Abstract
Rationale:
Comamonas species are rarely associated with human infections. Recent reports found that Comamonas kerstersii was associated with severe diseases such as abdominal infection and bacteremia. However, C. kerstersii maybe be confused with Comamonas testosteroni using the automatic bacterial identification systems currently available.
Patient concerns:
A 31-year-old man who had onset of left upper abdominal pain developed clinical manifestations of right lower abdominal pain and classic migration of pain at the temperature of 39°C. The positive strain of aerobic and anaerobic bottles of blood cultures was identified.
Diagnoses:
The patient was diagnosed as acute peritonitis and perforated appendix with abdominal abscess.
Interventions:
The bacterium was identified by routine methods, MALDI-TOF-MS and PCR amplification of the 16S rRNA. The patient was treated with exploratory laparotomy, appendectomy, tube drainage, and prescribing antibiotic treatment.
Outcomes:
The patients were discharged with complete recovery. The organisms were confirmed as C. kerstersii by MALDI-TOF-MS and a combination of the other results.
Lessons:
Our findings suggest that C. kerstersii infection occurs most often in association with perforated appendix and bacteremia. We presume that C. kerstersii is an opportunistic pathogen or commensal with the digestive tract and appendix bacteria.
Keywords: bacteremia, Comamonas kerstersii, perforated appendicitis
1. Introduction
The genus Comamonas was originally created in 1985, and it included a single species, Comamonas terrigena (C. terrigena).[1] In 1987, Comamonas testosteroni and Comamonas acidovorans were reclassified as members of the Comamonas genus. C. acidovorans was subsequently reclassified as Delftia acidovorans on the basis of its 16S rRNA gene sequence in 1999.[2]Comamonas kerstersii (C. kerstersii) was described as 1 of 3 genotypically separate groups of C. terrigena in 2003.[3] Now, Comamonas genus contains 17 species including C. terrigena,C. aquatica, C. kerstersii, C. testosteroni, C. denitrificans, C. nitrativorans, C. koreensis and others. Comamonas species have a wide geographic distribution and are commonly found in soil, plants, animal, water saprophytes, and in humidifier reservoir water.[4]
Comamonas species are rarely associated with human infections.[5] However, in recent years, several publications have incriminated C. testosteroni and C. kerstersii in human diseases, including severe invasive infections, such as abdominal infection and bacteremia.[6–14] However, C. kerstersii maybe be confused with C. testosteroni because of the difficulties in accurately identifying it using the automatic bacterial identification systems currently available. Some important biochemical tests, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF-MS) and gene sequencing by polymerase chain reaction (PCR) amplification of the 16S rRNA can confirm the specific Comamonas species. To the best of our knowledge, this is the first report of C. kerstersii bacteremia in a patient with acute perforated appendicitis.
2. Case presentation
A 31-year-old man presented to the emergency department of our hospital with onset of left upper abdominal pain followed by nausea and vomiting at a temperature of 37.5°C. His white blood cell was 11.6 × 109/L, and differential white blood count were: neutrophils 59.4%, lymphocytes 29.2%, monocytes 7.2%, eosinophils 4.1%. Serum C-reactive protein level was 23.5 mg/L. Elevated alanine transaminase level (101 U/L) was found. Other laboratory data were within the normal range. His chest and abdominal X-ray and ultrasonography were normal. The patient was treated with cefuroxime axetil according to empirical antibiotic routines. The next day, he had a temperature of 39°C with right lower abdominal pain and classic migration of pain. Aerobic and anaerobic bottles of blood cultures were drawn and incubated at 35°C; However, he refused to accept surgical intervention due to personal reason. A follow-up visit revealed that he was diagnosed with acute peritonitis and perforated appendix with abdominal abscess. He was discharged with complete recovery after exploratory laparotomy, appendectomy, tube drainage, prescribing antibiotic treatment for 14 days (Cefuroxime and metronidazole) and an observation period of 6 days.
As soon as the aerobic blood culture bottle became positive 18 hours after sampling and the anaerobic blood culture bottle became positive 20 hours after sample collection, they were transferred into Colombia blood agar, macconkey, nutrition agar and chocolate agar. After 24 hours of incubation at 35°C in ambient air, growth of a nonfermenting Gram-negative bacillus was observed. Other tests showed that oxidase and catalase activities were positive. 4-hour rapid urea hydrolysis was negative. Strains also grew on the 4 types of agar at 30°C and 42°C. The strain was initially identified as Bordetella bronchiseptica by SIEMENS MicroScan walkaway 96 plus system (Siemens, New York, NY). The minimum inhibitory concentration results showed that all the antibiotics except ciprofloxacin [(minimum inhibitory concentration, MIC) S≤1, R≥4], levofloxacin(MIC S≤2, R≥8) and trimethoprim-sulfamethoxazole (MIC S≤2/38, R≥4/36) were sensitive. The isolates were identified as C. testosteroni by VITEK 2, BD phoenix100 and ATB expression systems at different hospitals in Shanghai.
Identification was also carried by MALDI-TOF-MS. The spectral score for C. kerstersii was 1.815, followed by Comamonas aquatic of the score 1.673. Because the score was less than 2.0, we proceeded to extract DNA and performed PCR amplification of the 16s rRNA gene sequencing for bacterial identification, which showed it was closest with Comamonas kerstersii strain 8943 sequence (CP020121.1, complete genome, Max 2534, Total score 12673, Query cover 100%, Identity 99%) and C. kerstersii strain LMG 5323 sequence (AJ430348.1, partial 16S rRNA gene, Max 2525, Total score 2525, Query cover 100%, Identity 99%). Considered that the biochemical tests, the organisms were confirmed as C. kerstersii by MALDI-TOF-MS and a combination of the other results.
3. Discussion
Since 1987,[6–11] 34 patients infected with C. testosteroni around the world have been reported: 16 with bloodstream infections, 10 with abdominal cavity infections, 8 with other kinds of infections. Among these, Gul et al were the first to report C. testosteroni from the blood cultures of a 22-year-old man with a perforated appendix in Turkey, and the organism was identified by Mini API to be sensitive to all antibiotics tested.[9] Tsui et al presented 2 strains from bacteremia identified by the Phoenix100 System in 2011: a 54-year-old alcoholic patient with left leg cellulitis and a 73-year-old male with chronic hepatitis B infection, liver cirrhosis, and hepatocellular carcinoma after transarterial embolization. The 2 strains were sensitive to a broad range of antibiotics, including all tested cephalosporins and quinolones.[12] Opota et al also commented that there were 32 Comamonas sp. strains and 38 D. acidovorans strains isolated from 1997 to 2013 in his hospital, which were isolated primarily from respiratory tract samples (33%), urogenital tract samples (23%), and digestive tract samples (21%), while bacteremia represented 5% (3 patients) of the cases.[13]
However, it is possible that some of the isolates identified as C. testosteroni might have been C. kerstersii, as C. kerstersii is not found in the VITEK, ATB, API, Siemens, and BD system databases, in which there are only 2 types of Comamonadaceae: D. acidovorans and C. testosteroni. In the present case, the organisms were initially identified as B. bronchiseptica, which was obviously not correct because it is a strict aerobe that grows slowly and forms small colonies in 48 hours.[14]
To date, there are only 8 cases of C. kerstersii reported in the literature,[13,15,16] therefore, our patient represents the ninth reported case of C. kerstersii infection (Table 1). All of the C. kerstersii isolates were identified by MALDI-TOF-MS, which is a rapid and accurate method to differentiate between Comamonas species. Some tests can also differentiate C. kerstersii from other Comamonas species according to schemes proposed by Wauters et al,[17] such as sensitivity to colistin and deferoxamine, nonuse of testosterone, a negative pyrrolidone arylamidase test, growth at 42°C, and a positive tyrosine hydrolysis.
Table 1.
Clinical and microbiological characteristics of the 9 cases of C. kerstersii infections.
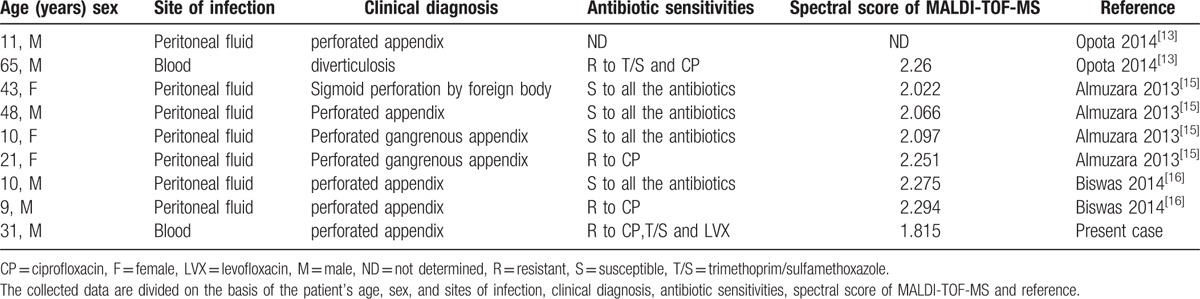
Drug sensitivity tests showed that the isolates were sensitive to a broad range of antibiotics. Among the 9 cases, 2 were identified in bacteremia patients with diverticulosis and perforated appendixes and the predominant source of infection were in the peritoneal fluid of the abdominal cavity(7/9). The main clinical diagnosis of these patients is perforated appendix(7/9), followed by sigmoid perforation and diverticulosis, which demonstrates the association of C. kerstersii with severe diseases. Aside from the previously reported cases of C. testosteroni infections, Opota et al[13] reported the first C. kerstersii bloodstream infection in a patient with diverticulosis. The present case is the first report of C. kerstersii bacteremia in a patient with acute perforated appendicitis. Comamonas species infection has been associated with exposure to contaminated fish tank water or exploration of the abdominal cavity.[18] Thus, we presume that C. kerstersii is an opportunistic pathogen or commensal with the digestive tract and appendix bacteria.
4. Conclusion
In summary, Comamonas kerstersii infection occurs most often in association with severe diseases, such as perforated appendix and bacteremia. This strain is always sensitive to a broad range of antibiotics. However, C. kerstersii is easily confused with C. testosteroni by automatic bacterial identification systems currently available on the market. Overall, MALDI-TOF-MS and gene sequencing are a more accurate approach to identify the species than others. Further research is required to clarify the origins of this organism.
Author contributions
Data curation: Y.H. Zhou.
Formal analysis: H.X. Ma.
Formal analysis: Y.H. Zhou.
Funding acquisition: M.H. Shen.
Methodology: H.X. Ma.
Resources: M.H. Shen.
Supervision: Z.Y. Dong.
Writing – original draft: Y.H. Zhou.
Writing – review & editing: Y.H. Zhou.
Acknowledgments
We are grateful to all of the contributors of the departments of laboratory medicine in different hospitals for their identification of this strain. This work was supported by the Key Programs of Science and Technology Commission Foundation of Changning District, Shanghai (CNKW2016Z05) and the National Nature Science Foundation of China (No. 81401855).
Footnotes
Abbreviations: C. kerstersii = Comamonas kerstersii, MALDI-TOF-MS = matrix-assisted laser desorption ionization–time of flight mass spectrometry, MIC = minimum inhibitory concentration, PCR = polymerase chain reaction.
YZ and HM contributed equally.
The authors declare no conflicts of interest.
Ethical Review: The case report is approved by Institutional Review Board of the Shanghai Provincial Crops Hospital of Chinese People's Armed Police Forces. Informed patient consent was obtained from the patient.
References
- [1].De Vos P, Kersters K, Falsen E, et al. Comamonas Davis and Park 1962 gen. nov., nom. rev. emend., and Comamonas terrigena Hugh 1962 sp. nov. Int J Syst Bacteriol 1985;35:443–53. [Google Scholar]
- [2].Wen A, Fegan M, Hayward C, et al. Phylogenetic relationships among members of the Comamonadaceae and description of Delftia acidovorans (den Dooren de Jong 1926 and Tamaoka et al 1987) gen. nov., comb. nov. Int J Syst Bacteriol 1999;49:567–76. [DOI] [PubMed] [Google Scholar]
- [3].Wauters G, De Baere T, Willems A, et al. Description of Comamonas aquatica comb. nov. and Comamonas kerstersii sp. nov. for two subgroups of Comamonas terrigena and emended description of Comamonas terrigena. Int J Syst Evol Microbiol 2003;53:859–62. [DOI] [PubMed] [Google Scholar]
- [4].Nakipoglu Y, Erturan Z, Buyukbaba-Boral O, et al. Evaluation of the contaminant organisms of humidifier reservoir water and investigation of the source of contamination in a university hospital in Turkey. Am J Infect Control 2005;33:62–3. [DOI] [PubMed] [Google Scholar]
- [5].Koneman EW, Allen SD, Janda WM, et al. Color atlas and Testbook of Diagnostic Microbiology. 1997;New York: J. B. Lippincott Company, 264–77. [Google Scholar]
- [6].Abraham JM, Simon GL. Comamonas testosteroni bacteremia: a case report and review of the literature. Infect Dis Clin Pract 2007;15:272–3. [Google Scholar]
- [7].Farshad S, Norouzi F, Aminshahidi M, et al. Two cases of bacteremia due to an unusual pathogen, Comamonas testosteroni in Iran and a review literature. J Infect Dev Ctries 2012;6:521–5. [DOI] [PubMed] [Google Scholar]
- [8].Bayhan Gi, Tanir G, Karaman I, et al. Comamonas testosteroni: an unusual bacteria associated with acute appendicitis. Balkan Med J 2013;30:447–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gul M, Ciragil P, Bulbuloglu E, et al. Comamonas testosteroni bacteremia in a patient with perforated acute appendicitis. Short communication. Acta Microbiol Immunol Hung 2007;54:317–21. [DOI] [PubMed] [Google Scholar]
- [10].Nseir W, Khateeb J, Awawdeh M, et al. Catheter-related bacteremia caused by Comamonas testosteroni in a hemodialysis patient. Hemodial Int 2011;15:293–6. [DOI] [PubMed] [Google Scholar]
- [11].Barbaro DJ, Mackowiak PA, Barth SS, et al. Pseudomonas testosteroni infections: eighteen recent cases and a review of the literature. Rev Infect Dis 1987;9:124–9. [DOI] [PubMed] [Google Scholar]
- [12].Tsui TL, Tsao SM, Liu KS. Comamonas testosteroni infection in Taiwan:reported two cases and literature review. J Microbiol Immunol Infect 2011;44:67–71. [DOI] [PubMed] [Google Scholar]
- [13].Opota O, Ney B, Zanetti G, et al. Bacteremia caused by Comamonas kerstersii in a patient with diverticulosis. J Clin Microbiol 2014;52:1009–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choy KW, Wulffraat NM, Wolfs TF, et al. Bordetella bronchiseptica respiratory infection in a child after bone marrow transplantation. Pediatr Infect Dis J 1999;18:481–3. [DOI] [PubMed] [Google Scholar]
- [15].Almuzara MN, Cittadini R, Vera Ocampo C, et al. Intra-abdominal infections due to Comamonas kerstersii. J Clin Microbiol 2013;51:1998–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Biswas JS, Fitchett J, O’Hara G. Comamonas kerstersii and the perforated appendix. J Clin Microbiol 2014;52:3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wauters G, Vaneechoutte M. Versalovic J, Carroll KC. Approaches to the identification of aerobic Gram-negative bacteria. Manual of Clinical Microbiology. Washington, DC: ASM Press; 2011. 539–58. [Google Scholar]
- [18].Smith MD, Gradon JD. Bacteremia due to Comamonas species possibly associated with exposure to tropical fish. South Med J 2003;96:815–7. [DOI] [PubMed] [Google Scholar]


