Abstract
Scavenger receptor class B type I (SR-B1) is highly expressed in a variety of cancers, including prostate, breast and ovarian. However, the relationship between SR-B1 and lung adenocarcinoma is unknown. We analyzed the expression of SR-B1 in a well-characterized lung adenocarcinoma tissue microarray by immunohistochemistry, in 90 cancerous and 90 adjacent normal lung tissues. Results showed that the positive expression rate of SR-B1 in cancer tissues (86/90, 96%) was significantly higher than that of adjacent tissues (50/90, 56%) (P < .001). And SR-B1 overexpression in lung adenocarcinoma tissue was significantly higher than that of adjacent normal tissue (P < .001), accounting for 67% of cases. This elevated SR-B1 expression was associated with AJCC stage (P < .001), T stage (P = .012), N stage (P = .002), and lymph node positivity (P < .001). The Kaplan–Meier survival analysis indicated that patients with high SR-B1 expression had a shorter overall survival (P < .001). On the multivariate analysis, SR-B1 was an independent prognostic factor for outcomes after adjustment for other prognostic factors (P = .038). In conclusion, high SR-B1 expression is associated with conventional pathologic parameters that represent tumor aggressiveness and may purport a poor clinical prognosis in lung adenocarcinoma.
Keywords: immunohistochemistry, lung adenocarcinoma, prognosis, scavenger receptor class B type I, tumor aggressiveness
1. Introduction
Lung cancer is common worldwide and carries a high mortality rate. In the United States, an estimated 222,500 new cases of lung cancer and 155,870 deaths are expected to occur in 2017.[1] Adenocarcinoma is the most common histologic type[2] and is associated with risk factors such as smoking, passive smoking, family history of lung cancer, air pollution, and chronic inflammation of the lungs.[3,4] However, the etiology of lung cancer is still not completely understood as carcinogenesis is a complex process. Previous studies have shown that cholesterol plays an important role in the development and progression of tumors. Under normal conditions, excess cholesterol is excreted by the liver as high-density lipoprotein (HDL). However, in carcinogenesis, excess cholesterol leads to tumor proliferation through metabolism of HDL. Thus, there is a negative correlation between serum HDL levels and cancer risk.[5,6] In addition, recent research has suggested that HDL is implicated in modulating normal lung health and disease, including lung cancer. Higher circulating HDL levels are a favorable prognostic factor in non-small cell lung carcinoma (NSCLC).[7,8]
Scavenger receptor class B type I (SR-B1) is an HDL receptor in many tissues and it is primarily expressed in the liver, adrenal glands, ovaries, and testes. One of its primary functions is to mediate the uptake of HDL-derived cholesterol and cholesteryl ester in the liver and steroidogenic tissues.[9–12] SR-B1 has been shown to be highly expressed in a variety of tumor cell lines, including liver, prostate, breast, colorectal, pancreatic, ovarian, and nasopharyngeal cancers.[13–16] Moreover, high SR-B1 expression is associated with tumor aggressiveness and poor clinical prognosis in breast cancer and prostate cancer. Additionally, the SR-B1 receptor is a crucial junction where breast tumor cells exploit HDL to ingest cholesterol for proliferative advantages. Thus, SR-B1 may be a potential target for cancer therapeutics.[17–19]
Little is known about the role of SR-B1 in lung adenocarcinoma. We evaluated SR-B1 expression using a high throughput tissue microarray containing 90 cases of lung adenocarcinoma tissue with paired adjacent normal lung tissue. We aimed to assess the correlation between SR-B1 expression and clinicopathological factors of lung adenocarcinoma, as well as its influence on overall survival (OS). We hypothesized that SR-B1 could be used as a new prognostic biomarker and a potential therapeutic target for lung adenocarcinoma.
2. Materials and methods
2.1. Human lung adenocarcinoma tissue microarray
The lung adenocarcinoma tissue microarray (Shanghai Outdo Biotech Co., Ltd., Shanghai, China) consisted of 90 cancer tissue samples and 90 paired adjacent normal lung tissue samples. Excluding metastasis and invasion of other primary tumors, all samples were obtained from patients with primary lung adenocarcinoma who underwent surgery between 2004 and 2009. None of the patients had received neoadjuvant chemotherapy or radiotherapy. Clinicopathologic parameters (age, tumor size, lymph node positivity, pathologic tumor-node-metastasis stage, pathologic T stage, pathologic N stage, and follow-up data) were reviewed. Tumor-node-metastasis (TNM) staging was based on the seventh edition of the AJCC staging system for lung cancer. OS was defined as the date of surgery to the date of death or last follow-up, which ranged from 1 to 121 (median 39) months. All patients whose tissue was used in this study underwent written informed consent prior to tissue collection. The study was approved by the Ethical Committee of Shandong Provincial Hospital, affiliated to Shandong University, People's Republic of China.
2.2. Immunohistochemistry
Immunohistochemistry was performed according to the following protocol. First, the tissue microarray slide was dewaxed and hydrated using a graded series of ethanol (100%, 95%, 80%, and 70%) and water. Then, antigen retrieval was performed with citrate buffer at a pH level of 6 for 5 minutes using a pressure cooker, followed by natural cooling to room temperature. In order to reduce nonspecific background staining caused by endogenous peroxidase, samples were incubated with 3% H2O2 for 15 minutes at room temperature. After washing with phosphate-buffered saline (PBS), nonspecific binding sites were blocked with normal goat serum for 20 minutes. Then, anti-SR-B1 antibody (EP1556Y, Abcam, Cambridge, MA) was used as the primary antibody, at a dilution rate of 1:75 at 4°C in a moist chamber overnight. The following day, biotin-labeled secondary antibody (goat anti-rabbit antibody, Zhongshan Biotechnology Co., Beijing, China) was added, followed by incubation at room temperature for 30 minutes. Then, streptavidin avidin biotin peroxidase complex (SABC) was added, followed by incubation at 37°C for 30 minutes. Following rinsing in PBS, the antibodies were visualized using diaminobenzidine before counterstaining with hematoxylin. Finally, the slices were sealed with a neutral gum. The stained tissue microarray was observed under the microscope. The positive reaction of the SR-B1 protein was visible as a yellow-brown staining of the cytoplasm and membrane and blue staining of the nuclei. Taking the place of the primary antibody, PBS was used as a negative control.
2.3. Immunohistochemical evaluation
The modified immunoreactive score (IRS) was used to evaluate immunohistochemical staining for SR-B1 expression. The staining intensity standard for evaluation was as follows: no coloring, 0; weak, 1; moderate, 2; strong, 3. The positive cell proportion standard for evaluation was as follows: no staining, 0; < 10%, 1; 10% to 50%, 2; 51% to 80%, 3; >80%, 4. Immunostaining intensity and percentages of stained cells were assessed independently by 2 pathologists who had no knowledge of clinicopathologic information or patient outcome. The final score (ranging from 0 to 12) was obtained by multiplying the two scores in each case. A score of <6 was deemed as low expression, whereas a score ≥6 was considered to be high expression.
2.4. Statistical analysis
The chi-squared test and CMH chi-squared test were used to analyze the correlation between SR-B1 expression and clinicopathologic characteristics, depending on the discrete or continuous nature of the parameters. Survival analysis was evaluated using the Kaplan–Meier method and differences in outcome for each variable were evaluated using the log-rank test. The multivariate survival analysis was performed using Cox's proportional hazard model to test significant parameters revealed through the univariate analysis. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were also calculated. A P-value of <.05 (two-tailed) was considered statistically significant. All statistical methods were performed using SPSS software, version 19.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Clinicopathologic characteristics of study patients
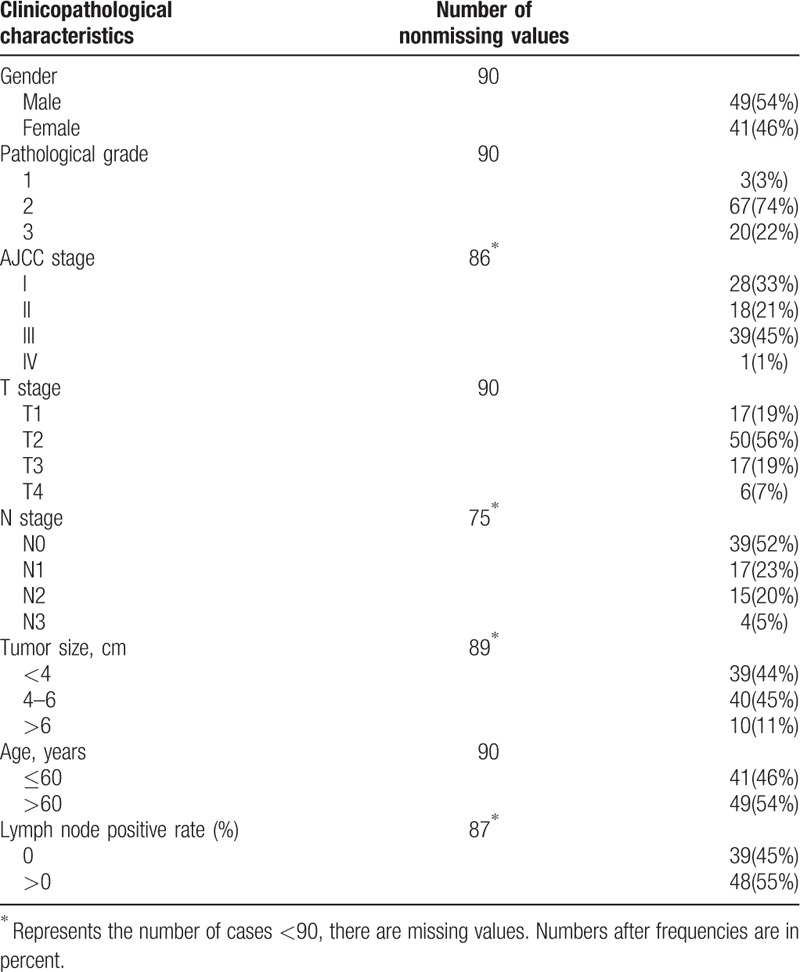
A total of 90 lung adenocarcinoma specimens with 90 paired adjacent normal lung specimens were analyzed for SR-B1. Clinicopathologic features are listed in Table 1. Of the 90 cases, there were 49 males and 41 females with a median age of 64 (range 30–84) years. The median tumor diameter was 4 (range 1.5 to 14) cm. Regarding TNM stage, the distribution was as follows: stage I, 33%; stage II, 21%; stage III, 45%; and stage IV,1%. The distribution of tumor pathologic grade was as follows: grade 1, 33%; grade 2, 65%; and grade 3, 22%. Pathologic T stage was: stage 1, 19%; stage 2, 55%; stage 3, 19%; and stage 4, 7%. A total of 39 cases had no lymph node metastasis (N0) whereas 36 cases demonstrated lymph node involvement (N+) and 15 cases were clinically undetermined. The postoperative follow-up period ranged from 1 to 121 (median 39) months. By the completion of this study, 67 (74%) patients had died.
Table 1.
Clinicopathological characteristics of the patient cohort (N = 90).
3.2. SR-B1 is overexpressed in lung adenocarcinoma tissue
Immunohistochemical analysis was performed to evaluate the expression of SR-B1 in lung adenocarcinoma tissues and adjacent normal lung tissues. Microscopic observation showed that SR-B1 was primarily localized in the cytoplasm and cell membranes. Representative images of SR-B1 staining are shown in Figure 1. Also, the positive expression rate of SRB1 in cancer tissues (86/90, 96%) was significantly higher than that of adjacent tissues (50/90, 56%) (P < .001, Table 2). Additionally, elevated SR-B1 expression was detected in 60 of 90 (67%) tumor tissues and 27 of 90 (30%) of normal tissues (P < .001, Table 3). Thus, SR-B1 was found to be overexpressed in lung adenocarcinoma compared to adjacent normal tissues.
Figure 1.
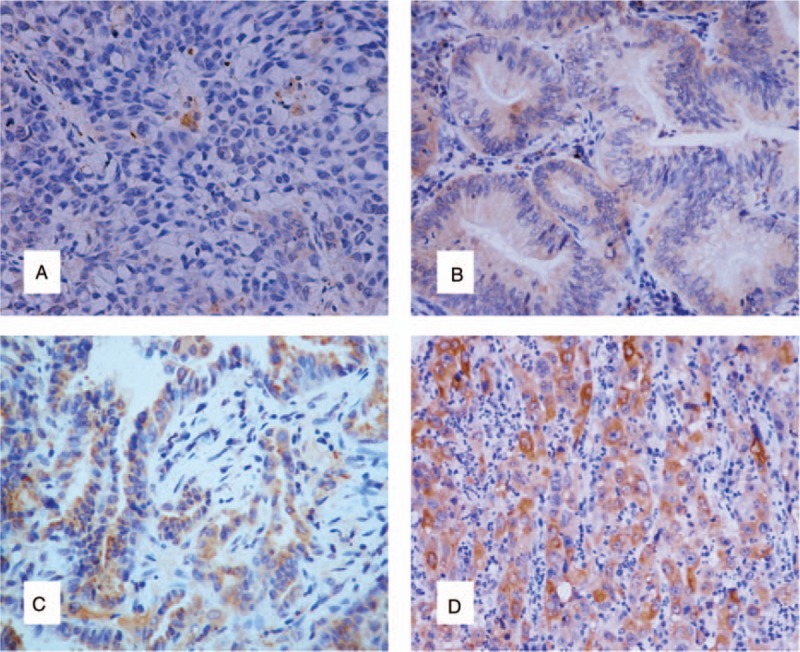
Immunohistochemical staining of scavenger receptor class B type I (SR-B1) in normal lung tissue and lung adenocarcinoma tissues (original magnification ×200). (A) Negative expression of SR-BI in normal lung tissue (intensity score = 0, final score = 0). (B) Weak expression of SR-BI in lung adenocarcinoma tissue (intensity score = 1, final score = 4). (C) Moderate expression of SR-BI in lung adenocarcinoma tissue (intensity score = 2, final score = 8). (D) Strong expression of SR-BI in lung adenocarcinoma tissue (intensity score = 3, final score = 12). SR-B1 = scavenger receptor class B type I.
Table 2.
Comparison of the positive expression rate of scavenger receptor class B type I in cancer and adjacent tissues.

Table 3.
Comparison of the expression levels of scavenger receptor class B type I in cancer and adjacent tissues.

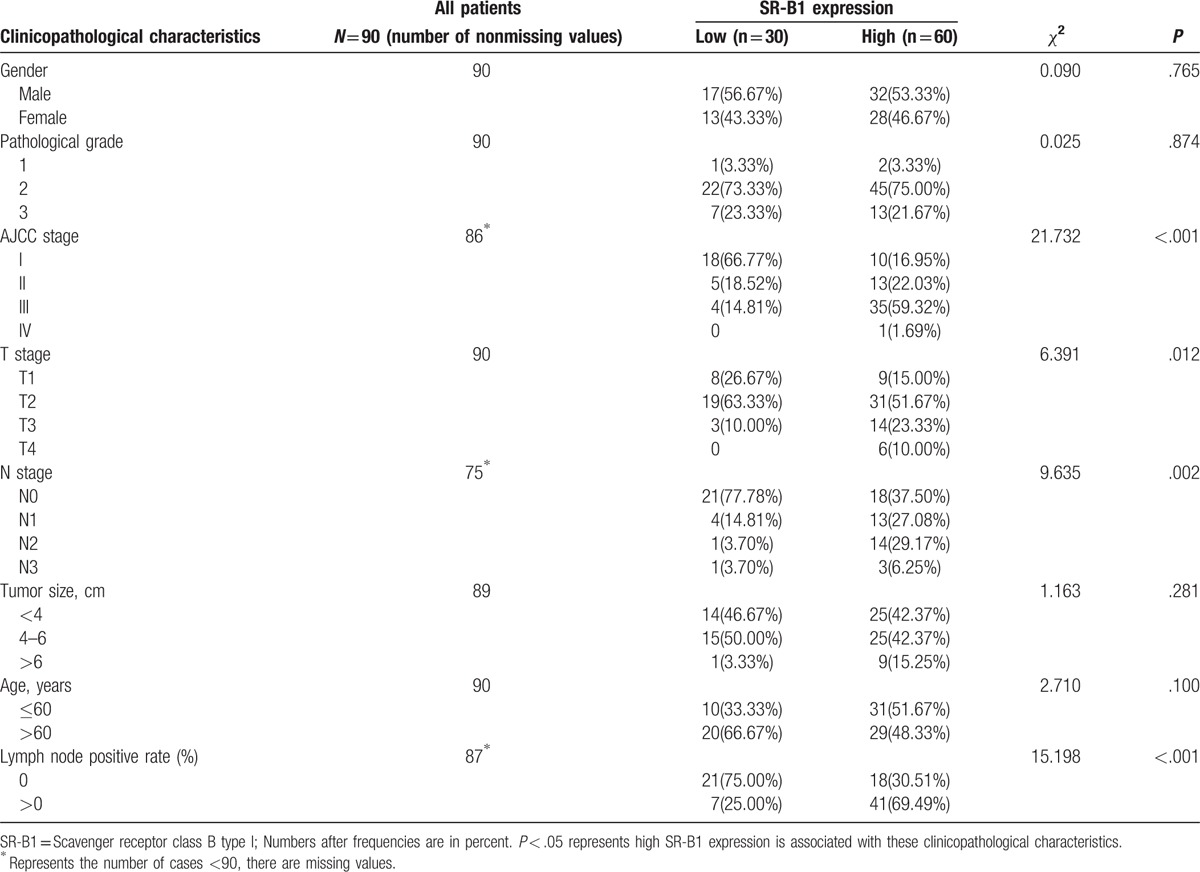
3.3. Correlation between SR-B1 expression and clinicopathologic characteristics
The association between SR-B1 expression and clinicopathologic factors is shown in Table 4. High SR-B1 expression significantly correlated with pTNM stage (P < .001) and lymph node positivity (P < .001). In addition, high SR-B1 expression was positively associated with T stage (P = .012) and N stage (P = .002), but was independent from sex (P = .765), age (P = .100), tumor size (P = .281), and pathologic grade (P = .874). Tumor aggressiveness was also related to these parameters (advanced TNM stage, higher T stage; higher N stage, high lymph node positivity). Accordingly, high SR-B1 expression was associated with tumor aggressiveness and may be an indicator of pathologic behavior and in lung adenocarcinoma.
Table 4.
Association of scavenger receptor class B type I expression with clinicopathological characteristics in lung adenocarcinoma.
3.4. SR-B1 expression and prognosis in lung adenocarcinoma
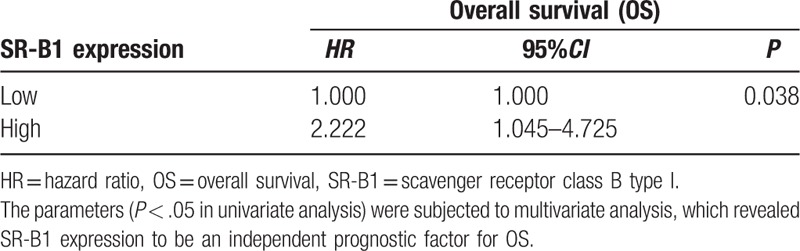
Figure 2 shows survival curves stratified according to SR-B1 expression. Survival analyses using the Kaplan–Meier method revealed an obviously lower 5-year survival rate in patients with high versus low SR-B1 expression (P < .001, log-rank test). The results of the univariate analyses of the factors related to prognosis are listed in Table 5. Univariate Cox analysis demonstrated that AJCC stage (P = .001), N stage (P = .001), tumor size (P = .043), lymph node positivity (P = .002), and SR-B1 expression (P < .001) significantly correlated with OS. These parameters were subjected to multivariate analysis, which revealed SR-B1 expression to be an independent prognostic factor for OS (P = .038, Table 6). Thus, we found high SR-B1 expression to be an independent prognostic factor for lung adenocarcinoma.
Figure 2.
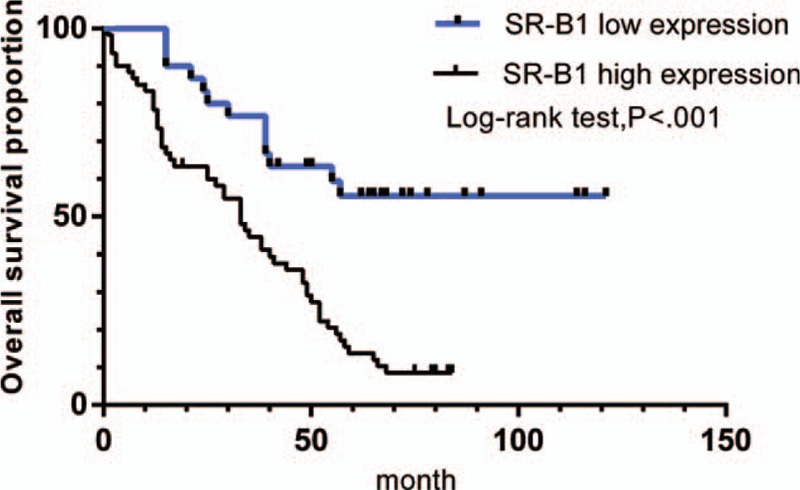
Kaplan–Meier curves showed that scavenger receptor class B type I expression was significantly associated with overall survival (P < .001).
Table 5.
Univariate Cox analysis (the relationship between several characteristics and prognosis of patients with lung adenocarcinoma).
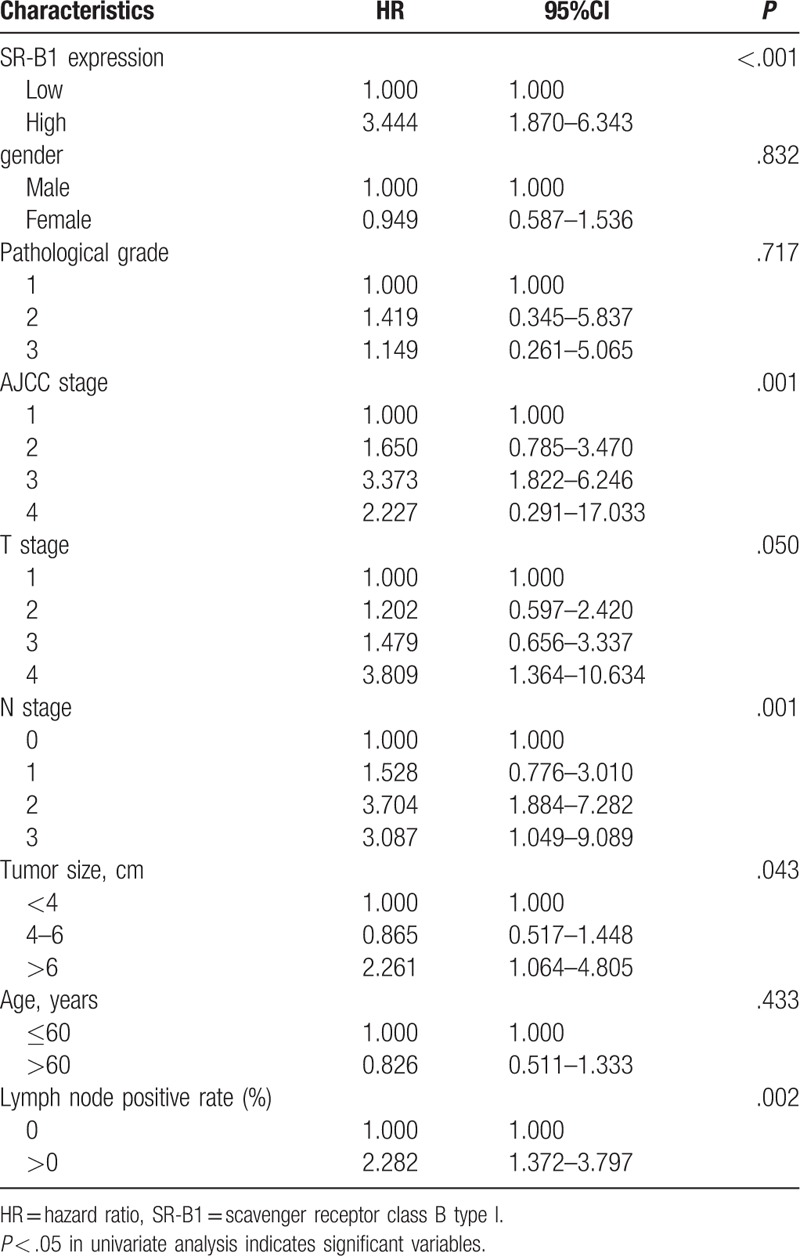
Table 6.
Multivariate Cox analysis.
4. Discussion
Numerous studies have discussed the role of SR-B1 as a transmembrane protein that is highly expressed in multiple carcinoma cells.[13–16] Zheng et al[13] suggested SR-B1 as a potential biomarker in human nasopharyngeal carcinoma. Li et al[18] reported that upregulated expression of SR-B1 related to malignant behavior and poor prognosis in breast cancer and Yuan et al[19] also showed that SR-B1 was highly expressed and was an independent prognostic factor in breast cancer. In the current study, we used immunohistochemistry to evaluate the expression of SR-B1 in 90 cases of lung adenocarcinoma, along with its relationship to pathologic factors and survival. We found SR-B1 to be widely expressed in lung adenocarcinoma specimens (60/90, 67%). Furthermore, we found SR-B1 to be closely related to tumor aggressiveness, as measured by TNM stage, T stage, N stage, and lymph node positivity. Most importantly, we found high SR-B1 expression to be an independent predictor of poor prognosis in lung adenocarcinoma.
It has been well established that serum HDL-cholesterol (HDL-C) is often downregulated in lung cancer and that HDL-C may stimulate lung cancer cell proliferation and migration.[20–25] Lv et al suggested that reduced serum of HDL-C levels may be mediated by a greater utilization of cholesterol for membrane biogenesis and by the accumulation of esterified cholesterol in tumor tissue.[26,27] SR-B1 is known to be a transmembrane protein that specifically binds to HDL to facilitate cellular transport of cholesterol.[28] In further support of a role for HDL in lung cancer growth, we have conjectured that SR-B1 is required for the proliferation and migration of lung cancer cells. This hypothesis is consistent with the results of this study that SR-B1 is highly expressed in lung adenocarcinoma and is related to tumor aggressiveness. Alpha-1 antitrypsin (A1AT) is an acute-phase protein which may inhibit inflammation, immune regulation, and cell apoptosis. High A1AT expression has been demonstrated in diverse cancer cell lines, including ovary, colon, lung, and cervical cancer cell lines.[29] New studies have begun to characterize the way that A1AT binds to HDL and how SR-B1 may be involved in A1AT uptake by binding and internalizing HDL in pulmonary endothelial cells.[30,31] Meanwhile, clinical research has revealed that the A1AT level in the serum of patients with lung cancer was significantly higher compared with healthy patients.[32,33] And A1AT is an essential protein for the migration and invasion of lung adenocarcinoma cells.[34] Hence, we inferred that SR-B1 could facilitate A1AT transfer from HDL to lung adenocarcinoma cells, which may be one of the mechanisms involved in tumorigenesis.
Early clinical studies have already suggested that patients with lung adenocarcinoma frequently overexpress epidermal growth factor receptor (EGFR), a 170 kD receptor tyrosine kinase (TK).[35] Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) are a class of novel biologically targeted agents widely used in lung adenocarcinoma. Activating the EGFR mutation could improve progression-free survival (PFS) and overall survival (OS).[36] Yang et al[27] reported that a low level of HDL-C could be used as a prognostic factor for lung adenocarcinoma patients treated with EGFR-TKI. As SR-B1 is a well-characterized HDL receptor involved in the uptake of cholesterol from HDL, we hypothesized that it may be a potential biomarker for lung adenocarcinoma patients treated with EGFR-TKI. This is consistent with our results that SR-B1 is overexpressed in lung adenocarcinoma and that high SR-B1 expression is a poor prognostic factor for lung adenocarcinoma. However, whether SR-B1 may be used as a potential therapeutic target for lung adenocarcinoma requires further investigation.
Currently, biologic carriers are widely used to improve therapeutic efficacy and reduce the adverse effects of anticancer drugs.[37] Reconstituted high density lipoprotein (rHDL), as a drug carrier, has been developed by a receptor (SR-B1) mediated mechanism in several human cancer cells. Zhang et al[38] indicated that reconstituted HDL with paclitaxel (PTX) enhances cellular uptake of PTX mediated by SR-B1 in multidrug-resistant MCF-7 breast cancer cells. PTX-loaded HDL nanoemulsions have been fabricated for the delivery of PTX to improve prostate cancer treatment via a selective (SR-B1 type) uptake mechanism.[39] As SR-B1 is widely overexpressed in lung adenocarcinoma, we suggest that rHDL may be used as a carrier for anticancer drugs, to enhance therapeutic efficacy and diminish drug resistance in lung adenocarcinoma.
This experiment has relative limitations. The company did not provide serum HDL levels in patients with lung adenocarcinoma, which may affect the relationship between SR-B1 expression and prognosis in patients. Secondly, the sample size should be expanded and the patient information should be improved.
In conclusion, we have demonstrated that SR-B1 is overexpressed in lung adenocarcinoma tissues and that high SR-B1 expression correlates significantly with classic clinicopathologic characteristics indicative of more aggressive lung tumors. Moreover, high SR-B1 expression is an independent factor for poor prognosis in lung adenocarcinoma. Accordingly, SR-B1 may be a potential biomarker and therapeutic target for lung adenocarcinoma.
Acknowledgments
This article was completed under the guidance of Professor Han Junqing. He has offered us valuable suggestions in the studies. In addition, I would also like to thank all my friends who helped me in my research process.
Author contributions
Conceptualization: J. Han, M. Wang.
Data curation: H. Feng, J. Ma, J. Han, M. Wang.
Formal analysis: J. Yu, J. Han, M. Wang.
Funding acquisition: C. Wu, H. Feng, J. Han.
Investigation: M. Wang.
Methodology: H. Feng, J. Ma, J. Han, M. Wang.
Project administration: C. Wu, H. Feng.
Resources: C. Wu, J. Ma.
Software: C. Wu, J. Yu, M. Wang.
Supervision: D. Wang.
Validation: D. Wang, J. Yu.
Visualization: D. Wang.
Writing – original draft: J. Ma, M. Wang.
Writing – review & editing: M. Wang.
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, A1AT = alpha-1 antitrypsin, EGFR-TKIs = epidermal growth factor receptor-tyrosine kinase inhibitors, HDL-C = high-density lipoprotein-cholesterol, HRs = hazard ratios, IRS = immunoreactive score, NSCLC = non-small cell lung carcinoma, OS = overall survival, PBS = phosphate-buffered saline, PFS = progression-free survival, PTX = paclitaxel, rHDL = reconstituted high density lipoprotein, SABC = streptavidin avidin biotin peroxidase complex, SR-B1 = scavenger receptor class B type I, TNM = tumor-node-metastasis.
HF and MW contributed equally to this study.
This work was supported by grants from the National Natural Science Foundation of China (No. 81201865), Natural Science Foundation of Shandong Province (ZR2017QH004), and the key research and development projects of Shandong Province (No. 2015GSF118169).
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Huang JY, Jian ZH, Nfor ON, et al. The effects of pulmonary diseases on histologic types of lung cancer in both sexes: a population-based study in Taiwan. BMC Cancer 2015;15:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Butler KM, Rayens MK, Wiggins AT, et al. Association of smoking in the home with lung cancer worry, perceived risk, and synergistic risk. Oncol Nurs Forum 2017;44:E55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lung cancer. Breathe (Sheff) 2016;12:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McMahon KM, Foit L, Angeloni NL, et al. Synthetic high-density lipoprotein-like nanoparticles as cancer therapy. Cancer Treat Res 2015;166:129–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cruz PM, Mo H, McConathy WJ, et al. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol 2013;4:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gordon EM, Figueroa DM, Barochia AV, et al. High-density lipoproteins and apolipoprotein A-I: potential new players in the prevention and treatment of lung disease. Front Pharmacol 2016;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chi PD, Liu W, Chen H, et al. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS One 2014;9:e91080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Acton SL, Scherer PE, Lodish HF, et al. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem 1994;269:21003–9. [PubMed] [Google Scholar]
- [10].Rhainds D, Bourgeois P, Bourret G, et al. Localization and regulation of SR-BI in membrane rafts of HepG2 cells. J Cell Sci 2004;117(pt 15):3095–105. [DOI] [PubMed] [Google Scholar]
- [11].Valacchi G, Sticozzi C, Lim Y, et al. Scavenger receptor class B type I: a multifunctional receptor. Ann N Y Acad Sci 2011;1229:E1–7. [DOI] [PubMed] [Google Scholar]
- [12].Landschulz KT, Pathak RK, Rigotti A, et al. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest 1996;98:984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zheng Y, Liu Y, Jin H, et al. Scavenger receptor B1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by HDL-mimetic nanoparticles. Theranostics 2013;3:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol 2008;295:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wadsack C, Hirschmugl B, Hammer A, et al. Scavenger receptor class B, type I on nonmalignant and malignant human epithelial cells mediates cholesteryl ester-uptake from high density lipoproteins. Int J Biochem Cell Biol 2003;35:441–54. [DOI] [PubMed] [Google Scholar]
- [16].Gutierrez-Pajares JL, Ben Hassen C, Chevalier S, et al. SR-BI: linking cholesterol and lipoprotein metabolism with breast and prostate cancer. Front Pharmacol 2016;7:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mooberry LK, Sabnis NA, Panchoo M, et al. Targeting the SR-B1 receptor as a gateway for cancer therapy and imaging. Front Pharmacol 2016;7:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li J, Wang J, Li M, et al. Up-regulated expression of scavenger receptor class B type 1 (SR-B1) is associated with malignant behaviors and poor prognosis of breast cancer. Pathol Res Pract 2016;212:555–9. [DOI] [PubMed] [Google Scholar]
- [19].Yuan B, Wu C, Wang X, et al. High scavenger receptor class B type I expression is related to tumor aggressiveness and poorprognosis in breast cancer. Tumour Biol 2016;37:3581–8. [DOI] [PubMed] [Google Scholar]
- [20].Shields PG. Molecular epidemiology of smoking and lung cancer. Oncogene 2002;21:6870–6. [DOI] [PubMed] [Google Scholar]
- [21].Siemianowicz K, Gminski J, Stajszczyk M, et al. Serum HDL cholesterol concentration in patients with squamous cell and small cell lung cancer. Int J Mol Med 2000;6:307–11. [DOI] [PubMed] [Google Scholar]
- [22].Dessi S, Batetta B, Pulisci D, et al. Altered pattern of lipid metabolism in patients with lung cancer. Oncology 1992;49:436–41. [DOI] [PubMed] [Google Scholar]
- [23].Fiorenza AM, Branchi A, Sommariva D. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int J Clin Lab Res 2000;30:141–5. [DOI] [PubMed] [Google Scholar]
- [24].Umeki S. Decreases in serum cholesterol levels in advanced lung cancer. Respiration 1993;60:178–81. [DOI] [PubMed] [Google Scholar]
- [25].Kucharska-Newton AM, Rosamond WD, Schroeder JC, et al. HDL-cholesterol and the incidence of lung cancer in the Atherosclerosis Risk in Communities (ARIC) study. Lung Cancer 2008;61:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Borgquist S, Butt T, Almgren P, et al. Apolipoproteins, lipids and risk of cancer. Int J Cancer 2016;138:2648–56. [DOI] [PubMed] [Google Scholar]
- [27].Lv Y, Miao LY, Chen QF, et al. Monitoring of high-density lipoprotein cholesterol level is predictive of EGFR mutation and efficacy of EGFR-TKI in patients with advanced lung adenocarcinoma. Onco Targets Ther 2016;9:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Saddar S, Carriere V, Lee WR, et al. Scavenger receptor class B type I is a plasma membrane cholesterol sensor. Circ Res 2013;112:140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shakya R, Tarulli GA, Sheng L, et al. Mutant p53 upregulates alpha-1 antitrypsin expression and promotes invasion in lung cancer. Oncogene 2017;36:4469–80. [DOI] [PubMed] [Google Scholar]
- [30].Lockett AD, Petrusca DN, Justice MJ, et al. Scavenger receptor class B, type I-mediated uptake of A1AT by pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 2015;309:L425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Janciauskiene S, Welte T. Well-known and less well known functions of alpha-1 antitrypsin. Its role in chronic obstructive pulmonary disease and other disease developments. Ann Am Thorac Soc 2016;13(suppl 4):S280–288. [DOI] [PubMed] [Google Scholar]
- [32].Daddi G, Mancini PA, Parola D, et al. Alfa-antitrypsin increase in lung cancer. Boll Ist Sieroter Milan 1976;55:510–2. [PubMed] [Google Scholar]
- [33].Di Martino G, di Matteo L, De Bellis G, et al. Serum levels of GPI, AAT and CEA in primary lung neoplasms and chronic bronchopneumopathies. Boll Ist Sieroter Milan 1979;58:344–55. [PubMed] [Google Scholar]
- [34].Chang YH, Lee SH, Liao IC, et al. Secretomic analysis identifies alpha-1 antitrypsin (A1AT) as a required protein in cancer cellmigration, invasion, and pericellular fibronectin assembly for facilitating lung colonization of lung adenocarcinoma cells. Mol Cell Proteomics 2012;11:1320–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Arteaga CL. Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol 2002;29(5 suppl 14):3–9. [DOI] [PubMed] [Google Scholar]
- [36].Huang CH, Powers BC. The evolving role of maintenance therapy using epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in the management of advanced non-small-cell lung cancer. Clin Med Insights Oncol 2012;6:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Danhier F, Vroman B, Lecouturier N, et al. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release 2009;140:166–73. [DOI] [PubMed] [Google Scholar]
- [38].Zhang F, Wang X, Xu X, et al. Reconstituted high density lipoprotein mediated targeted co-delivery of HZ08 and paclitaxel enhances the efficacy of paclitaxel in multidrug-resistant MCF-7 breast cancer cells. Eur J Pharm Sci 2016;92:11–21. [DOI] [PubMed] [Google Scholar]
- [39].Mooberry LK, Nair M, Paranjape S, et al. Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. J Drug Target 2010;18:53–8. [DOI] [PubMed] [Google Scholar]


