Abstract
While both sepsis-induced myocardial dysfunction (SIMD) and stress-induced cardiomyopathy (SICMP) are common in patients with sepsis, the pathogenesis of the 2 diseases is different, and they require different treatment strategies. Thus, we aimed to investigate risk factors and outcomes between the 2 diseases.
This retrospective study enrolled patients diagnosed with sepsis or septic shock, admitted to intensive care unit via emergency department in Korea University Anam Hospital, and who underwent transthoracic echocardiography within the first 24 hours of admission.
In all, 25 patients with SIMD and 27 patients with SICMP were enrolled. Chronic obstructive pulmonary disease and a history of heart failure (HF) were more prevalent in both the SIMD and SICMP groups than in the control group. In the SIMD and SICMP groups, levels of inflammatory cytokines were similar. Serum troponin level was significantly elevated in the SICMP and SIMD group compared to the control group. N-terminal pro-brain natriuretic peptide (NT pro-BNP) level was significantly elevated in the SIMD group compared to the SICMP group or control group. The in-hospital mortality rate in the SIMD and SICMP group was about 40%, showing increased trends compared with the control group. The in-hospital mortality rate was significantly increased in SIMD group with EF<30% than in SICMP group with EF<30%. In multiple logistic regression analysis, a past history of diabetes mellitus (DM) and HF was significantly associated with the incidence of SIMD. Younger age, elevated levels of NT pro-BNP, and positive result of blood culture also showed significant odds ratio regard to the occurrence of SIMD. However, only elevated lactate and troponin level were positively associated with the incidence of SICMP.
The SIMD and SICMP had different risk factors. The risk factors of SIMD were younger age, history of DM, history of HF, elevated NT pro-BNP, and positive result of blood culture. The elevated levels of lactate and troponin were identified as risk factors of SICMP. More importantly, in-hospital mortality rate from SIMD and SICMP showed increased trend and worse outcome in SIMD group with reduced EF<30%. Thus, developing SIMD or SICMP reflected poor prognosis in sepsis or septic shock.
Keywords: intensive care unit, mortality, sepsis, sepsis-induced myocardial dysfunction, stress-induced cardiomyopathy
1. Introduction
Sepsis-induced myocardial dysfunction (SIMD) is a reversible myocardial depression caused by sepsis and characterized by left ventricular dilation, depressed ejection fraction (EF), and a recovery period of seven to 10 days.[1,2] Although several studies have reported that endotoxins, inflammatory cytokines, and nitric oxide are related to the pathogenesis of SIMD, the condition is still not completely understood.[3,4] The incidence of SIMD has been reported at 18% to 65%, and the mortality rate is 40% to 70%.[1] As response to fluid resuscitation and inotropic agents decreases, SIMD is considered to be a major risk associated with sepsis but long-term outcome is favorable.[5]
Stress-induced cardiomyopathy (SICMP), also known as Takotsubo cardiomyopathy, is a dysfunction of the left ventricular apex that presents as hypokinesia, akinesia, or dyskinesia of the midsegments, with or without apical involvement beyond a single coronary vascular distribution.[6] The etiology of SICMP is elevated catecholamine release, and most cases are preceded by instances of emotional or physical stress or acute medical conditions[7] SICMP is prevalent in patients with sepsis, and a previous analysis reported that sepsis is the most frequent cause of SICMP.[8] Although the in-hospital mortality rate due to SICMP is favorable in patients without sepsis, the mortality in patients with sepsis increases by more than 20% depending on the underlying patient condition.[9,10]
The natural courses of SIMD and SICMP are somewhat similar in that most patients exhibit complete recovery over several days to weeks.[11] However, while both SIMD and SICMP are common in patients with sepsis, the pathogenesis of these 2 diseases is different, and each may need a different treatment strategy. First of all, myocardial injury detected by cardiac troponin enzyme in both diseases or hypotension can mimic acute coronary syndrome and be unnecessarily connected to invasive strategy.[12] In addition, some investigators considered that SIMD is a partially protective process and inotropes are potentially harmful.[13] Consequently, recognition of SIMD or SICMP in early stage is important to manage properly. To date, their clinical features remain unclear, and there are currently no known clinical characteristics that can be used to distinguish between the 2 diseases. Therefore, we retrospectively compared the risk factors and outcomes of SIMD and SICMP in patients with sepsis or septic shock.
2. Methods
2.1. Study patients and design
Patients aged 20 to 100 years were eligible for this study if they were: diagnosed with sepsis or septic shock and admitted to the intensive care unit (ICU) via the emergency department (ED). A total of 730 patients admitted to the ICU via the ED at Korea University Anam Hospital between January 2012 and February 2015 were screened for inclusion (Fig. 1). The following patients were excluded: patients whose diagnostic criteria did not fulfill sepsis or septic shock; those whose final diagnosis was lung disease; those with myocardial infarction; those with valvular heart diseases; those with structural heart diseases; or those with a lack of transthoracic echocardiography (TTE) data or TTE that was obtained only after the first 24 hours of admission. This study was a retrospective study approved by the Korea University Hospital Institute Review Board.
Figure 1.
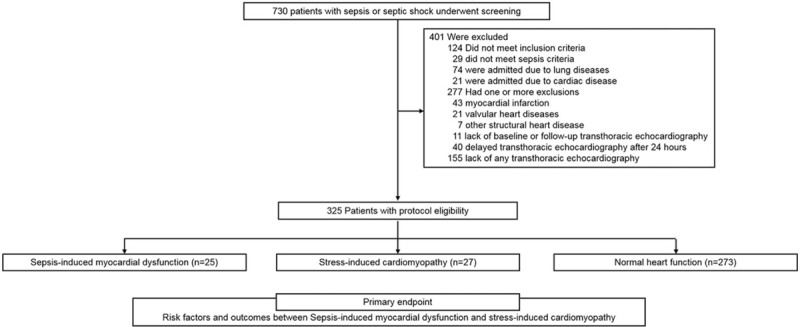
Study protocol. Patients diagnosed with sepsis or septic shock and admitted to the ICU via the ED were eligible. A total of 730 patients admitted to the ICU via the ED were screened for inclusion at Korea University Anam Hospital between January 2012 and February 2015. ED = emergency department, ICU = intensive care unit.
2.2. Endpoints
The primary endpoint of this study was to compare risk factors between SIMD and SICMP. The secondary endpoints were length of in-hospital stay, in-hospital mortality rate, and readmission rate for SIMD or SICMP. Factors contributing to the incidence of SIMD or SICMP were also analyzed.
2.3. Variable definitions
Sepsis was defined according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).[14] In the emergency department, the quick sepsis-related organ failure assessment (qSOFA) was used to identify patients with sepsis, who were defined as those with an infection who had at least 2 of the following criteria: a respiratory rate of 22 breaths/min or greater, altered mentality, or systolic blood pressure of 100 mm Hg or less. Septic shock was defined as the need for a vasopressor in order to maintain a mean arterial blood pressure of 65 mm Hg or greater and a serum lactate level higher than 2 mmol/L in the absence of hypovolemia.
SIMD was defined as an EF <50% and a ≥10% decrease in the patient's baseline EF; this conditions usually resolves within 2 weeks in patients with sepsis or septic shock.[15,16] If the baseline EF was unknown, we defined SIMD as an EF <50% and a ≥10% decrease in the patient's initial EF assessed on admission. The definition of recovery was an improvement in EF to the baseline level within 2 weeks. SICMP was defined as the presence of hypokinesia of the mid-to-apical segments, apical ballooning, and hyperkinesia of the basal walls. SICMP was diagnosed according to the criteria suggested by Mayo Clinic or Kawai et al.[17,18] The control group included patients who did not show SIMD, SICMP, or any structural/valvular heart diseases. Inotropic agents, such as norepinephrine and epinephrine, were administered if the patient's vital signs were unstable before TTE, in accordance with the Sepsis-3 consensus.
All clinical data were collected from the electronic medical records written by the residents or the attending physicians in each patient's department. TTE was performed by 4 well-trained technicians who had each performed at least 1000 such procedures in the past. EF was measured using the modified Simpson method. Two independent echocardiologists blinded to clinical data reviewed TTE images and diagnosed the SIMD or SICMP. Atypical cases who both SIMD and SICMP were possible were excluded from the study and the patients who were clearly differentiated from SIMD or SICMP were enrolled.
2.4. Propensity score matching
To balance the distribution of baseline characteristics, we used propensity score matching. We estimated a propensity score for each study patient using the multivariable logistic regression model. In the model, potential confounders and variables associated with SIMD and SICMP, such as age, sex, hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), coronary artery disease, history of heart failure (HF), and use of inotropes were included. We then created an exchangeable comparison group of the patients with SIMD by matching each patients with SICMP. Our propensity score model discriminated well between the SIMD and SICMP groups. The model was fit to the data during all steps of the regression analyses (relative multivariate imbalance L1 after matching = 0.48). We then used the propensity score to match each patients with SIMD to another SICMP patients. We matched 25 of SIMD patients to another 25 of SICMP patients who had a similar propensity score. Our assessment of the covariate balance after matching focused on these standardized differences. After matching, the mean propensity score for the patients with SIMD was 0.48 and SICMP was 0.48.
2.5. Statistical analysis
Data were expressed as mean ± standard deviation for continuous variables and as the number and percentage of patients for categorical variables. Fisher's exact test or chi-square test was used for categorical variables, and Wilcoxon signed-rank test was used for continuous variables. We calculated the odds ratios (ORs) and 95% confidence intervals (95% CIs) for SIMD or SICMP compared to control group according to various risk factors using multiple logistic regression analysis. All possible confounding variables such as age, sex, source of infection, underling comorbidities, heart rate, level of white blood count, erythrocyte sedimentation rate, C-reactive protein, procalcitonin, lactate, troponin, and NT pro-BNP, atrial fibrillation on admission, cardiomegaly, use of inotropic agents, and positive blood culture were included for association with SIMD or SICMP. SPSS ver. 23.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses. A P value < .05 was considered to be statistically significant.
3. Results
3.1. Patient characteristics
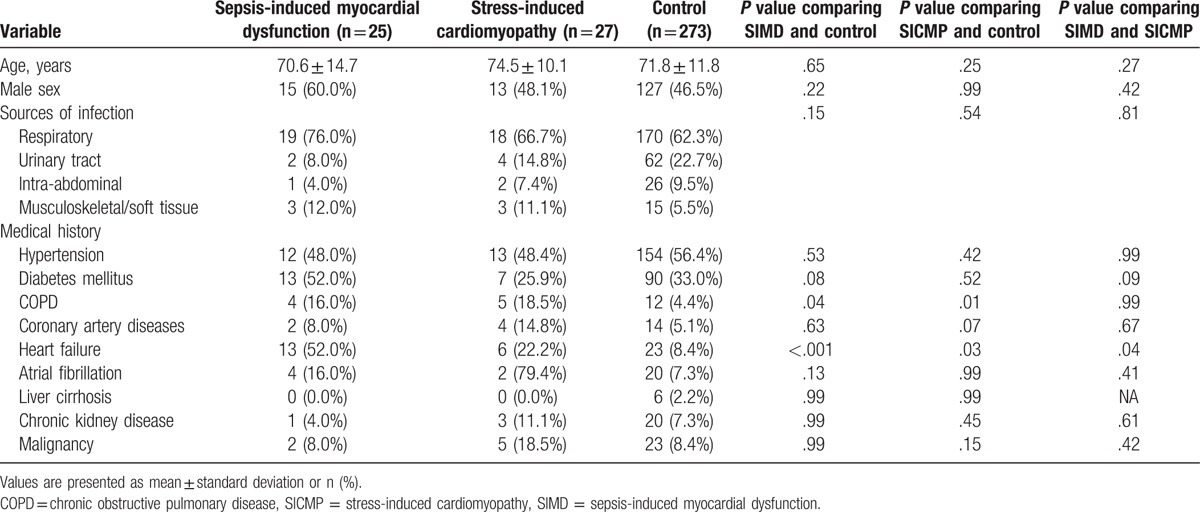
Among the 451 patients who underwent TTE and who were diagnosed with sepsis in this study, 25 were diagnosed with SIMD and 27 were diagnosed with SICMP. Therefore, the incidence of SIMD and SICMP was 5.5% and 6.0%, respectively, in the eligible patients. The baseline patient characteristics of mean age, sex, source of infection, and medical histories were similar between the SIMD and SICMP groups (Table 1). COPD and history of HF were more prevalent in both the SIMD and SICMP groups than in the control group. Furthermore, history of HF in SIMD group was significantly prevalent than in SICMP group.
Table 1.
Baseline patient characteristics.
3.2. Comparisons of clinical parameters
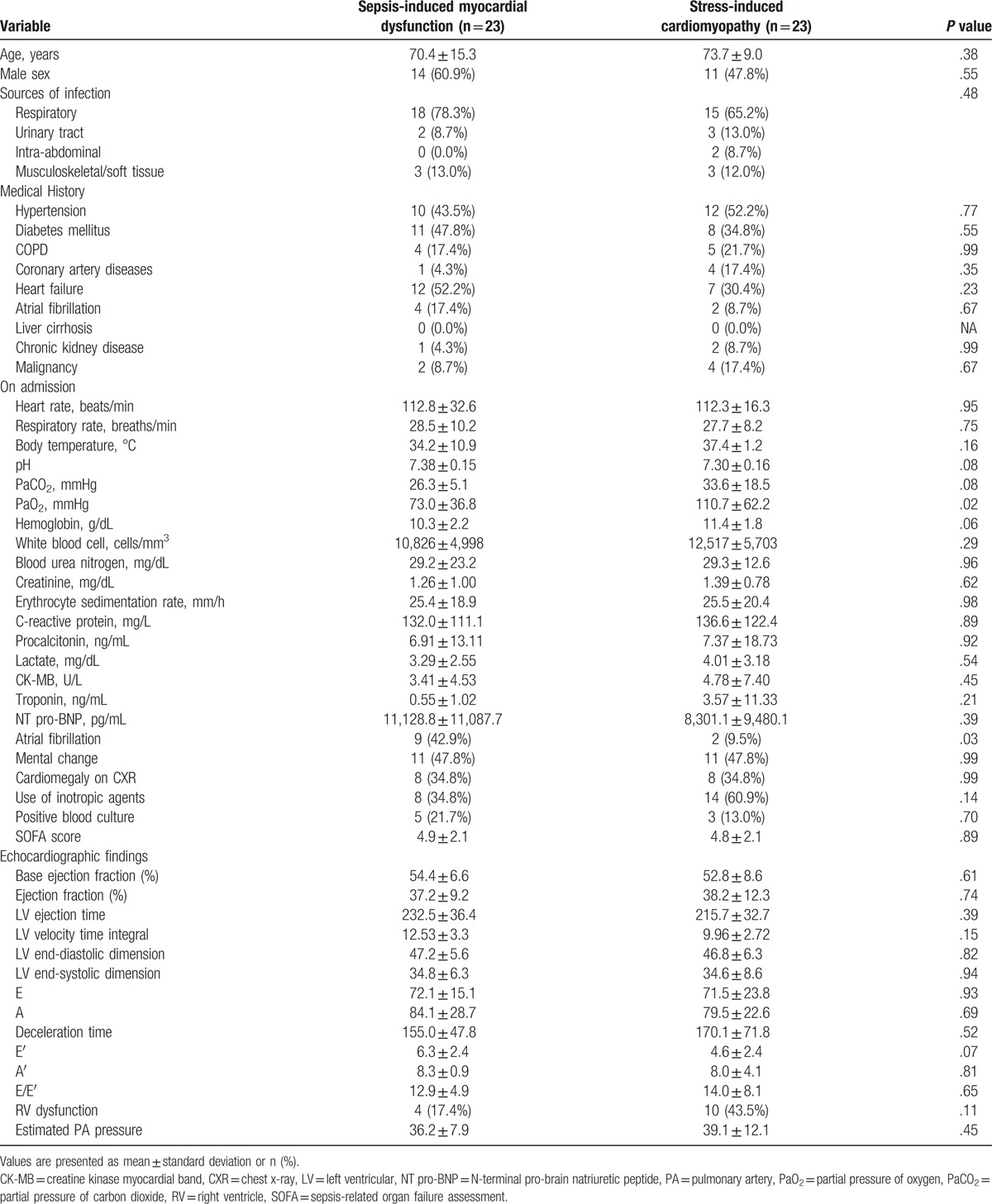
In the SIMD and SICMP groups, levels of inflammatory cytokines such as erythrocyte sedimentation rate, C-reactive protein, procalcitonin, and lactate on admission were similar. (Table 2). The pH and PaO2 were significantly lower in SICMP group than in SIMD. Serum troponin level was significantly elevated in the SICMP and SIMD group compared to the control group. There was no significant difference in troponin level between the SICMP and SIMD groups. N-terminal pro-brain natriuretic peptide (NT pro-BNP) level was significantly elevated in the SIMD group compared to the SICMP group or control group. Additionally, the number of patients who needed inotropic agents was higher in the SICMP group than in the SIMD group. The incidence of atrial fibrillation on admission was higher in the SIMD group than in the SICMP group or control. After propensity score matching, baseline characteristics between the 2 groups were similar (Table 3). There were no significantly different parameters on admission except PaO2 level and incidence of atrial fibrillation on admission in matched population.
Table 2.
Comparison of parameters among the sepsis-induced myocardial dysfunction, stress-induced cardiomyopathy, and control groups.
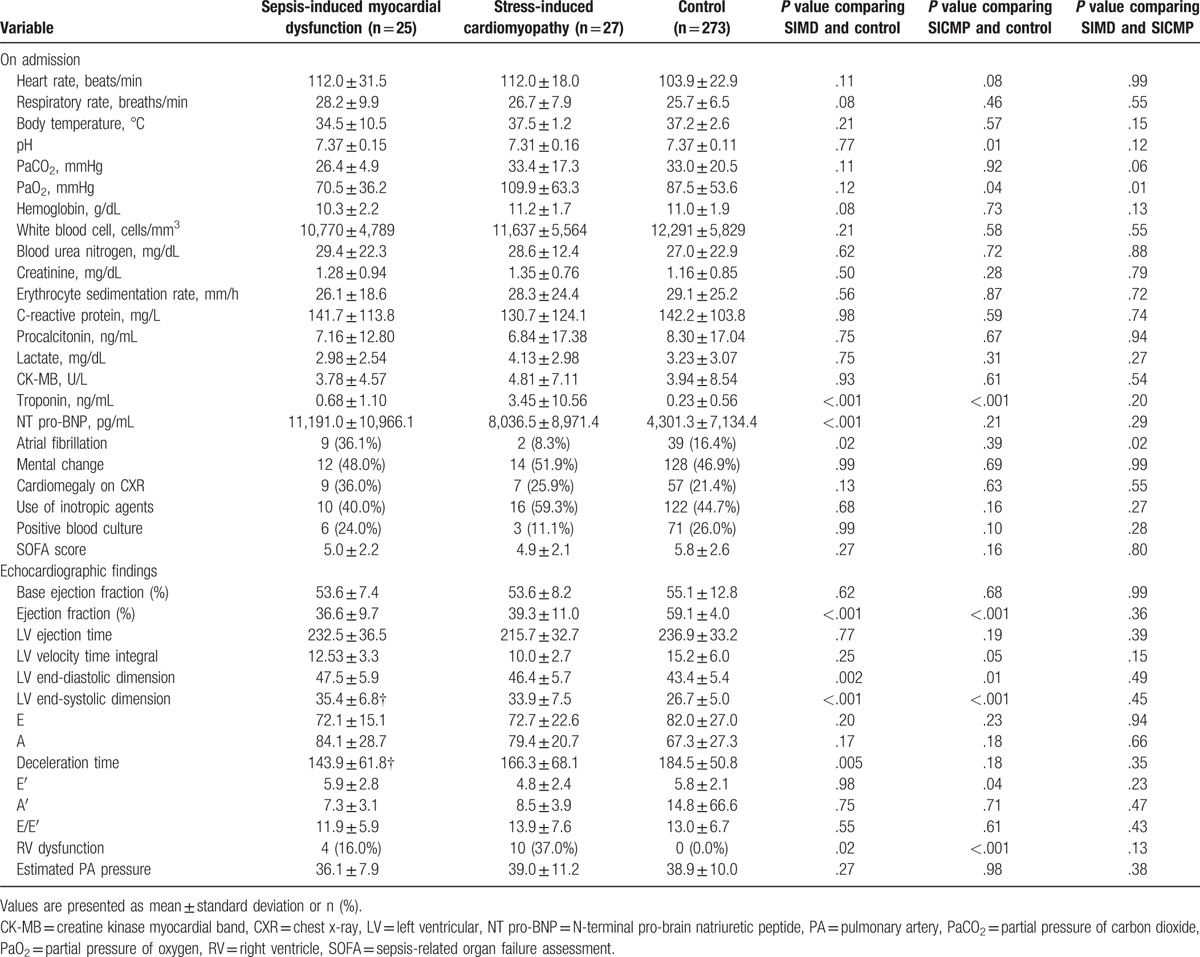
Table 3.
Comparison of characteristics among the sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in propensity score-matched group.
3.3. Comparisons of clinical outcomes
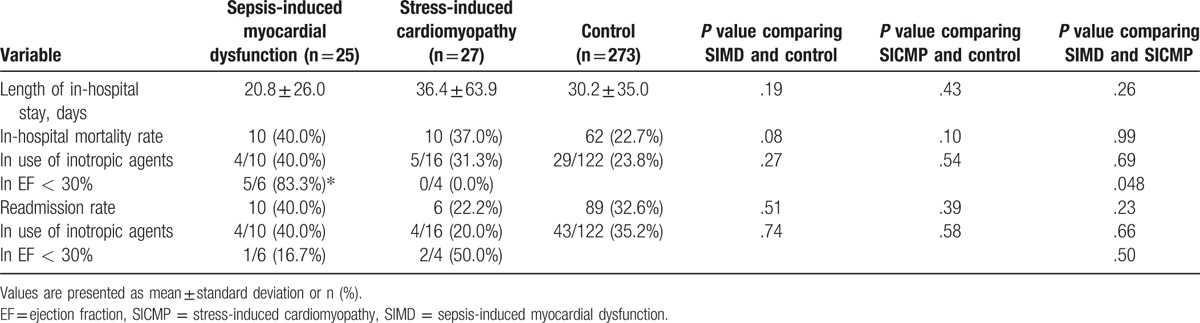
The in-hospital mortality rate in the SIMD group was 40%, which was an increased trend compared with the rate in the control group (P = .08) (Table 4). Also, the in-hospital mortality rate in the SICMP group was 37.0%, which was also an increased trend compared with the rate in the control group (P = .10). The in-hospital mortality rate was significantly increased in SIMD group with EF < 30% than in SICMP group with EF < 30%.
Table 4.
Comparison of outcomes among the sepsis-induced myocardial dysfunction, stress-induced cardiomyopathy, and control groups.
3.4. Predictors for SIMD or SICMP
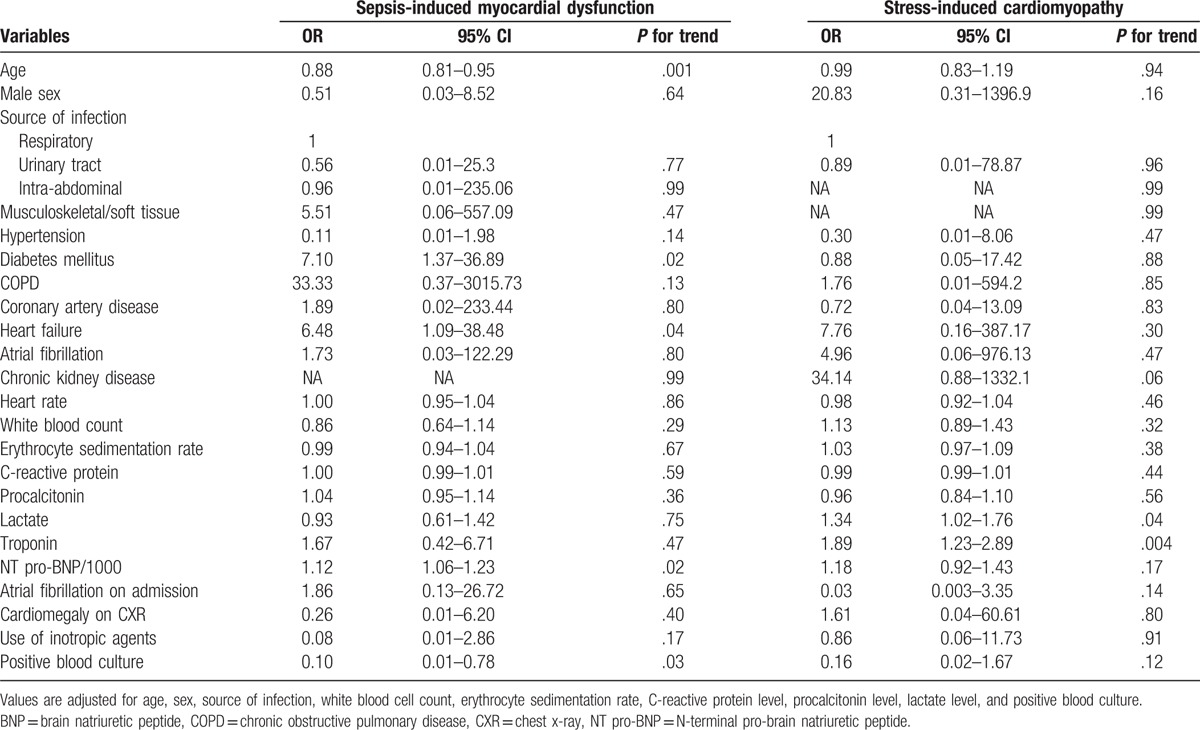
In multiple logistic regression analysis, a past history of DM and HF was significantly associated with the incidence of SIMD (Table 5). Younger age, elevated levels of NT pro-BNP, and positive result of blood culture also showed significant ORs regard to the occurrence of SIMD. However, only elevated lactate and troponin level were positively associated with the incidence of SICMP.
Table 5.
Predictors for sepsis-induced myocardial dysfunction or stress-induced cardiomyopathy compared to control group.
4. Discussion
This retrospective study investigated the risk factor and outcomes of SIMD and SICMP in patients with sepsis or septic shock. The risk factors of SIMD were younger age, a history of DM and HF, elevated NT pro-BNP, and positive result of blood culture. The elevated levels of lactate and troponin were identified as risk factors of SICMP. Propensity score-matching analysis showed that prediction for developing SIMD or SICMP in patients with similar comorbidities was difficult. Inflammatory cytokines and SOFA score were similar among SIMD, SICMP, and control groups. More importantly, in-hospital mortality rate from SIMD and SICMP showed increased trends and worse outcome in SIMD group with reduced EF < 30%. From these findings, SIMD and SICMP had different risk factors and developing SIMD or SICMP reflected poor prognosis.
Several studies have investigated the clinical parameters of SIMD or SICMP. Known risk factors for SICMP include female gender, elevated troponin levels, and prior use of catecholaminergic drugs.[19] Elevated troponin levels are especially associated with SICMP, with a sensitivity of nearly 100% in diagnosing SICMP.[20] In addition, B-type natriuretic peptide levels have predictive value in identifying regional wall motion abnormalities in iatrogenic catecholamine-induced cardiomyopathy.[21] In cases of SIMD, elevated levels of NT pro-BNP and troponin are known risk factors in patients with septic shock, but other parameters are not well recognized.[22,23] The increase in the troponin levels is not clearly understood in SIMD but is probably due to a loss of cardiomyocyte membrane integrity and a subsequent troponin leakage.[24] In this study, younger age, a history of DM, or HF, elevated NT pro-BNP, and positive result of blood culture were considered candidate risk factors for SIMD. Obviously, a history of HF contributes to future cardiac events, particularly to myocardial dysfunction, in the context of both diseases.[16,25] A history of DM can be a preceding risk of SIMD in that these diseases are well-known risk factors for cardiomyopathies.[25] In contrast, only elevated of lactate and troponin levels were significantly associated with the occurrence of SICMP. In a previous study, younger age, a history of HF and coronary artery disease were suggested as risk factors for SIMD.[16] The findings of younger age and a history of HF were consistent with our study. Of note, our findings had a strength in that we included all possible cofoundings and analyzed risk factors for SIMD or SICMP. With knowledge of these risk factors, the prediction of SIMD and SIMCP could be made in order to avoid unnecessary, invasive diagnostic methods, such as coronary angiography. However, although there were significant differences in underlying co-morbidities for SIMD and SICMP, propensity score-matching analysis showed that prediction for developing SIMD or SICMP in patients with similar comorbidities was difficult.
In our study, inflammatory cytokines such as erythrocyte sedimentation rate, C-reactive protein, procalcitonin, lactate, and even SOFA score were similar among 3 groups. Previous study showed that C-reactive protein and Acute Physiology and Chronic Health Evaluation II score (APACHE II) were higher in the SIMD group than in the SIMD group, suggesting that SIMD could low the mortality rate and induce a paradoxical result.[16] An increased incidence of SIMD in younger and normotensive patients might be related to the protective adaptation seen in SIMD. Myocardial dysfunction in sepsis can develop due to compensatory measures of the heart to reduce the increased energy consumption caused by dysfunctional mitochondria.[26] These results and theories were opposite findings from our study. Of note, in-hospital mortality rates in the SIMD and SICMP groups showed increased trends compared with the control group, in accordance with the results of previous studies.[1,10,27–29] A meta-analysis demonstrated that lower EF of SIMD did not have a protective role.[30] The mortality rate in patients with SIMD has reported to be 40% to 70%, and that in patients with SICMP was more than 20%. Even though SIMD develops to protect heart function via reducing the response to catecholamine in sepsis, the presence of SIMD itself reflects the severity of the disease and is one of the contributing factors to increased mortality. In addition, patients with decreased EF < 30% in SIMD showed significantly increased in-hospital morality. Therefore, meaning of developing SIMD should be further confirmed. Similarly, SICMP in patients with sepsis is not favorable and should be attended to. Because the etiology of SIMD is not fully understood, definite treatment strategy was not validated. Inotropic agents, such as dobutamine, have been highlighted for their ability to alleviate SIMD. Nonetheless, dobutamine should be administered cautiously in patients with sepsis. Because the bacterial growth and virulence are associated with catecholamines from inotropes, dobutamine can adversely affect patient outcomes.[2,31] As there is no proven treatment for SIMD, further study is needed to investigate the efficacy of levosimendan or mechanical support with extracorporeal membrane oxygenation.[32] A meta-analysis reported that levosimendan reduced mortality compared to standard inotropic therapy possibly because it does not stimulate β-adrenergic receptor.[33] In SICMP, supportive care is a crucial strategy during the acute phase.[25] Hypotension developed with a mid-ventricular obstruction caused by the systolic anterior motion of the anterior mitral leaflet and the juxtaposition of the septum to the mitral chordal apparatus might need to be treated with beta-blockers and phenylephrine.[34] There is also a concern that excessive increases in catecholamine level due to inotropic agents during sepsis can create adverse effects, but patients with severe left ventricular dysfunction might need inotropic agents. In fact, the in-hospital mortality rate in SIMD group treated with inotropes was similar with in SIMD group treated without inotropes or SICMP group in this study. Although need for inotropes partly reflected the severity of cardiac dysfunction, it did not increase the in-hospital mortality. Because patients of this study were treated according to early goal directed therapy, all the patients treated with inotropes received norepinephrine at first. Accordingly, the influence of different inotropes to clinical outcomes could not be assessed. To confirm our findings, a larger prospective cohort study is warranted.
4.1. Study limitations
The present study has several limitations. First, its retrospective design precluded conclusions about causal relationships. Second, the size of the study population was small. Small population made the wide confidence intervals and big odds ratio to be cautiously interpreted. Thus, further larger prospective studies and intervention trials should be undertaken to establish a causal association between risk factors and sepsis/stress-induced cardiomyopathy.
5. Conclusions
The SIMD and SICMP had different risk factors. The risk factors of SIMD were younger age, history of DM, history of HF, elevated NT pro-BNP, and positive result of blood culture. The elevated levels of lactate and troponin were identified as risk factors of SICMP. Inflammatory cytokines and SOFA score were similar among SIMD, SICMP, and control groups. More importantly, in-hospital mortality rate from SIMD and SICMP showed increased trend and worse outcome in SIMD group with reduced EF < 30%. Thus, developing SIMD or SICMP reflected poor prognosis in sepsis or septic shock.
Author contributions
Conceptualization: H.S. Jeong, S.J. Hong.
Data curation: H.S. Jeong, J-H. Kim, T.H. Lee.
Formal analysis: C.H. Bang, H.S. Jeong, J-H. Kim, T.H. Lee.
Funding acquisition: C.H. Bang, J-H. Kim.
Investigation: C.H. Bang, H.S. Jeong, J-H. Kim, T.H. Lee.
Methodology: C.H. Bang, H.S. Jeong, J-H. Kim, T.H. Lee.
Project administration: C.H. Bang, H.S. Jeong, J-H. Kim.
Resources: C.H. Bang, H.S. Jeong, J-H. Kim.
Supervision: S.J. Hong.
Validation: H.S. Jeong.
Writing – original draft: H.S. Jeong.
Writing – review & editing: S.J. Hong.
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, EF = ejection fraction, HF = heart failure, NT pro-BNP = N-terminal pro-brain natriuretic peptide, PaO2 = partial pressure of oxygen, PaCO2 = partial pressure of carbon dioxide, SICMP = stress-induced cardiomyopathy, SIMD = sepsis-induced myocardial dysfunction, SOFA = sepsis-related organ failure assessment, TTE = transthoracic echocardiography.
Ethics approval and consent to participate: This research was approved by the Korea University Anam Institute Review Board, and consent for research participation was deemed to be waived.
Competing interests: The authors declare that they have no competing interest.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Vieillard-Baron A, Caille V, Charron C, et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008;36:1701–6. [DOI] [PubMed] [Google Scholar]
- [2].Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, et al. Sepsis-induced cardiomyopathy. Curr Cardiol Rev 2011;7:163–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kumar A, Thota V, Dee L, et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996;183:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zaky A, Deem S, Bendjelid K, et al. Characterization of cardiac dysfunction in sepsis: an ongoing challenge. Shock 2014;41:12–24. [DOI] [PubMed] [Google Scholar]
- [5].Vallabhajosyula S, Jentzer JC, Geske JB, et al. New-onset heart failure and mortality in hospital survivors of sepsis-related left ventricular dysfunction. Shock 2017;49:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kurowski V, Kaiser A, von Hof K, et al. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest 2007;132:809–16. [DOI] [PubMed] [Google Scholar]
- [7].Guglin M, Novotorova I. Neurogenic stunned myocardium and takotsubo cardiomyopathy are the same syndrome: a pooled analysis. Congest Heart Fail 2011;17:127–32. [DOI] [PubMed] [Google Scholar]
- [8].Cappelletti S, Ciallella C, Aromatario M, et al. Takotsubo cardiomyopathy and sepsis. Angiology 2017;68:288–303. [DOI] [PubMed] [Google Scholar]
- [9].Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–9. [DOI] [PubMed] [Google Scholar]
- [10].Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J 2012;164:215–21. [DOI] [PubMed] [Google Scholar]
- [11].Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005;111:472–9. [DOI] [PubMed] [Google Scholar]
- [12].Henning DJ, Kearney KE, Hall MK, et al. Identification of hypotensive emergency department patients with cardiogenic etiologies. Shock 2017;49:131–6. [DOI] [PubMed] [Google Scholar]
- [13].Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994;330:1717–22. [DOI] [PubMed] [Google Scholar]
- [14].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care 2015;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sato R, Kuriyama A, Takada T, et al. Prevalence and risk factors of sepsis-induced cardiomyopathy: a retrospective cohort study. Medicine (Baltimore) 2016;95:e5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–17. [DOI] [PubMed] [Google Scholar]
- [18].Kawai S, Kitabatake A, Tomoike H, et al. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J 2007;71:990–2. [DOI] [PubMed] [Google Scholar]
- [19].Kothavale A, Banki NM, Kopelnik A, et al. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care 2006;4:199–205. [DOI] [PubMed] [Google Scholar]
- [20].Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart 2003;89:1027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee JW, Kim JY, Youn YJ, et al. Clinical characteristics and prognostic factors of stress-induced cardiomyopathy. Korean Circ J 2010;40:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med 2003;29:1696–702. [DOI] [PubMed] [Google Scholar]
- [23].Mehta NJ, Khan IA, Gupta V, et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004;95:13–7. [DOI] [PubMed] [Google Scholar]
- [24].Jozwiak M, Persichini R, Monnet X, et al. Management of myocardial dysfunction in severe sepsis. Semin Respir Crit Care Med 2011;32:206–14. [DOI] [PubMed] [Google Scholar]
- [25].Boland TA, Lee VH, Bleck TP. Stress-induced cardiomyopathy. Crit Care Med 2015;43:686–93. [DOI] [PubMed] [Google Scholar]
- [26].Kakihana Y, Ito T, Nakahara M, et al. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 2016;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Etchecopar-Chevreuil C, Francois B, Clavel M, et al. Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med 2008;34:250–6. [DOI] [PubMed] [Google Scholar]
- [28].Blanco J, Muriel-Bombin A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 2008;12:R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med 2004;32:660–5. [DOI] [PubMed] [Google Scholar]
- [30].Huang SJ, Nalos M, McLean AS. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit Care 2013;17:R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lyte M, Freestone PP, Neal CP, et al. Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet 2003;361:130–5. [DOI] [PubMed] [Google Scholar]
- [32].Hajjej Z, Meddeb B, Sellami W, et al. Effects of levosimendan on cellular metabolic alterations in patients with septic shock: a randomized controlled pilot study. Shock 2017;48:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zangrillo A, Putzu A, Monaco F, et al. Levosimendan reduces mortality in patients with severe sepsis and septic shock: a meta-analysis of randomized trials. J Crit Care 2015;30:908–13. [DOI] [PubMed] [Google Scholar]
- [34].Previtali M, Repetto A, Scuteri L. Dobutamine induced severe midventricular obstruction and mitral regurgitation in left ventricular apical ballooning syndrome. Heart 2005;91:353. [DOI] [PMC free article] [PubMed] [Google Scholar]


