Supplemental Digital Content is available in the text
Keywords: blood platelets, mucinous carcinoma, ovarian cancer, patient survival
Abstract
Smouldering inflammation, thrombocytosis, and platelet hyper-reactivity are linked to malignancy. The relationships between preoperative diagnostic blood morphology parameters and cancer have been the focus of much interest, because some of these parameters are correlated with advanced cancer stages and poor patient survival rates. This study aimed to perform an observational, retrospective analysis of the intradiversity of blood platelet parameters in patients with different International Federation of Gynaecology and Obstetrics (FIGO) stages and different histological types of epithelial ovarian carcinomas (EOC), and also an analysis of the overall survival rate.
In all, 94 EOC patients were included in this analysis (23 mucinous, 33 serous, 20 undifferentiated, 14 endometrioid, and 4 clear cell carcinoma cases). Peripheral blood samples were collected and analyzed before drug or surgical treatment.
The platelet-to-neutrophil ratio (PNR) was related to the histological type of EOC, particularly mucinous carcinoma. In patients with mucinous cancer, the PNR was significantly lower compared with patients with nonmucinous cancer, and this parameter distinguished between mucinous and nonmucinous groups of patients (area under receiver-operating characteristic [ROC] curve 0.721 ± .056; sensitivity 82.6%; specificity 61%; P < .001; ROC analysis), regardless of the FIGO stage. Moreover, elevated PNR values were correlated with lower survival rate of EOC patients.
The reduced PNR, similar to the lower level of cancer antigen 125, is characteristic for mucinous ovarian carcinoma patients. Moreover, elevated PNR index might correlate with poor survival of patients.
1. Introduction
It has been proven that smouldering inflammation, thrombocytosis, and platelet hyper-reactivity are linked to malignancy.[1,2] Disturbances in blood platelet function and an imbalance of their absolute or relative number results from ongoing inflammation and contributes to coagulopathy, that is, venous thromboembolism, in cancer patients, including ovarian cancer.[3–6]
Considerable diversity in blood parameters has been reported in different groups of ovarian cancer patients, in terms of the severity of the disease. However, there are few data that clearly demonstrate the relationship between preoperative blood platelets parameters and disease progression in ovarian cancer or the health outcomes of patients. It has been demonstrated earlier by others that high platelet, high neutrophil, and low leukocyte counts in peripheral blood are related to advanced stages of ovarian cancer and are accompanied by poor patient prognosis.[7–9] Differentiation of the neutrophil-to-leukocyte ratio (NLR) has been shown to correlate with invasive and borderline ovarian carcinomas.[9,10] A balance between platelets and leukocytes (ie, the platelet-to-lymphocyte ratio [PLR]) may correlate with the stage of ovarian cancer and patient prognosis.[11,12] In our previous papers, we have shown that the neutrophil activation stage might be related to more aggressive ovarian cancer.[13,14] In the present article, the diversity of platelet parameter values, especially in terms of histological type, is considered. Particular attention has been paid to mucinous carcinoma, as an entity separate from other histopathologic subtypes, for which the survival of patients is worse compared with other subtypes of epithelial ovarian carcinomas (EOC).[15,16]
The study aimed to analyze the intradiversity of blood platelets parameters in EOC patients with different International Federation of Gynaecology and Obstetrics (FIGO) stages and different histological types of cancer. The platelet count, morphology, and platelet and leukocytes ratios in EOC and noncancer patients were compared, and selected parameters were analyzed in details. The newly proposed parameter, that is, platelet-to-neutrophil ratio (PNR), and also the quantitative relationships between the PNR values and the histological types of EOC, were considered.
2. Material and methods
2.1. Patients
The 1-center retrospective study was conducted at the Polish Mother's Memorial Hospital Research Institute, Lodz, Poland. Patients were admitted to the gynaecologic departments due to a pelvic mass. Patients with a history of any previous malignant neoplasia or gynaecologic operation, transplantation, autoimmune disease, diabetes, thyroid problems, or any signs of infection were excluded from the study. We finally included 94 patients with epithelial ovarian cancer into our analysis. The patients ranged in age from 24 to 88 years, and the ovaries or fallopian tubes were the primary sites of malignancy for all women. The clinical stage of ovarian cancer was established after laparotomy and pathologic examination, and it was based on the FIGO classification system.[17,18] Tissues removed during surgery were examined by a pathologist and were assessed for the following factors: tumor grade, histological type, and the presence of metastases, which were determined according to the FIGO and World Health Organization classifications. In accordance with a paradigm of the pathogenesis and origin of EOC, all cases were classified into 2 broad categories of ovarian cancer, according to the clinical, pathological, and molecular/genetic-based classification system proposed by Kurman and Shih[19]: type I tumors included mucinous carcinoma, low-grade serous, low-grade endometrioid, and clear cell cancers; and type II included high-grade serous, high-grade endometrioid, and undifferentiated carcinomas. The clinical and pathological characteristics of the patients are shown in Supplemental Table 1. Most patients were at advanced FIGO stages III to IV (70.2%). Serous carcinomas (35.1%) predominated in our patients, and type II ovarian cancer was established in 54.3% of the patients. Different cancer groups were age-matched (Supplemental Table 1). The diagnostic parameters of peripheral blood were presented in comparison to the “noncancer” group of patients. The “noncancer” group consisted of 50 age-matched generally healthy women who were admitted to the gynaecologic department because of static disorders of the vagina (cystocoele, rectocoele), without history or signs of endometriosis or inflammation, and without blood coagulation abnormalities.[14] Institutional review board of Polish Mother's Memorial Hospital Research Institute approved the study. Ethical approval was not necessary for the analysis of database of diagnostic parameters.
2.2. Blood collection
Peripheral blood samples were taken on the day of admission, before any drug or surgical treatment, and analyzed as previously described.[14] The blood samples were analyzed using an automatic blood cell counter Vitros 5.1/Vitros 350 (Ortho Clinical Diagnostics, Inc., Raritan, NJ) at the Diagnostic Laboratory at the Polish Mother's Memorial Hospital Research Institute, Lodz, Poland, during preoperative workups of the patients.
2.3. Statistical analysis
Descriptive statistics were performed for each tumor type. The incidence of the same histological type was considered separately and then combined, when defensible. Endometrioid and clear cell carcinomas were included in 1 group (“other”). The FIGO I, II, III, and IV stages were divided into 2 groups “I to II” and “III to IV” for analysis. Differences between the clinical parameter values were calculated for cancer and noncancer patients through nonparametric Mann–Whitney U tests or chi-square tests. Relationships between the clinical parameters and clinicopathological features were analyzed through the Fisher exact test, chi-square tests, and the Kruskal–Wallis test. Significant data for clinical parameters were converted into 2 groups (dichotomizing) based on significant cut-off values calculated in the receiver-operating characteristic (ROC) analysis. The area under the ROC curve (AUC), sensitivity, selectivity, and statistical significance of the cut-off values were calculated.
Compliance of data distribution with normal distribution was evaluated using the Shapiro-Wilk test. Homogeneity of variance was verified by Levene test. The data values were log-transformed to fully meet the requirements of distribution normality and variance homogeneity, as necessary. Univariate and multivariate analyses of variance (ANOVA/MANOVA) and Newman-Keuls test for multiple comparisons were performed to examine differences between the different EOC groups. Wilks test was used in MANOVA. Multiple linear regression and multiple logistic regression analyses were employed for more than 2 variables, and P values under 0.05 were considered to be statistically significant. The Statistica software package, version 13.0 (StatSoft, Cracow, Poland), was used for the calculations.
3. Results
3.1. EOC patients versus noncancer patients
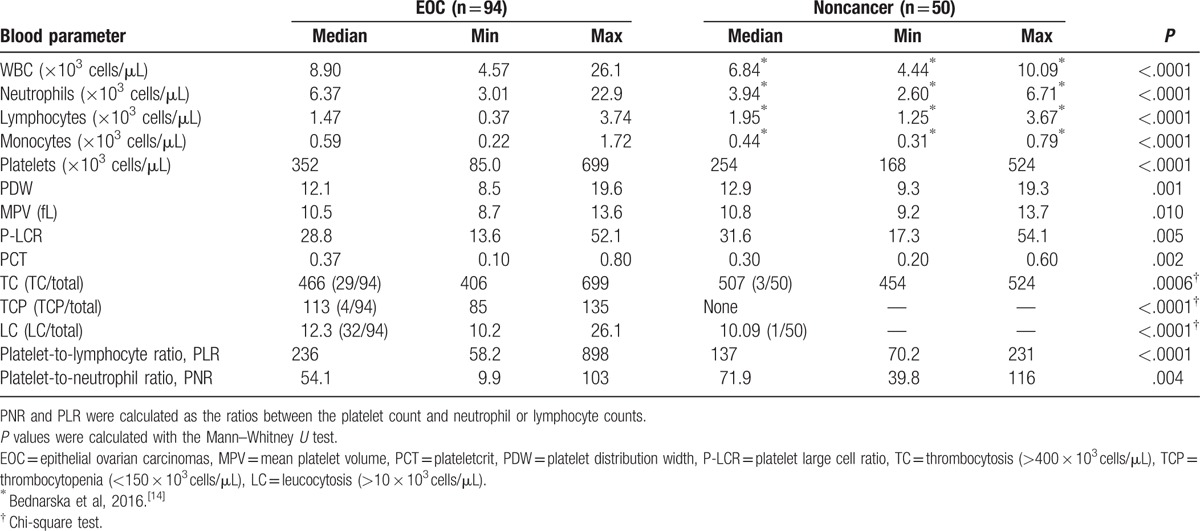
Epithelial ovarian carcinoma patients were characterized by an increased number of platelets, neutrophils, and monocytes, and significantly decreased lymphocyte numbers in their peripheral blood, compared with the control group (Table 1). Thrombosis and leucocytosis occurred in one-third of the EOC patients. The evident increase in the platelet count and decrease in the number of lymphocytes in the EOC patients resulted in an approximately 2-fold higher PLR value compared with the noncancer patients (Table 1). The most noticeable (approximately 2-fold) increase occurred in the number of neutrophils in EOC patients versus noncancer patients. Although the number of platelets increased, the ratio of platelets to neutrophils in EOC patients significantly decreased compared with noncancer patients. The differences in the platelet morphology parameters (eg, mean platelet volume, platelet large cell ratio, and platelet distribution width) of the cancer and noncancer patients, although statistically significant, were virtually irrelevant (Table 1). The selected platelet parameters, related to platelet count, were then analyzed in relationship to the different cancer patient groups.
Table 1.
Parameters of cell counts in the peripheral blood of EOC and noncancer patients.
3.2. Platelet parameter diversity in different groups of EOC
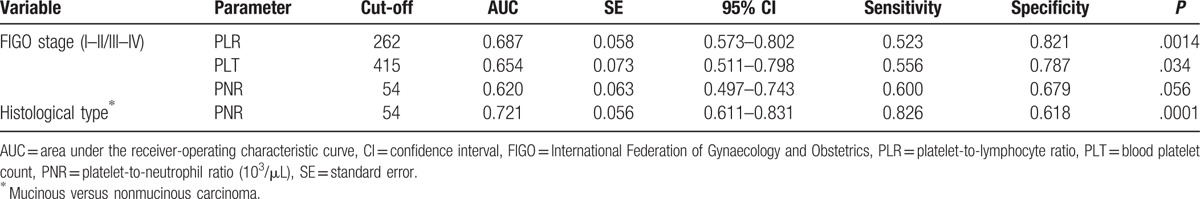
The ROC analysis of platelet parameters was performed for different FIGO and histological cancer groups. The significant cut-off values for certain parameter is shown in Table 2. The AUCs were significant for the platelet indices (ie, PLR, PLT), and it was large enough (0.653–0.687) to analyze the EOC, according to early and advanced FIGO stages. The specificities of classifications were good (range 0.786–0.821); however, the sensitivities were poor (0.523–0.556). The AUC was at the edge of significance for PNR, in terms of the FIGO stage (P = .056). However, the PNR cut-off value of 54, which distinguished between mucinous and nonmucinous carcinomas, had the largest AUC (0.721), with good sensitivity (0.826) and acceptable specificity (0.618), and it was significant (P < .001; Table 2).
Table 2.
ROC analysis results.
On the basis of the ROC analysis results, the clinical parameters data were assigned to 2 groups: equal or above (“high”); and below (“low”) the cut-off values. For example, PNR values ≥54 were identified as “high,” and PNR values <54 were “low.” The median “low” PNR was 40.8 (range 9.9–54), the median “high” PNR was 66.9 (range 54.2–103; P < .001, Mann–Whitney U test), the median “low” PLT (<415 × 103/μL) was 276 × 103/μL (range 85–411 × 103/μL), and the median “high” PLT (>415 × 103/μL) was 505 (range 416–699 × 103/μL; P < .001, Mann–Whitney U test). Relationship between the platelet parameters, and clinical and histological features of the EOC patients are shown in Table 3.
Table 3.
Relationship between the platelet parameters and characteristics of EOC.
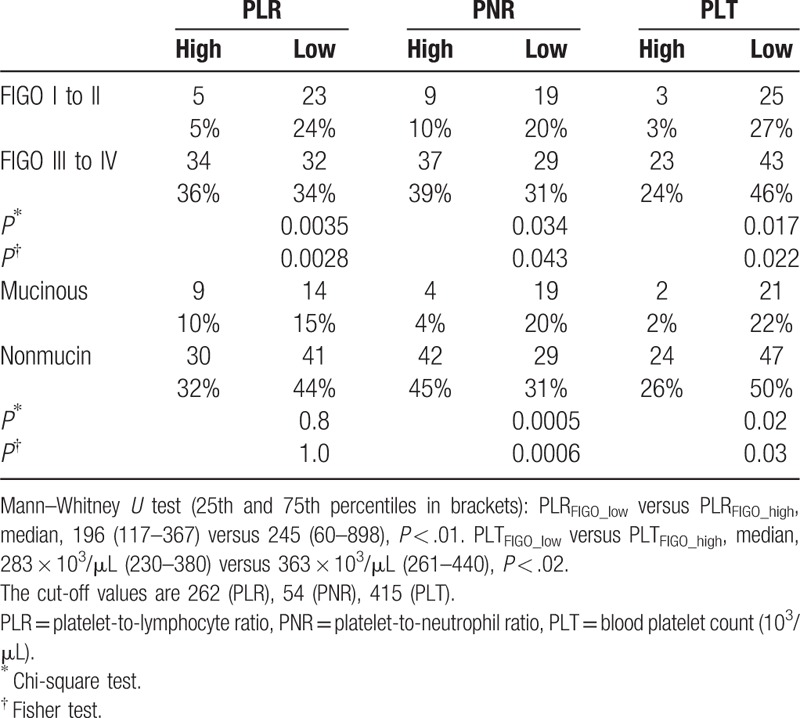
All tested blood platelet parameters (ie, PLT, PLR, PNR) were significantly correlated with the FIGO stage (Table 3). In early ovarian cancer (FIGO I–II) cases, the number of patients with high PLT was reduced compared with the FIGO III to IV cases, which is understandable because the number of platelets was also an indicator of inflammation in cancer patients. Low platelet count was correlated with early FIGO stages. Similar to PLT, low PLR and PNR values were correlated with early FIGO stages (Table 3). The platelet count and PNR values were also correlated with the histological type of cancer (Table 3). A significantly reduced number of patients with high PLT and high PNR values was observed in the group of mucinous cancer patients, regardless of whether the patients were divided into 2 (mucinous and nonmucinous; Table 3) or 4 groups (mucinous, serous, undifferentiated, and others; Supplemental Table 2). Nonetheless, the Kruskal–Wallis test revealed that PNR was the only parameter significantly related to the histological type of cancer. Indeed, the PNR values for mucinous and nonmucinous carcinomas differed significantly: PNRmucinous versus PNRnon-muc, median, 41.5 (25.1–57.8) versus 57.5 (44.8–72.0) (P < .001) (25th and 75th percentiles in brackets).
In accordance with a paradigm of the pathogenesis and origin of EOC that divides EOC into 2 categories (type 1 and 2), the platelet parameters were also compared in both groups. Although the occurrence of high and low platelet parameter values (PLR and PNR) significantly correlated with the type of cancer (P = .02 and P = .01, respectively, Supplemental Table 3), the Mann–Whitney U test calculated for continuous values of the parameters did not confirm this relationship (P values .13–.43, data not shown). Hence, it could be concluded that the platelet parameters were not related to the type of cancer.
The parametric approach to data analysis confirmed a significant relationship between the PNR and histological type of EOC, regardless of the FIGO stage. The PNR was a significant parameter that discriminated mucinous carcinoma in the univariate (P = .002; Supplemental Table 4) and multivariate analyses (P = .014; Wilks lambda test, Supplemental Table 4). These results are consistent with the ROC analysis, as described above. Moreover, PLR and PLT were not related to mucinous cancer. The univariate analysis showed that PLR and PLT significantly discriminated FIGO stages I to II and III to IV (Supplemental Table 4), but the multivariate analysis results did not reach the level of statistical significance (data not shown). The regression approach confirmed the analyses results, showing that low PNR values are related to mucinous carcinoma (P = .004, data not shown). In summary, as the only among a variety of analyzed platelet parameters, the PNR parameter has been shown to be statistically significant in terms of the histological type of the cancer, regardless of the disease stage (ie, FIGO stage).
3.3. PNR values versus cancer antigen 125
The analysis of the cancer marker cancer antigen 125 (CA 125) was assessed through 2-factor ANOVA, including histological types of cancer and FIGO stages. In most primary ovarian mucinous cancers, CA 125 is not elevated,[20,21] which is their characteristic feature. In our study, the serum CA 125 levels were significantly lower in patients with mucinous carcinomas (median, 285 × 103 U/mL, range 10–899 103 U/mL) compared to patients with non-mucinous carcinomas (median, 788 × 103 U/mL, range, 58–19,250 × 103 U/mL; P < .001, ANOVA, log-normalized values). The PNR values were significantly lower in mucinous cancer patients, similar to CA 125; therefore, we compared these 2 parameters. However, the PNR values, similar to CA 125, were lower in mucinous patients versus nonmucinous patients (P = .034, ANOVA), but this finding was not depended on the FIGO stage. MANOVA confirmed statistical significance. Thus, the relationships between CA 125 and PNR were convergent/similar only in respect to the histological type of cancer (Fig. 1A and B, right graphs), but not in the case of FIGO I to II and III to IV stages (Fig. 1A and B, left graphs). In summary, PNR was exclusively related to mucinous carcinomas, regardless of the FIGO stage. The relationships between the CA 125 or PNR and the other platelet parameters are discussed in details in the Supplemental material (Supplemental Figs. 1–3 and Supplemental text).
Figure 1.
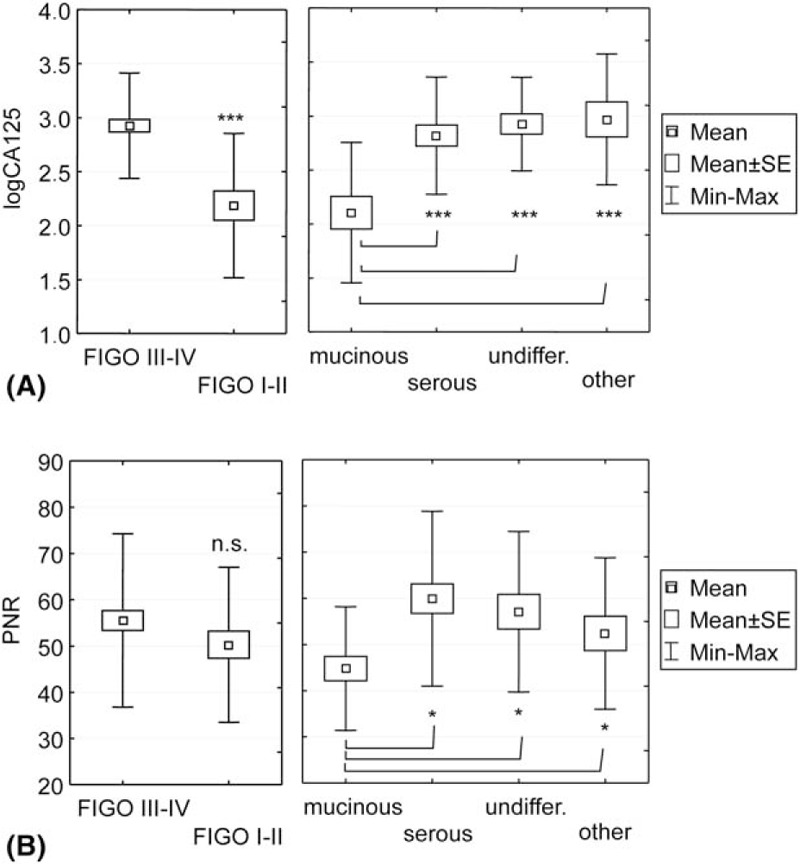
Comparison of the mean PNR (A) and CA 125 (B) values in different FIGO stages and certain histological types of EOC. SE, standard error. The CA 125 values were log-normalized. The CA 125 level in the blood samples was measured using the Elecsys CA 125 II assay (Roche Diagnostics, IN), in accordance with the manufacturer's instructions, and expressed in 103 U/mL. The comparisons were performed through 2-factor MANOVA followed by the Newman–Keuls test (∗P < .05, ∗∗∗P < .001). The main effect of the FIGO stage and histological type was significant (respectively, P = .0003 and P = .003, Wilks test). The FIGO stage and histological type were considered to be independent variables, there were no significant interaction between these variables (P = .6, Wilks test). CA 125 = cancer antigen 125, EOC = epithelial ovarian carcinomas, FIGO = International Federation of Gynaecology and Obstetrics, MANOVA = multivariate analysis of variance, PNR = platelet-to-neutrophil ratio.
3.4. Analysis of patient survival
It was well-documented that FIGO stages III to IV were related to poor survival in the EOC patients and were regarded as an independent prognostic factor. A 5-year overall survival rate for the FIGO III to IV patients (47.4%) was approximately half of the value for FIGO stage I to II patients (90.5%) in our study (Cox–Mantel test: P < .001; log-rank test: P < .001). The Kaplan–Meier analysis also showed a considerable difference between the survival rate of patients with elevated versus low PNR (cut-off 54, log-rank test: P = .011), especially in the early FIGO stage group (log-rank test: P < .001). Cox proportional-hazards regression revealed significantly increased hazard ratio (HR) for patients with elevated PNR values (HR 1.643, confidence interval [CI] 0.904–2.985). Moreover, HR was more considerable in FIGO I to II group of patients (HR 2.419, CI 0.964–6.069). Statistical inference of HR calculation would be more significant for a higher number of patients (according to sample size test). However, the results shows tendency that elevated PNR index might correlate with poor survival of patients. On the contrary, platelet number alone was not significant parameter related to patients survival.
4. Discussion
The high mortality rate and poor overall prognosis of patients are related to the late diagnosis of ovarian cancer. There is still a pressing need to analyze the inter-relationships between the diagnostic parameters before and after treatment to determine useful algorithms for clinical approaches and to understand cancer biology. In our work, we specifically attempted to evaluate the relationships between the platelet parameters in the blood of EOC patients, measured before treatment, in terms of histological heterogeneity of ovarian cancer and the disease severity. In our study, the overall analysis showed that the blood platelet parameters varied in EOC patients, not only compared with noncancer patients but also compared with different EOC groups. Regardless of some platelet count increases, the PNR was significantly lower in the EOC patients. The results of the detailed analysis of the platelet parameters in different groups of EOC patients are interesting. Certain analyzed blood platelet parameters were significantly associated with the histological type and/or FIGO stage of EOC.
4.1. PNR
The PNR is less known and less used as a parameter associated with the pathogenesis of cancer. In this work, we analyzed the PNR values in different EOC groups. Our results revealed that low PNR values are characteristic of mucinous carcinoma, regardless of whether early or advanced FIGO stages are considered. While high platelet counts and an increased PLR are clearly associated with advanced FIGO stages, decreased PNR values are observed for FIGO stages I to II and III to IV in mucinous carcinoma patients. The ROC analysis showed fine sensitivity and good specificity for PNR. Although this descriptive research was not intended to explain the cause of the unbalanced proportion of platelets and leukocytes in patients with different types of EOC, the evident occurrence of low PNR values in patients with mucinous cancer is interesting and worthy of further discussion. Numerous reports have indicated that the mucins produced by mucinous adenocarcinoma might be present in the sera of patients, where they interact with the respective receptors on platelets, endothelial cells, or leukocytes (ie, P, E, or L-selectin, respectively), which could mediate undesirable interactions between platelets and leukocytes.[22–24] Mucins of mucinous carcinomas of the ovary, heavily glycosylated proteins that carry specific ligands (sLea and sLex), are recognized by leukocyte and platelet receptors,[25] and help to form platelet–leukocyte aggregates. It can be assumed that by forming platelet–leukocyte aggregates, enhanced interactions between platelets and leukocytes may explain the apparently reduced number of circulating blood platelets in patients with mucinous carcinoma.
Mucinous carcinomas are relatively rare among other types of EOC, and they are included in EOC type 1. However, at advanced stages, these tumors become lethal, and the survival rate among mucinous patients is worse than that among serous patients.[15,16,26] Prognosis of this heterogeneous type of cancer is highly dependent on stage and morphology.[16,27] It has been proposed that mucinous should be considered as a separate entity, and also because of its specific treatment.[15] In our study, the survival rate of mucinous patients with advanced EOC was considerably lower than that of patients with early mucinous EOC, and even lower than the survival rate of patients with other histopathologic EOC subtypes (Cox–Mantel test or log-rank test: P < .05; data not shown). However, the reduced platelet-to-peripheral neutrophil ratio, similar to the lower level of CA 125, was characteristic for all mucinous EOC patients, regardless of patient survival. On the contrary, high PNR values (>54, a cut-off in our study) was related to poor survival of EOC patients. This tendency was clearly visible, especially in the group of FIGO I to II patients. Because mucinous carcinoma is relatively rare, multicenter retrospective studies for larger group of patients would be valuable to confirm this conclusion.
4.2. Other parameters
Other authors have shown that a high PLR value correlated with an advanced stage of ovarian cancer, which provided a better predictive value than the broadly described neutrophil-to-lymphocyte ratio (NLR) and might influence patient survival times.[12] Our research showed that the relationship between PLR values and FIGO stages was highly significant, although the diagnostic importance of PLR should be viewed with caution. However, despite high specificity (82.1%), the ROC analysis showed low sensitivity (52.3%) for the PLR parameter, meaning that even though most cancers will truly be classified as advanced, based on the PLR values, many slowly advancing cancers could be falsely included into this group. In their work investigating CA 125 parameters, Kim et al have reported that in terms of the discrimination of PLR values, the sensitivity and specificity of FIGO staging was not satisfactory (60.5% and 57%, respectively).[28] However, this result does not change the fact that enhanced PLR, similar to broadly described NLR, is a considerable indicator of systemic inflammation and significantly related to an advanced FIGO stage in ovarian cancer patients. Moreover, other researchers have shown that the high preoperative values of NLR and PLR are associated with poor patient survival rates.[29–33] On the contrary, EOC patients with lower PLR or NLR values have an extended period of remission and survival.[30,31] The relationship between these parameters and CA 125 in EOC patients is slight, and they are not satisfactorily specific in the ROC analysis (Supplementary material). We assumed that PLR and NLR, even combined with CA 125 levels, are not important as diagnostic parameters.
5. Conclusions
In conclusion, analysis of the available clinical data (in this case, blood morphology) has shown that EOC histological heterogeneity is accompanied by a specific pattern of an impaired ratio of platelets and leukocytes. The elevated values of platelet-to-peripheral neutrophil ratio might correlate with poor survival of EOC patients. On the contrary, the reduced PNR, similar to the decreased level of CA 125, is characteristic of mucinous EOC patients, which may be due to the biological properties of the diseased tissue. Further research is needed to investigate whether this basic feature is related to the clinical aspects of the haemostatic and blood coagulation disorders found in patients with this type of EOC.
6. Author contributions
Conceptualization: K. Bednarska.
Data curation: E. Głowacka, E. Król, H. Romanowicz, K. Bednarska, K. Szyllo, M. Nowak.
Formal analysis: K. Bednarska.
Investigation: K. Bednarska, M. Klink, Z. Sulowska.
Methodology: E. Głowacka, E. Król, H. Romanowicz, K. Bednarska, K. Szyllo, M. Klink, M. Nowak, Z. Sulowska.
Software: K. Bednarska.
Supervision: K. Bednarska, M. Nowak.
Validation: E. Głowacka, E. Król, H. Romanowicz.
Visualization: K. Bednarska.
Writing – original draft: K. Bednarska.
Writing – review & editing: M. Klink, M. Nowak, Z. Sulowska.
Supplementary Material
Footnotes
Abbreviations: AUC = area under ROC curve, CA 125 = cancer antigen 125, CI = confidence interval, EOC = epithelial ovarian carcinomas, FIGO = International Federation of Gynaecology and Obstetrics, HR = hazard ratio, MANOVA = multivariate analysis of variance, NLR = neutrophil-to-leukocyte ratio, PLR = platelet-to-lymphocyte ratio, PNR = platelet-to-neutrophil ratio, ROC curve/analysis = receiver-operating characteristic curve/analysis.
Funding: This work was supported by the Institute of Medical Biology of Polish Academy of Sciences and the Polish Mother's Memorial Hospital Research Institute.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Danckwardt S, Hentze MW, Kulozik AE. Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond. J Mol Med (Berl) 2013;91:1257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res 2016;118:1392–408. [DOI] [PubMed] [Google Scholar]
- [3].Young A, Chapman O, Connor C, et al. Thrombosis and cancer. Nat Rev Clin Oncol 2012;9:437–49. [DOI] [PubMed] [Google Scholar]
- [4].Satoh T, Oki A, Uno K, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer 2007;97:1053–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Graul A, Latif N, Zhang X, et al. Incidence of venous thromboembolism by type of gynecologic malignancy and surgical modality in the National Surgical Quality Improvement Program. Int J Gynecol Cancer 2017;27:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wysokinska EM, Hodge D, McBane RD. Ovarian vein thrombosis: incidence of recurrent venous thromboembolism and survival. Thromb Haemost 2006;96:126–231. [PubMed] [Google Scholar]
- [7].Absenger G, Szkandera J, Pichler M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer 2013;109:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang D, Yang JX, Cao DY, et al. Preoperative neutrophil-lymphocyte and platelet-lymphocyte ratios as independent predictors of cervical stromal involvement in surgically treated endometrioid adenocarcinoma. Onco Targets Ther 2013;6:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yildirim M, Demir Cendek B, Filiz Avsar A. Differentiation between benign and malignant ovarian masses in the preoperative period using neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. Mol Clin Oncol 2015;3:317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Williams KA, Labidi-Galy SI, Terry KL, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol 2014;132:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, et al. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol 2012;23:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kokcu A, Kurtoglu E, Celik H, et al. May the platelet to lymphocyte ratio be a prognostic factor for epithelial ovarian cancer? Asian Pac J Cancer Prev 2014;15:9781–4. [DOI] [PubMed] [Google Scholar]
- [13].Klink M, Jastrzembska K, Nowak M, et al. Ovarian cancer cells modulate human blood neutrophils response to activation in vitro. Scand J Immunol 2008;68:328–36. [DOI] [PubMed] [Google Scholar]
- [14].Bednarska K, Klink M, Wilczyński JR, et al. Heterogeneity of the Mac-1 expression on peripheral blood neutrophils in patients with different types of epithelial ovarian cancer. Immunobiology 2016;221:323–32. [DOI] [PubMed] [Google Scholar]
- [15].Hess V, A’Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol 2004;22:1040–4. [DOI] [PubMed] [Google Scholar]
- [16].Simons M, Massuger L, Bruls J, et al. Relatively poor survival of mucinous ovarian carcinoma in advanced stage: a systematic review and meta-analysis. Int J Gynecol Cancer 2017;27:651–8. [DOI] [PubMed] [Google Scholar]
- [17].Benedet JL, Bender H, Jones H, 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209–62. [PubMed] [Google Scholar]
- [18].Prat J. FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124:1–5. [DOI] [PubMed] [Google Scholar]
- [19].Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Duffy MJ, Bonfrer JM, Kulpa J, et al. CA 125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int J Gynecol Cancer 2005;15:679–91. [DOI] [PubMed] [Google Scholar]
- [21].Zorn KK, Tian C, McGuire WP, et al. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: a Gynecologic Oncology Group study. Cancer 2009;115:1028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Elsenberg EH, van Werkum JW, van de Wal RM, et al. The influence of clinical characteristics, laboratory and inflammatory markers on ’high on-treatment platelet reactivity’ as measured with different platelet function tests. Thromb Haemost 2009;102:719–27. [DOI] [PubMed] [Google Scholar]
- [23].Slavka G, Perkmann T, Haslacher H, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol 2011;31:1215–8. [DOI] [PubMed] [Google Scholar]
- [24].Wahrenbrock M, Borsig L, Le D, et al. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 2003;112:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim YJ, Borsig L, Han HL, et al. Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am J Pathol 1999;155:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hanski C, Hanski ML, Zimmer T, et al. Characterization of the major sialyl-Lex-positive mucins present in colon, colon carcinoma, and sera of patients with colorectal cancer. Cancer Res 1995;55:928–33. [PubMed] [Google Scholar]
- [27].Ricardo S, Marcos-Silva L, Valente C, et al. Mucins MUC16 and MUC1 are major carriers of SLe(a) and SLe(x) in borderline and malignant serous ovarian tumors. Virchows Arch 2016;468:715–22. [DOI] [PubMed] [Google Scholar]
- [28].Zaino RJ, Brady MF, Lele SM, et al. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer 2011;117:554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol 2005;24:4–25. [PubMed] [Google Scholar]
- [30].Kim HS, Choi HY, Lee M, et al. Systemic inflammatory response markers and CA 125 levels in ovarian clear cell carcinoma: A two center cohort study. Cancer Res Treat 2016;48:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13:499–503. [DOI] [PubMed] [Google Scholar]
- [32].Miao Y, Yan Q, Li S, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark 2016;17:33–40. [DOI] [PubMed] [Google Scholar]
- [33].Cummings M, Merone L, Keeble C, et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer 2015;113:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


