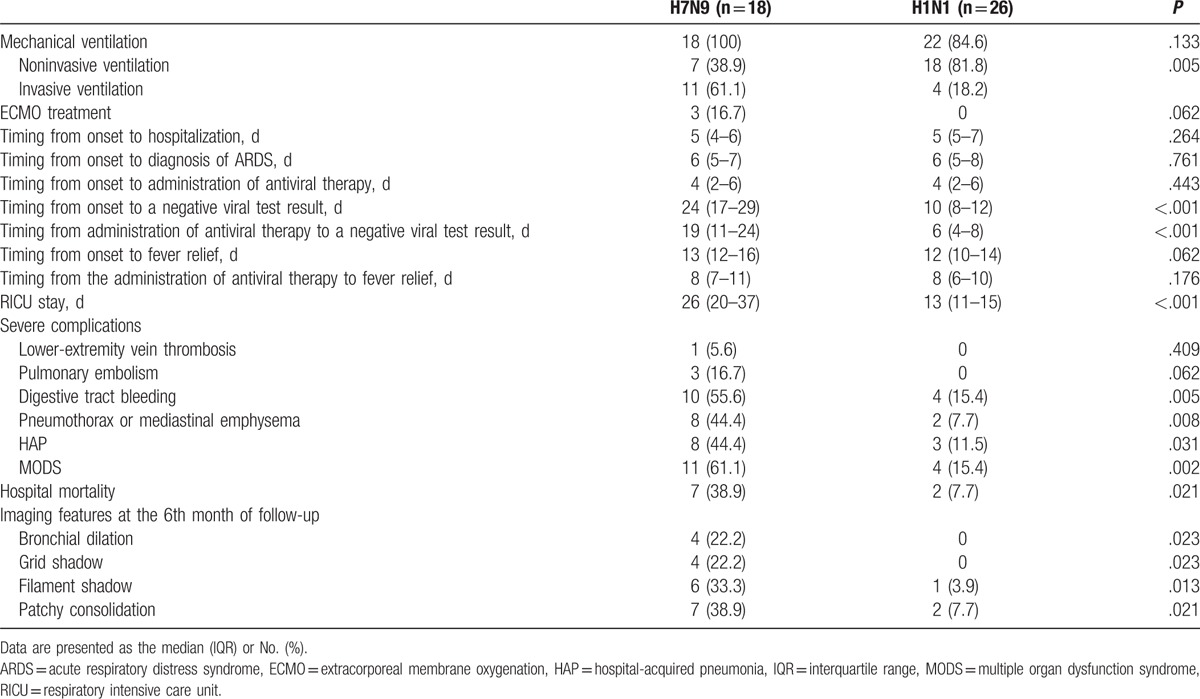
Over the course of the viral infections, the 2 groups presented with a variety of serious accompanying complications, of which the occurrence rates of alimentary tract hemorrhage, pneumothorax or septum emphysema, hospital-acquired pneumonia (HAP) and multiple organ dysfunction syndrome (MODS) were significantly higher in the H7N9 group than in the H1N1 group (P < .05). The hospital mortality rate of the H7N9 group was significantly higher than that of the H1N1 group (P < .05).
During follow-up, residual lesions on chest CT could be observed in both groups. At the 6th month of follow-up, the rates of bronchiectasia, reticular opacities, fibrous stripes, and patchy opacities in the H7N9 group were significantly higher than those in the H1N1 group (P < .05). Most residual lesions were adjacent to the pleura and obvious pulmonary fibrosis changes with collapsed lung could be detected in some survivors in the H7N9 group.
3.4. Different subtypes of influenza viral infection correlate with the presence of severe ARDS and death
After the adjustments for baseline variables (age, sex, and coexisting diabetes), H7N9 influenza viral infection was associated with a higher risk of the presence of severe ARDS than H1N1 influenza viral infection (odds ratio [OR] 8.29, 95% confidence interval [CI] 1.53–44.94; P < .05; Table 4). The differences remained significant when the corrections for only age and sex were made and when no corrections for baseline variables were made (OR 10.20, 95% CI 1.97–52.40; P < .05 and OR 4.50, 95% CI 1.24–16.28; P < .05, respectively; Table 4).
Table 4.
The association between the different subtypes of influenza viral infection and the risk of the presentation of severe ARDS and death.
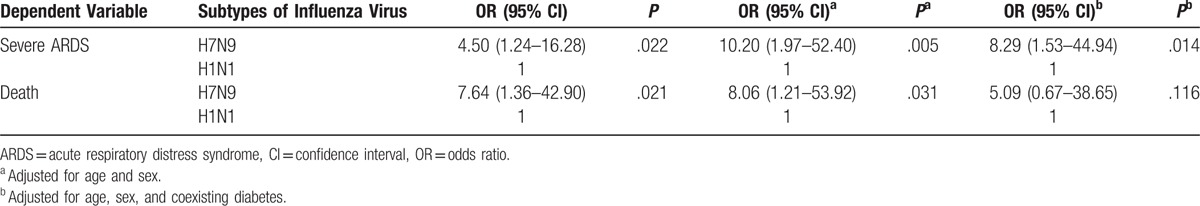
After the adjustments for baseline variables (age, sex, and coexisting diabetes), the risk of death between the 2 subtypes of influenza viral infection (H7N9 and H1N1) was not significantly different (OR 5.09, 95% CI 0.67–38.65; P > .05; Table 4), though the differences were significant when corrections for only age and sex were made and when no corrections for baseline variables were made (OR 8.06, 95% CI 1.21–53.92; P < .05; OR 7.64, 95% CI 1.36–42.90; P < .05; Table 4).
4. Discussion
In our study, the proportions of underlying diseases in the patients with H7N9 and those with H1N1 complicated by ARDS were high at 50.0% and 42.3%, respectively, which indicated that the combination of underlying diseases had a significant effect on the severities of H7N9 and H1N1.[5,15] Interestingly, 3 cases (16.7%) with coexisting schizophrenia were found in the H7N9 group. To our knowledge, there was no specific association between schizophrenia and the presence of H7N9 influenza viral infection, and the coexistence of the 2 diseases was considered coincidental.
The presentation of ARDS in both the H7N9 and H1N1 patients was rapid after viral infection, along with a series of clinical symptoms. Notably, large amounts of bloody sputum production showing diffuse alveolar hemorrhage were more common among the H7N9 patients, which indicated that the viral injury to the H7N9 patients was more severe.[16] In addition, H7N9 patients had similar patterns of leukopenia, lymphopenia, and thrombocytopenia and similar levels of creatine kinase, lactic dehydrogenase, alanine aminotransferase, and aspartic transaminase to those observed in the H1N1 patients. These experimental indexes were considered to be closely related to the severities of the H7N9 and H1N1 infections.[12,17]
The main manifestations on chest CT for both diseases were characterized by consolidations, ground-glass opacities (GGOs), and air bronchograms. Pleural effusion, interlobular septal thickening, and mediastinal or hilar lymphadenectasis were the second most common imaging abnormalities. The pleural effusion rate in the H7N9 group reached 88.9%, while the pleural effusion rate in the H1N1 group was only 7.7%, suggesting that the combination with pleural effusion might have a certain value in the differential diagnosis of the 2 diseases. During the follow-up period of the survivors, chest CT showed that the resolution of the lesions among the H7N9 patients was slow, which was consistent with the previously reported rate in the literature[18] and which was similar to the rate among the patients with avian influenza A (H5N1) viral infection.[19] In addition, pulmonary fibrosis changes were much more commonly observed in the H7N9 survivors. The main cause might have been the obviously prolonged viral shedding time during the H7N9 infection, which may have produced more severe damage to the lung tissue.[20] More observations are needed to clarify further imaging changes in such patients.
Notably, H7N9 influenza viral infection was associated with a significantly higher risk of the presence of severe ARDS. Specifically, as observed in our study, ARDS in the H1N1 group was mainly light and moderate, while ARDS in the H7N9 group was mainly severe, suggesting the relatively moderate conditions of the H1N1 patients.[21] Since ARDS in the H7N9 group was much more severe, the necessity for mechanical ventilation was much higher, but the treatments for mild and moderate ARDS of the 2 groups were mainly based on noninvasive positive pressure ventilation (NPPV). A multiple-center survey[22] indicated that when NPPV was applied as the first-line intervention for ARDS, intubation was avoided for 54% of treated patients, with less ventilator-associated pneumonia and a lower intensive care unit mortality rate. In addition, the multicenter trial reported by Zhan et al[23] showed that the early application of NPPV for mild ARDS improved PaO2/FiO2 with time and decreased the proportion of patients requiring intubation with a lower number of organ failures and a trend towards reducing inhospital mortality. In our research, most of the patients with mild and moderate ARDS and some with severe ARDS in both groups were treated with NPPV and ultimately cured, indicating that the use of NPPV for mild and moderate ARDS had a good curative effect. However, additional prospective and comparative trials that address the need for intubation and the mortality rate as the outcomes of interest for patients with viral pneumonia and ARDS are required.
5. Conclusions
The patients with H7N9 complicated by ARDS had much more severe diseases with worse outcomes than the patients with H1N1. More changes in pulmonary were observed in the patients with H7N9 at the convalescent stage, and thus, these patients should be carefully followed up to further clarify their outcomes. Additional studies are needed to further the understanding of ARDS in patients with influenza.
6. Author contributions
Conceptualization: J. Huang.
Data curation: C. Lan, H. Zhang, X. Wang, J. Pan.
Formal analysis: H. Zhang, L. Chen, J. Huang.
Methodology: H. Weng.
Project administration: H. Li.
Writing – original draft: H. Li, C. Lan, J. Huang.
Writing – review & editing: H. Weng, J. Huang.
Footnotes
Abbreviations: APACHE-II = acute physiology and chronic health evaluation-II, ARDS = acute respiratory distress syndrome, CI = confidence interval, CPAP = continuous positive airway pressure, CT = computed tomography, FiO2 = fraction of inspiration oxygen, GGOs = ground-glass opacities, HAP = hospital-acquired pneumonia, IQR = interquartile range, MODS = multiple organ dysfunction syndrome, NPPV = noninvasive positive pressure ventilation, OR = odds ratio, PaO2/FiO2 = the ratio of arterial partial pressure of oxygen to inspiratory oxygen fraction, PCT = procalcitonin, PEEP = positive end expiratory pressure, RICU = respiratory intensive care unit, RT-PCR = reverse transcription polymerase chain reaction.
HL, HW, and CL have contributed equally to this work.
The authors declare that they have no conflict of interest.
References
- [1].Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 2013;368:1888–97. [DOI] [PubMed] [Google Scholar]
- [2].Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009;361:680–9. [DOI] [PubMed] [Google Scholar]
- [3].Takayama K, Kuramochi J, Oinuma T, et al. Clinical features of the 2009 swine-origin influenza A (H1N1) outbreak in Japan. J Infect Chemother 2011;17:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ramsey C, Kumar A. H1N1: viral pneumonia as a cause of acute respiratory distress syndrome. Curr Opin Crit Care 2011;17:64–71. [DOI] [PubMed] [Google Scholar]
- [5].Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013;368:2277–85. [DOI] [PubMed] [Google Scholar]
- [6].Webb SA, Aubron C, Bailey M, et al. ANZIC Influenza Investigators. Critical care services and the H1N1 (2009) influenza epidemic in Australia and New Zealand in 2010: the impact of the second winter epidemic. Crit Care 2011;15:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaber S, Conseil M, Coisel Y, et al. (H1N1): patients’ characteristics and management in intensive care unit. A literature review. Ann Fr Anesth Reanim 2010;29:117–25. [DOI] [PubMed] [Google Scholar]
- [8].Wang C, Yu H, Horby PW, et al. Comparison of patients hospitalized with influenza A subtypes H7N9, H5N1, and 2009 pandemic H1N1. Clin Infect Dis 2014;58:1095–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang J, Xu H, Yang X, et al. Cardiac complications associated with the influenza viruses A subtype H7N9 or pandemic H1N1 in critically ill patients under intensive care. Braz J Infect Dis 2017;21:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu SH, Li HJ, Li N, et al. Comparative study of CT findings and clinical course of patients with severe pneumonia due to avian influenza H7N9 and swine influenza H1N1 infection. Radiol Pract 2014;29:756–9. [Google Scholar]
- [11].Pan QW, Chen Z, Luo JQ, et al. Comparative study of avian influenza A (H7N9) and influenza A (H1N1). IMHGN 2015;21:2578–80. [Google Scholar]
- [12].National Health and Family Planning Commission (NHFPC). The diagnosis and treatment protocol for human infections with Avian Influenza A (H7N9) (2014). Chin J Clin Infect Dis 2014;7:1–3. [Google Scholar]
- [13].Chinese Ministry of Health. The diagnosis and treatment protocol for human infections with influenza A (H1N1) (2010). Int J Respir 2011;31:81–4. [Google Scholar]
- [14].Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- [15].Tan W, Dai B, Sun LF, et al. Clinical analysis of survival and death cases in 289 patients with novel influenza A (H1N1). Int J Respir 2011;31:411–4. [Google Scholar]
- [16].Pan JG, Huang JB, Li HY, et al. Clinical analysis of 13 severe cases of influenza A (H7N9) virus infection. Chin J Clin Infect Dis 2016;9:363–6. [Google Scholar]
- [17].Li NN, Dai B, Wen H, et al. Analysis of serum myocardial enzymes in 63 H1N1 patients. J China Med Univ 2010;39:873–4. [Google Scholar]
- [18].Tang XJ, Xi XH, Chen CC, et al. Long-term follow-up of 5 survivors after the first outbreak of human infections with avian influenza A (H7N9) virusin Shanghai, China. Chin Med J (Engl) 2016;129:2128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lu PX, Wang YX, Zhou BP, et al. Radiological features of lung changes caused by avian influenza subtype A H5N1 virus: report of two severe adult cases with regular follow-up. Chin Med J (Engl) 2010;123:100–4. [PubMed] [Google Scholar]
- [20].Huang JB, Li HY, Liu JF, et al. Histopathological findings in a critically ill patient with avian influenza A(H7N9). J Thorac Dis 2015;7:E672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vaillant L, La Ruche G, Tarantola A, et al. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009;14:pii: 19309. [DOI] [PubMed] [Google Scholar]
- [22].Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007;35:18–25. [DOI] [PubMed] [Google Scholar]
- [23].Zhan Q, Sun B, Liang L, et al. Early use ofnoninvasive positive pressure ventilation for acute lung injury: a multicenter randomized controlled trial. Crit Care Med 2012;40:455–60. [DOI] [PubMed] [Google Scholar]


