Abstract
Background:
The relationship between vitamin intake and pancreatic cancer (PC) risk is disputed. We aimed to investigate the association between vitamin intake and the risk of PC via meta-analysis.
Methods:
We conducted a meta-analysis of studies concerning vitamin intake and the risk of PC from EMBASE, MEDLINE, and Cochrane Library. The search yielded 25 correlative studies including 1,214,995 individuals. The relative risks (RR) were examined by a random-effect model or fixed-effect model. Subgroup analysis, dose–response analysis, sensitivity analysis, meta-regression, and publication bias analysis were used to analyze studies.
Results:
The RR of PC in the highest vitamin intake group was 0.90 (95% confidence interval, 0.83–0.98) compared with that in the lowest vitamin intake in the prospective studies. Different increments of vitamin intake and the risk of PC were examined with dose–response analysis, and a decrease in the risk of PC was observed with vitamin D (25%) and vitamin B12 (27%).
Conclusions:
This meta-analysis found that vitamin intake can decrease the risk of PC, particularly vitamin D and vitamin B12.
Keywords: meta-analysis, pancreatic cancer, vitamin B12, vitamin D, vitamin intake
1. Introduction
Pancreatic cancer (PC) is one of the most malignant cancers with a 5-year survival rate of about 5%.[1] Almost 80% of PC patients are in the late stage at the first diagnosis in China,[2] and incidence has been increasing in recent years.[3] Therefore, efficacious preventive methods for PC, such as vitamin intake, have attracted worldwide attention. Vitamins have been suggested to prevent PC via several mechanisms.[4] The preventive effects might be via up-regulation of p21 and p27 expression,[5] increased activity of superoxide dismutase,[6] cell cycle arrest at the G1 phase,[7] suppression of NF-κB-mediated inflammatory pathways,[8] down-regulation of Her2/ErbB2 expression,[9] increased caspase-3 activity,[10] or induction of Bax expression and activation EGR-1.[11]
Vitamin intake and the risk of PC have previously been reported. However, retrospective case–control studies, cohort studies, randomized placebo-controlled trials (RCTs), and some meta-analyses[12–22] have had various results for the relationship between vitamin intake and the risk of PC. Therefore, we aimed to investigate the association between vitamin intake and the risk of PC via meta-analysis.
2. Methods
2.1. Search strategy
Studies investigating vitamin intake and PC were searched in EMBASE, MEDLINE, and Cochrane Library through March 30, 2015. Search terms were (pancreas OR pancreatic) AND (cancer OR carcinoma OR neoplasm) AND (vitamin OR food OR diet OR nutrition). References of the retrieved papers were hand-searched for potentially correlative papers. Two authors searched the studies and retrieved papers independently. Disagreements were solved by deliberation with other authors. This study was approved by the Ethics Committee of Qiqihar Medical University.
2.2. Study selection
The inclusion criteria of retrieved papers were case–control, placebo–control, or cohort design; vitamin intake as the independent variable of interest; PC as the dependent variable of interest plus reported PC incidence; and reported odds ratio (OR), relative risk (RR), or hazard ratio with the corresponding 95% confidence interval (CI). Nonhuman studies, mechanistic research, and review articles were excluded.
2.3. Data extraction
Two authors read the retrieved papers and extracted data independently from the studies according to the selection criteria. Disagreements were solved by deliberation with other authors. The following information was extracted from each paper: first author's last name, year of publication, study design, geographic location, the age and sex of participants, follow-up period, the size of study, type and doses of vitamins, RR or OR with 95% CI for vitamin intake, and PC risk. When 2 or more papers concerned the same study, the paper with the most data was used in this study.
2.4. Quality assessment
Two authors independently evaluated the quality of retrieved studies according to the Newcastle–Ottawa scale. The retrieved papers were evaluated based on selection of cohorts (0–4 points), comparability of cohorts (0–2 points), and exposure/outcome of the participant (0–3 points). Studies with 7 to 9 points were marked as “high quality.”
2.5. Statistical analysis
RRs or ORs with 95% CI and their standard errors were obtained from the studies to assess the relationship between vitamin intake and the risk of PC. The random-effect model was used to combine RRs or ORs with 95% CI concerning both intra- and inter-study variation (τ2). I2 was used to evaluate heterogeneity among studies including here, and I2 values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively. A fixed-effect model was utilized if I2 values <50%, otherwise a random-effect model was selected. Meta-regression of the variables of study design, vitamin dose, and geographic area of study was employed to assess heterogeneity among all included studies. The influence of grouping on total results was evaluated by subgroup stratification analysis. Potential causes of heterogeneity were estimated by the sensitivity analysis. Publication bias was evaluated by means of funnel plots and Egger test. This meta-analysis was carried out with Rev Man 5.3 or Stata 12.1, and P < .05 was considered statistically significant.
3. Results
3.1. Search results and study characteristics
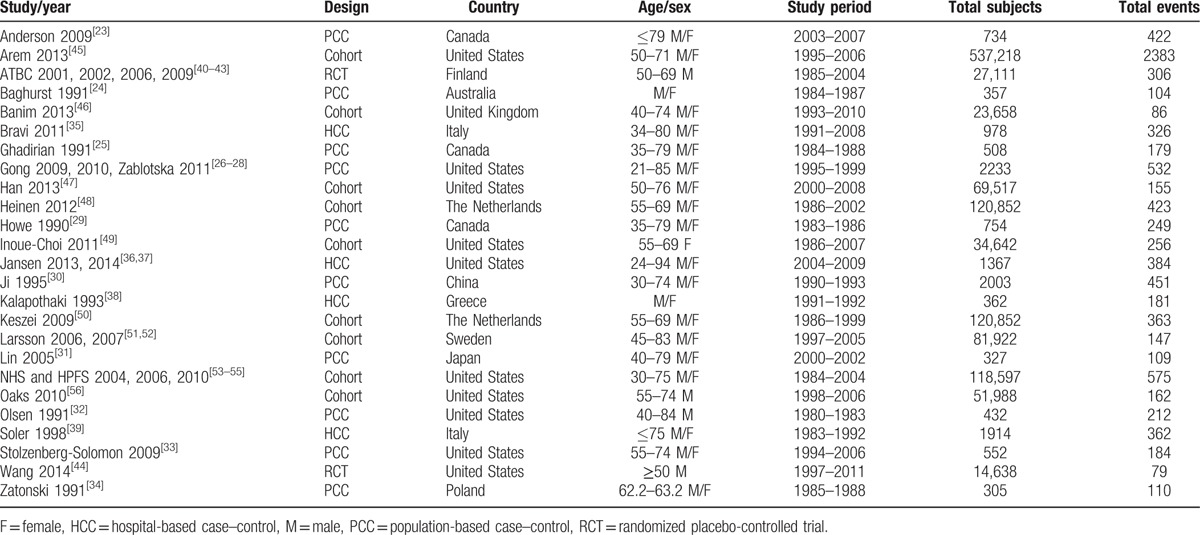
In this meta-analysis, we retrieved 25 studies including 1,213,821 participants published from 1991 to 2014 (Fig. 1). In the identified studies (Table 1), 10 were population-based case–control studies,[23–34] 4 were hospital-based case–control studies,[35–39] 2 were RCTs,[40–44] 9 were cohort studies,[45–56] 11 were prospective studies,[40–56] and 14 were retrospective studies.[23–39] The number of participants ranged from 305[34] to 537,218[45] and PC cases ranged from 79[44] to 2383.[45] Quality scores of included case–control and cohort studies ranged from 7 to 9 with an average score of about 8. The quality of RCTs was also estimated (data not shown).
Figure 1.
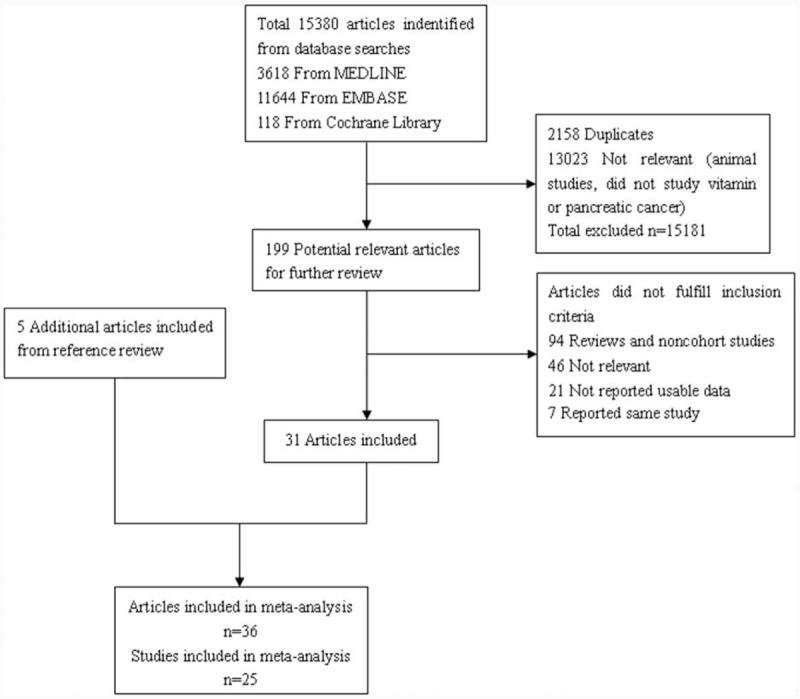
Flow diagram of study selection.
Table 1.
Characteristics of the included studies.
3.2. Vitamin intake and pancreatic cancer risk
A fixed-effect model was used and the combined multivariable-adjusted RR were 0.90 (95% CI: 0.83–0.98) and 0.79 (95% CI: 0.73–0.85) for the highest vitamin intake group compared with the lowest intake group in the prospective studies and retrospective ones, respectively (Fig. 2). Among the 25 studies, an opposite association between vitamin intake and PC risk was observed in 19 studies[23–28,30–32,34–38,45–49,51–56] and was statistically significant in 7 studies.[23,30,34–38,45] No significant heterogeneity was observed among included studies (P < .00001, I2 = 36% among retrospective studies; P < .05, I2 = 11% among prospective studies). These results demonstrate that moderate vitamin consumption can reduce the risk of PC.
Figure 2.
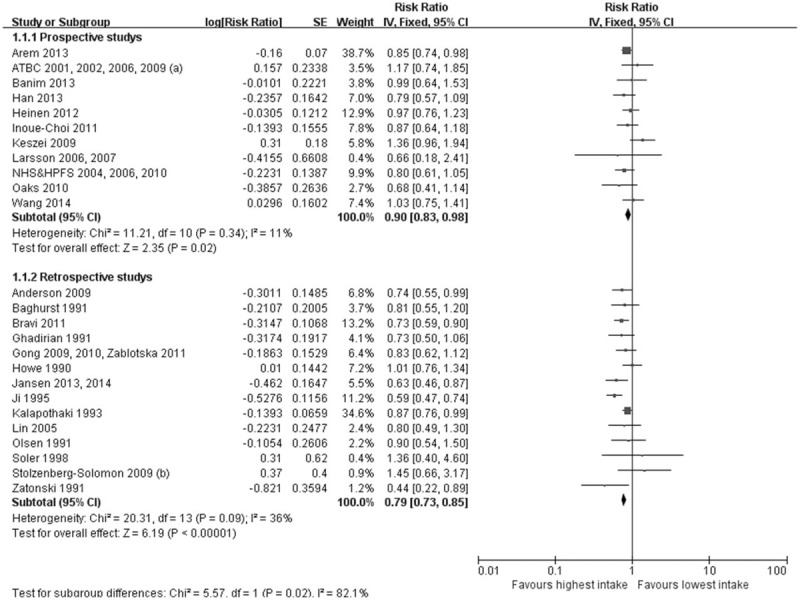
Forest plot of vitamin intake and risk of pancreatic cancer. Squares or diamonds to the left of the solid vertical line indicate benefit with vitamin intake.
3.3. Dose–response meta-analysis
In prospective studies, the multivariable-adjusted RR of vitamin D (10 μg/d) intake was 0.75 (95% CI: 0.60–0.93) with moderate heterogeneity (P = .008, I2 = 59%) in the 3 included studies. The multivariable-adjusted RR of vitamin B12 (10 μg/d) intake was 0.73 (95% CI: 0.44–1.22) in 1 included study. Figure 3 details the dose–response meta-analysis data.
Figure 3.

Forest plot of dose–response meta-analysis. Squares or diamonds to the left of the solid vertical line indicate benefit with vitamin intake. Left forest plot indicates prospective studies and the right one indicates retrospective studies.
In retrospective study, the multivariable-adjusted RR of vitamin E (10 mg/d) intake was 0.75 (95% CI: 0.57–0.98) with moderate heterogeneity (P = .04, I2 = 66%) in the 5 included studies. The multivariable-adjusted RR of vitamin B12 (10 μg/d) intake was 0.67 (95% CI: 0.44–1.01) in 1 included study. The multivariable-adjusted RR of nicotinic acid (30 mg/d) intake was 0.52 (95% CI: 0.36–0.76) without heterogeneity (P = .0006, I2 = 0%) in the 2 included studies. The multivariable-adjusted RR of riboflavin (3 mg/d) intake was 0.75 (95% CI: 0.43–1.33) without significant heterogeneity (P = .33, I2 = 31%) in the 2 included studies. The multivariable-adjusted RR of the thiamine (2 mg/d) intake was 0.65 (95% CI: 0.45–0.95) without heterogeneity (P = .02, I2 = 0%) in the 2 included studies. Figure 3 details the dose–response meta-analysis data.
3.4. Subgroup analysis
3.4.1. Study design
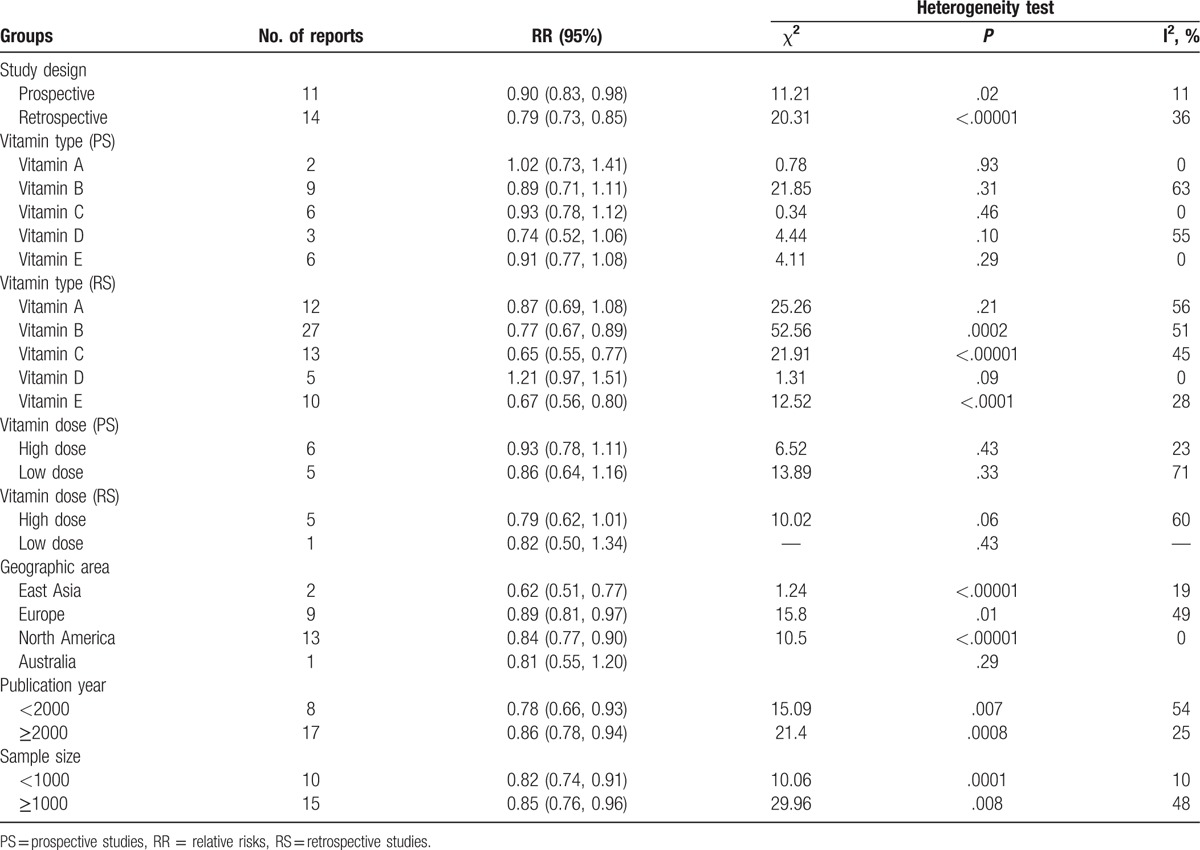
The multivariable-adjusted RR of the prospective studies was 0.90 (95% CI: 0.83–0.98), which demonstrated that vitamin intake can moderately reduce the risk of PC. The multivariable-adjusted RR of the retrospective studies was 0.79 (95% CI: 0.73–0.85), which suggested that vitamin intake can significantly reduce the risk of PC. Details of the subgroup analysis are shown in Table 2.
Table 2.
Subgroup analyses of vitamin intake and pancreatic cancer risk.
3.4.2. Geographic area
The combined RR was 0.84 (95% CI: 0.77–0.90) for research carried out in North America,[23,25–29,32,33,36,37,44,45,47,49,53–56] 0.89 (95% CI: 0.81–0.97) for research carried out in Europe,[34,35,38–43,46,48,50–52] 0.62 (95% CI: 0.51–0.77) for research carried out in East Asia,[30,31] and 0.81 (95% CI: 0.55–1.20) for research carried out in Australia.[24] These results demonstrated that vitamin intake can moderately decrease the risk of PC (Table 2).
3.4.3. Vitamin dose
In prospective studies, there was no significant difference in PC risk in the high-dose group compared with the low-dose (Fig. 4; Table 2). The combined RR was 0.93 (95% CI: 0.78–1.11) in participants who were given 2 or more times the vitamin dosage than the standard vitamin intake level in 6 studies (high-dose group). The combined RR was 0.86 (95% CI: 0.64–1.16) in participants who were given doses under the standard vitamin intake level in 5 studies (low-dose group).
Figure 4.
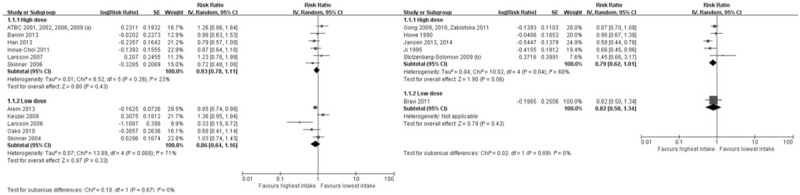
Forest plot of high-dose versus low-dose vitamin intake and risk of pancreatic cancer. Squares or diamonds to the left of the solid vertical line indicate benefit with vitamin intake. Left forest plot indicates prospective studies and the right one indicates retrospective studies.
In retrospective studies, there was no significant difference in PC risk in the high-dose group compared with the low-dose (Fig. 4; Table 2). The combined RR was 0.79 (95% CI: 0.62–1.01) in participants who were given 2 or more times the vitamin dosage than the standard vitamin intake level in 5 studies (high-dose group). The RR was 0.82 (95% CI: 0.50–1.34) in participants who were given doses under the standard vitamin intake level in 1 study (low-dose group).
3.4.4. Vitamin type
In prospective studies, the combined RR of vitamin A or retinol intake and PC risk was 1.02 (95% CI: 0.73–1.41).[41,54] The combined RR of B family vitamin intake and PC risk was 0.89 (95% CI: 0.71–1.11).[40,45,50–53,56] The combined RR of vitamin C intake and PC risk was 0.93 (95% CI: 0.78–1.12).[41,44,46–49] The combined RR of vitamin D intake and PC risk was 0.74 (95% CI: 0.52–1.06).[42,54,55] The combined RR of vitamin E intake and PC risk was 0.91 (95% CI: 0.77–1.08).[43,44,46–49] These findings are summarized in Table 2.
In retrospective studies, the combined RR of vitamin A or retinol intake and PC risk was 0.87 (95% CI: 0.69–1.08).[24,25,28–32,34–36,38,39] The combined RR of B family vitamin intake and PC risk was 0.77 (95% CI: 0.67–0.89).[23–26,32,35–38] The combined RR of vitamin C intake and PC risk was 0.65 (95% CI: 0.55–0.77).[23–25,27,29–32,34–36,38] The combined RR of vitamin D intake and PC risk was 1.21 (95% CI: 0.97–1.51).[24,28,33,35,37] The combined RR of vitamin E intake and PC risk was 0.67 (95% CI: 0.56–0.80).[24,25,27,29–32,35–37] These findings are summarized in Table 2.
3.5. Sensitivity analyses and meta-regression
In prospective study group, the combined RR was 0.91 (95% CI: 0.82–1.00) after 3 studies[44,49,55] were excluded owing to not adjusting for dietary factors or total energy intake with a moderate level of heterogeneity (P = .06, I2 = 28%). The combined RR was 0.89 (95% CI: 0.82–0.98) among 10 studies adjusted for smoking with a moderate level of heterogeneity (P = .01, I2 = 14%).[40–43,45–56] The combined RRs were 0.88 (95% CI: 0.81–0.96) to 0.94 (95% CI: 0.84–1.04) after any single study was excluded, which did not affect the final result.
In retrospective study group, the combined RR was 0.79 (95% CI: 0.73–0.85) after 1 study[39] was excluded owing to not adjusting for dietary factors or total energy intake with a moderate level of heterogeneity (P < .00001, I2 = 39%). The combined RR was 0.78 (95% CI: 0.72–0.84) among 13 studies adjusted for smoking with a moderate level of heterogeneity (P < .00001, I2 = 33%).[23–32,34–39] The combined RRs were 0.75 (95% CI: 0.68–0.82) to 0.82 (95% CI: 0.75–0.88) after any single study was excluded, which did not affect the final result.
Meta-regression analysis demonstrated that study design (P = .005) included significant sources of heterogeneity. Study design alone explained 44.52% of the τ2 in the meta-regression analyses.
3.6. Publication bias
No unambiguous asymmetry was detected in the funnel plot (Fig. 5) and no publication bias was observed in the Egger test (P = .764).
Figure 5.
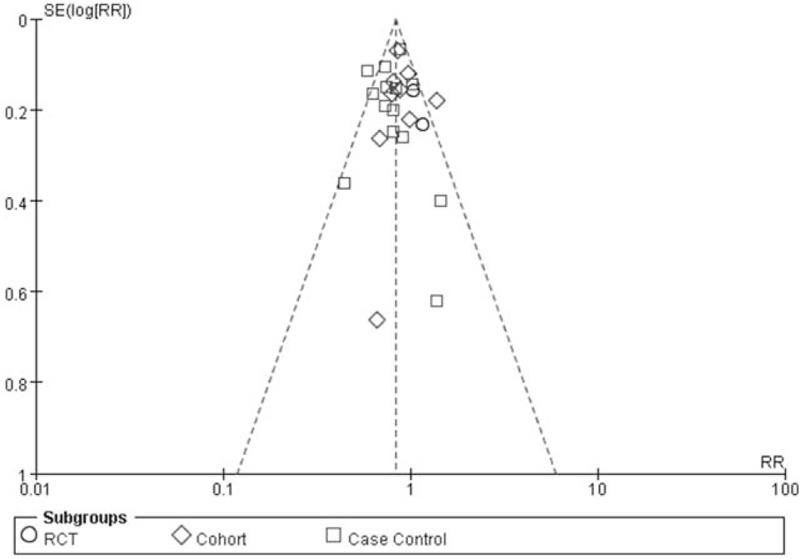
Funnel plot of relative risk of studies.
4. Discussion
This meta-analysis included more than 1.2 million human participants and 8000 PC cases. We found that vitamin consumption can moderately decrease the risk of PC. Daily consumption of 10 μg/d of vitamin B12 or vitamin D can dramatically reduce the incidence of PC, 27% for vitamin B12 and 25% for vitamin D in the dose–response meta-analysis.
Several RCTs and observational studies have explored the association of vitamin consumption and the risk of PC. Some studies reported that vitamin consumption may be correlated with PC incidence.[26,27,30–32,34–37,40,43,51,54,56] However, others found that vitamin consumption had no influence on the incidence of PC.[38,44,48,50,53] Vitamin intake may also have a negative effect on the prevention of PC.[28,39,42] The discrepancy of study design, type and dosage of vitamin intake, method used to estimate vitamin intake, and the time of follow-up may contribute to the different results among the studies.
Some meta-analyses have reported a preventive effect of vitamins on PC.[12–22] The results of some of them suggested that vitamin intake can reduce the risk of PC,[13,17,19–21] which echo this study. Nevertheless, several other studies reported that vitamins cannot decrease the risk of PC and may increase the risk.[12,14–16,18,22] Differences in vitamin dosages used in the latter studies, inclusion of retrospective case–control studies, and inclusion of high-risk individuals, such as long-time chronic smokers, may contribute to discrepancies between their conclusions and ours.
Many studies[23–26,28,32,33,35–38,40,42,45,50–56] have found that nonantioxidant vitamins may help prevent PC. However, some studies[23–25,27–32,34–39,41,43,44,46–49,54] have suggested that antioxidant vitamins, such as vitamins A, C, and E, may influence the prevention of PC. Nevertheless, it is difficult to separate antioxidant vitamins from nonantioxidant vitamins in the daily diet. Therefore, we combined antioxidant and nonantioxidant vitamins together in this meta-analysis.
This meta-analysis demonstrated that vitamins can moderately reduce the incidence of PC. We found that the RR was 0.79 (95% CI: 0.73–0.85) in retrospective studies; however, it was 0.90 (95% CI: 0.83–0.98) in the prospective ones. It suggested that vitamin consumption can moderately decrease the risk of PC. In retrospective study, the RR of vitamin E intake was 0.75 (95% CI: 0.57–0.98), the RR of vitamin B12 intake was 0.67 (95% CI: 0.44–1.01), the RR of nicotinic acid intake was 0.52 (95% CI: 0.36–0.76), the RR of riboflavin intake was 0.75 (95% CI: 0.43–1.33), and the RR of the thiamine intake was 0.65 (95% CI: 0.45–0.95). Nevertheless, the prospective studies suggested that the consumption of vitamin D (10 μg/d; RR: 0.75; 95% CI: 0.60–0.93) and vitamin B12 (10 μg/d; RR: 0.73; 95% CI: 0.44–1.22) can decrease the risk of PC. These dose–response meta-analysis data recommended that daily consumption of 10 μg/d of vitamin B12 or vitamin D can dramatically reduce the incidence of PC, 27% for vitamin B12 and 25% for vitamin D. Some in vitro studies have suggested that nicotinic acid, thiamine, and vitamin B12 can prevent PC. Pour and Lawson[57] suggested that nicotinic acid can inhibit pancreatic carcinogenesis in a hamster model. Zhang et al[58] reported that nicotinamide prohibits proliferation and enhances chemosensitivity in PC cells; and Hanberry et al[10] reported that high-dose vitamin B1 reduces proliferation in Panc-1 PC cell lines. However, whether or not the consumption of vitamin E, vitamin B12, nicotinic acid, riboflavin, and thiamine can reduce the incidence of PC need more evidence from prospective studies.
Several studies have investigated the mechanism of how vitamins might inhibit PC. Vitamin D can up-regulate p21 and p27 during growth inhibition of PC cell lines.[5] Vitamins A, C, and E can increase the activity of superoxide dismutase to decrease the incidence of PC in hamsters.[6] Vitamin E can induce cell cycle arrest at the G1 phase, induce apoptosis in human PC cells,[7] induce Bax expression, and activate EGR-1 in PC cells.[11] Another study found that vitamin E can inhibit the growth of human PC cells by suppressing NF-κB-mediated inflammatory pathways.[8] Vitamin E can also induce apoptosis in PC cells by suppressing signaling pathways such as the PI3K/AKT and ERK/MAPK pathways via down-regulation of Her2/ErbB2,[9] and inhibit the proliferation of PC cells dependent on p27 (Kip1) induction.[59] Vitamin K can inhibit PC cell survival via a caspase-dependent pathway.[60] Thiamine can increase caspase-3 activity and reduce proliferation in PC cell lines.[10] Nevertheless, the mechanisms of vitamins reducing the risk of PC needs further investigation.
5. Conclusion
In conclusion, this meta-analysis suggested that vitamin intake can moderately reduce the risk of PC, particularly the consumption of vitamin D and vitamin B12.
Author contributions
Data curation: S. Lu, X. Sun.
Funding acquisition: X. Wang.
Investigation: S. Liu, X. Wang, Y. Liu.
Methodology: X. Sun.
Software: S. Lu, X. Sun.
Supervision: Y. Liu.
Writing – original draft: S. Liu.
Writing – review & editing: S. Liu.
Footnotes
Abbreviations: CI = confidence interval, OR = odds ratio, PC = pancreatic cancer, RCT = randomized placebo-controlled trial, RR = relative risks.
Ying Liu, Shengnan Lu, and Shi Liu designed the study; Xiaojie Wang, Xuejia Sun, Shengnan Lu, and Shi Liu collected the data; Ying Liu, Shengnan Lu, and Shi Liu wrote the manuscript; all authors reviewed the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut 2007;56:1134–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang Y, Shi X. Progress in the chemotherapy of pancreatic carcinoma. World Chin J Digestol 2009;17:1422–6. [Google Scholar]
- [3].Saif MW. New developments in the treatment of pancreatic cancer. Highlights from the “44th ASCO Annual Meeting” Chicago, IL, USA, May 30–June 3, 2008, JOP 2008;9:391–7. [PubMed] [Google Scholar]
- [4].Davis-Yadley AH, Malafa MP. Vitamins in pancreatic cancer: a review of underlying mechanisms and future applications. Adv Nutr 2015;6:774–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kawa S, Nikaido T, Aoki Y, et al. Vitamin D analogues up-regulate p21 and p27 during growth inhibition of pancreatic cancer cell lines. Br J Cancer 1997;76:884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wenger FA, Kilian M, Ridders J, et al. Influence of antioxidative vitamins A, C and E on lipid peroxidation in BOP-induced pancreatic cancer in Syrian hamsters. Prostaglandins Leukot Essent Fatty Acids 2001;65:165–71. [DOI] [PubMed] [Google Scholar]
- [7].Hussein D, Mo H. d-δ-Tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas 2009;38:e124–36. [DOI] [PubMed] [Google Scholar]
- [8].Kunnumakkara AB, Sung B, Ravindran J, et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res 2010;70:8695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shin-Kang S, Ramsauer VP, Lightner J, et al. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med 2011;51:1164–74. [DOI] [PubMed] [Google Scholar]
- [10].Hanberry BS, Berger R, Zastre JA. High-dose vitamin B1 reduces proliferation in cancer cell lines analogous to dichloroacetate. Cancer Chemother Pharmacol 2014;73:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang C, Husain K, Zhang A, et al. EGR-1/Bax pathway plays a role in vitamin E δ-tocotrienol-induced apoptosis in pancreatic cancer cells. J Nutr Biochem 2015;26:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bjelakovic G, Nikolova D, Simonetti RG, et al. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2004;364:1219–28. [DOI] [PubMed] [Google Scholar]
- [13].Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology 2006;131:1271–83. [DOI] [PubMed] [Google Scholar]
- [14].Waterhouse M, Risch HA, Bosetti C, et al. Vitamin D and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Ann Oncol 2015;26:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Druesne-Pecollo N, Latino-Martel P, Norat T, et al. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer 2010;127:172–84. [DOI] [PubMed] [Google Scholar]
- [16].Bao Y, Michaud DS, Spiegelman D, et al. Folate intake and risk of pancreatic cancer: pooled analysis of prospective cohort studies. J Natl Cancer Inst 2011;103:1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin HL, An QZ, Wang QZ, et al. Folate intake and pancreatic cancer risk: an overall and dose-response meta-analysis. Public Health 2013;127:607–13. [DOI] [PubMed] [Google Scholar]
- [18].Liu SL, Zhao YP, Dai MH, et al. Vitamin D status and the risk of pancreatic cancer: a meta-analysis. Chin Med J 2013;126:3356–9. [PubMed] [Google Scholar]
- [19].Tio M, Andrici J, Cox MR, et al. Folate intake and the risk of upper gastrointestinal cancers: a systematic review and meta-analysis. J Gastroenterol Hepatol 2014;29:250–8. [DOI] [PubMed] [Google Scholar]
- [20].Peng L, Liu X, Lu Q, et al. Vitamin E intake and pancreatic cancer risk: a meta-analysis of observational studies. Med Sci Monit 2015;21:1249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fan H, Kou J, Han D, et al. Association between vitamin C intake and the risk of pancreatic cancer: a meta-analysis of observational studies. Sci Rep 2015;5:13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hua YF, Wang GQ, Jiang W, et al. Vitamin C intake and pancreatic cancer risk: a meta-analysis of published case-control and cohort studies. PLoS ONE 2016;11:e0148816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control 2009;20:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baghurst PA, McMichael AJ, Slavotinek AH, et al. A case–control study of diet and cancer of the pancreas. Am J Epidemiol 1991;134:167–79. [DOI] [PubMed] [Google Scholar]
- [25].Ghadirian P, Simard A, Baillargeon J, et al. Nutritional factors and pancreatic cancer in the francophone community in Montréal, Canada. Int J Cancer 1991;47:1–6. [DOI] [PubMed] [Google Scholar]
- [26].Gong Z, Holly EA, Bracci PM. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control 2009;20:1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gong Z, Holly EA, Bracci PM. Intake of fatty acids and antioxidants and pancreatic cancer in a large population-based case-control study in the San Francisco Bay Area. Int J Cancer 2010;127:1893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zablotska LB, Gong Z, Wang F, et al. Vitamin D, calcium, and retinol intake, and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control 2011;22:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Howe GR, Jain M, Miller AB. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. Int J Cancer 1990;45:604–8. [DOI] [PubMed] [Google Scholar]
- [30].Ji BT, Chow WH, Gridley G, et al. Dietary factors and the risk of pancreatic cancer: a case-control study in Shanghai China. Cancer Epidemiol Biomarkers 1995;4:885–93. [PubMed] [Google Scholar]
- [31].Lin Y, Tamakoshi A, Hayakawa T, et al. Nutritional factors and risk of pancreatic cancer: a population-based case-control study based on direct interview in Japan. J Gastroenterol 2005;40:297–301. [DOI] [PubMed] [Google Scholar]
- [32].Olsen GW, Mandel JS, Gibson RW, et al. Nutrients and pancreatic cancer: a population-based case-control study. Cancer Causes Control 1991;2:291–7. [DOI] [PubMed] [Google Scholar]
- [33].Stolzenberg-Solomon RZ, Hayes RB, Horst RL, et al. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res 2009;69:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zatonski W, Przewozniak K, Howe GR, et al. Nutritional factors and pancreatic cancer: a case-control study from south-west Poland. Int J Cancer 1991;48:390–4. [DOI] [PubMed] [Google Scholar]
- [35].Bravi F, Polesel J, Bosetti C, et al. Dietary intake of selected micronutrients and the risk of pancreatic cancer: an Italian case-control study. Ann Oncol 2011;22:202–6. [DOI] [PubMed] [Google Scholar]
- [36].Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J Gastrointest Cancer 2013;44:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jansen RJ, Robinson DP, Frank RD, et al. Fatty acids found in dairy, protein and unsaturated fatty acids are associated with risk of pancreatic cancer in a case-control study. Int J Cancer 2014;134:1935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kalapothaki V, Tzonou A, Hsieh CC, et al. Nutrient intake and cancer of the pancreas: a case-control study in Athens, Greece. Cancer Causes Control 1993;4:383–9. [DOI] [PubMed] [Google Scholar]
- [39].Soler M, Chatenoud L, La Vecchia C, et al. Diet, alcohol, coffee and pancreatic cancer: final results from an Italian study. Eur J Cancer Prev 1998;7:455–60. [DOI] [PubMed] [Google Scholar]
- [40].Stolzenberg-Solomon RZ, Pietinen P, Barrett MJ, et al. Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. Am J Epidemiol 2001;153:680–7. [DOI] [PubMed] [Google Scholar]
- [41].Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol 2002;155:783–92. [DOI] [PubMed] [Google Scholar]
- [42].Stolzenberg-Solomon RZ, Vieth R, Azad A, et al. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res 2006;66:10213–9. [DOI] [PubMed] [Google Scholar]
- [43].Stolzenberg-Solomon RZ, Sheffler-Collins S, Weinstein S, et al. Vitamin E intake, alpha-tocopherol status, and pancreatic cancer in a cohort of male smokers. Am J Clin Nutr 2009;89:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang L, Sesso HD, Glynn RJ, et al. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians’ Health Study II randomized trial. Am J Clin Nutr 2014;100:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Arem H, Reedy J, Sampson J, et al. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP study. J Natl Cancer Inst 2013;105:1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Banim PJ, Luben R, McTaggart A, et al. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut 2013;62:1489–96. [DOI] [PubMed] [Google Scholar]
- [47].Han X, Li J, Brasky TM, et al. Antioxidant intake and pancreatic cancer risk. Cancer 2013;119:1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Heinen MM, Verhage BA, Goldbohm RA, et al. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands Cohort Study. Int J Cancer 2012;130:147–58. [DOI] [PubMed] [Google Scholar]
- [49].Inoue-Choi M, Flood A, Robien K, et al. Nutrients, food groups, dietary patterns, and risk of pancreatic cancer in postmenopausal women. Cancer Epidem Biomar 2011;20:711–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Keszei AP, Verhage BA, Heinen MM, et al. Dietary folate and folate vitamers and the risk of pancreatic cancer in the Netherlands cohort study. Cancer Epidem Biomar 2009;18:1785–91. [DOI] [PubMed] [Google Scholar]
- [51].Larsson SC, Hakansson N, Giovannucci E, et al. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst 2006;98:407–13. [DOI] [PubMed] [Google Scholar]
- [52].Larsson SC, Giovannucci E, Wolk A. Methionine and vitamin B6 intake and risk of pancreatic cancer: a prospective study of Swedish women and men. Gastroenterology 2007;132:113–8. [DOI] [PubMed] [Google Scholar]
- [53].Skinner HG, Michaud DS, Giovannucci EL, et al. A prospective study of folate intake and the risk of pancreatic cancer in men and women. Am J Epidemiol 2004;160:248–58. [DOI] [PubMed] [Google Scholar]
- [54].Skinner HG, Michaud DS, Giovannucci E, et al. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev 2006;15:1688–95. [DOI] [PubMed] [Google Scholar]
- [55].Bao Y, Ng K, Wolpin BM, et al. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer 2010;102:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Oaks BM, Dodd KW, Meinhold CL, et al. Folate intake, post-folic acid grain fortification, and pancreatic cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr 2010;91:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pour PM, Lawson T. Modification of pancreatic carcinogenesis in the hamster model. XV. Preventive effect of nicotinamide. J Natl Cancer Inst 1984;73:767–70. [PubMed] [Google Scholar]
- [58].Zhang JG, Zhao G, Qin Q, et al. Nicotinamide prohibits proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatology 2013;13:140–6. [DOI] [PubMed] [Google Scholar]
- [59].Hodul PJ, Dong Y, Husain K, et al. Vitamin E δ-tocotrienol induces p27 (Kip1)-dependent cell-cycle arrest in pancreatic cancer cells via an E2F-1-dependent mechanism. PLoS ONE 2013;8:e52526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Showalter SL, Wang Z, Costantino CL, et al. Naturally occurring K vitamins inhibit pancreatic cancer cell survival through a caspase-dependent pathway. J Gastroenterol Hepatol 2010;25:738–44. [DOI] [PubMed] [Google Scholar]


