Abstract
An Arabidopsis cDNA (AtPGMp) encoding the plastidic phosphoglucomutase (PGM) predicted a 623-amino acid protein with an N-terminal sequence typical of a plastid signal peptide. Expression of a recombinant protein in Escherichia coli confirmed its enzyme activity. The recombinant enzyme had an apparent Km value of 98.5 μm and a Vmax of 4.48 μmol min−1 (mg protein)−1. The Calvin cycle intermediates fructose-1,6-bisphosphate and ribulose-1,5-bisphosphate exerted an inhibitory effect on PGM activity, supporting its proposed involvement in controlling photosynthetic carbon flow. A point mutation was identified in the AtPGMp gene of the Arabidopsis pgm-1 mutant. The mutation in the mutant transcript generated a stop codon at about one third of the wild-type open reading frame, and thus rendered the polypeptide nonfunctional. Storage lipid analysis of the pgm-1 mutant seeds showed a 40% reduction in oil content compared with that of wild type. Our results indicate that plastidic PGM is an important factor affecting carbon flux in triacylglycerol accumulation in oilseed plants, most likely through its essential role in starch synthesis.
Phosphoglucomutase (PGM, EC 2.7.5.1) is a widely distributed enzyme that catalyzes the readily reversible interconversion of Glc-1-P and Glc-6-P. There are two PGM isoforms in plants, one localized in the plastids and the other in the cytosol (Muhlbach and Schnarrenberger, 1978). Both isoforms require Glc-1,6-bisphosphate (Glc-1,6-bisP) as a cofactor and the enzymes have been shown to be phosphoproteins in vivo (Salvucci et al., 1990). The cytosolic isoform is involved in Suc catabolism to provide intermediates for glycolysis and substrate for the synthesis of a variety of cellular constituents (Manjunath et al., 1998). The plastidic PGM is essential for starch synthesis to store net photosynthate in leaves during the day (Dietz, 1987), and also plays an essential role in the degradation of assimilatory starch (Hattenbach and Heineke, 1999). Studies with spinach chloroplasts indicated that PGM activity may be regulated by light, stromal pH, and Glc-1,6-bisP concentration (Sicher, 1989).
Plastidic PGM mutants have been isolated from Arabidopsis (Caspar et al., 1985), Nicotiana sylvestris (Hanson and McHale, 1988), and pea (Pisum sativum) (Harrison et al., 1998). Deficiency in the plastidic PGM activity resulted in a “starchless” phenotype in Arabidopsis and N. sylvestris. The P. sativum PGM mutant, rug3, has a wrinkled seed phenotype with only 1% of the seed dry weight as starch, compared with 60% in the wild type (Harrison et al., 1998). The reduction on starch synthesis in rug3 is also accompanied by a significant increase in seed lipid content, and a decreased legumin to vicilian ratio (Casey et al., 1998). Both the Arabidopsis and the N. sylvestris mutants accumulate relatively high levels of soluble carbohydrates in the leaf and stem tissue (Huber and Hanson, 1992). The increase in sugar concentration in rug3 seeds is believed to affect the accumulation of storage proteins, at least in part through changes in the osmotic status of the developing seeds (Casey et al., 1998). These studies indicated that plastidic PGM not only plays an essential role in starch synthesis, but also has a significant impact on the deposition of other storage products in seeds.
The synthesis of a number of commercially important products in seeds, including starch and fatty acids, is restricted to the same intracellular compartment, namely, the plastids. In Brassicaceae, Glc-6-P has been demonstrated to be an efficient substrate imported by the plastids for both starch and fatty acid synthesis (Kang and Rawsthorne, 1994; Harrison et al., 1998). The carbon precursors supplied from the cytosol are usually shared and competed for by various pathways. Due to the readily reversible nature of the PGM reaction, the plastidic enzyme was generally considered not to be involved in metabolic control. However, studies with amyloplasts of developing wheat endosperm indicated that the ratio of Glc-6-P to Glc-1-P may be significantly displaced from the equilibrium predicted by the PGM reaction, leading to the conclusion that PGM is pivotal in partitioning carbon between starch synthesis and carbohydrate oxidation in heterotrophic tissues (Tetlow et al., 1998). In this paper, we report the identification of a cDNA encoding the plastidic PGM (PGMp) from Arabidopsis and describe the properties of a recombinant enzyme expressed in Escherichia coli. Using the Arabidopsis PGM mutant pgm-1, we present data to show that plastidic PGM is a crucial factor affecting seed oil content, which thus supports the concept that accumulated starch in young embryos plays an important role in providing carbon resources for seed storage lipid biosynthesis in oilseed plants.
MATERIALS AND METHODS
Plant Materials
The Arabidopsis (L.) Heynh. PGM mutant, pgm-1, was obtained from the Arabidopsis Biological Resource Center (The Ohio State University, Columbus). Both the wild-type (ecotype Columbia) and the mutant Arabidopsis were grown in controlled-environment chambers at 20°C to 22°C under long-day conditions (16 h of cool-white fluorescent light). Leaf, shoot, flower, and silique tissues for northern analysis were sampled from plants growing in soil. Root material was collected from seedlings growing on the surface of vertically positioned plates with ½-strength Murashige and Skoog medium (Murashige and Skoog, 1962).
DNA Sequencing and Sequence Analysis
Double-strand sequencing of plasmid or PCR-amplified DNA was performed on an Automated Sequencer (PE-Applied Biosystems, Foster City, CA) by the DNA Technology Group of the Plant Biotechnology Institute, National Research Council of Canada. Sequences were analyzed using DNASTAR software (DNASTAR, Madison, WI), and databank searches were conducted through the BLAST program (Altschul et al., 1990).
Identification of the cDNA and PCR Cloning of the Gene
Arabidopsis expressed sequence tags (ESTs) with sequence similarity to the rabbit muscle PGM1 were identified from BLAST database searches. Clone G3G5T7 (accession no. N96760) was obtained from the Arabidopsis Resources Center and fully sequenced. Two primers, TGGCAGGAGAGGAATTTGGGCTTCAATAAG and GGAACAAGGGAGATATGTGCTTAATCGAGAG, designed according to the sequence of the At-PGMp gene, were synthesized to perform PCR amplification of the DNA fragments encompassing the PGM gene by using wild-type and pgm-1 Arabidopsis genomic DNA as templates. The PCR products were purified with a PCR purification kit (QIAquick, Qiagen USA, Valencia, CA) and sequenced. The point mutation in the pgm-1 gene was identified by comparing sequences of the PCR products from the wild type and the pgm-1 mutant.
Escherichia coli Expression and Purification of the Recombinant Enzyme
A primer (TTGGAATTCAGCATTGAGATTAAATCGTTG) designed according to sequences surrounding the putative signal peptide cleavage site of the At-PGMp and the T7 primer (located on the cDNA cloning vector) were used in a PCR amplification of the cDNA to generate a fragment that has an EcoRI restriction site at both ends. This fragment was subsequently gel-purified and inserted in frame into the EcoRI site of the pMALC2 vector (New England Biolabs, Beverly, MA) to generate the chimeric construct encoding for a fusion protein between the maltose-binding protein and the At-PGMp omitting the putative signal peptide. The integrity of the construct was confirmed through sequencing. An E. coli (PGM) mutant (Adhya and Schwartz, 1971) defective in PGM activity was obtained from the E. coli Stock Center (Department of Biology, Yale University, New Haven, CT). Transformation of E. coli was performed according to standard protocols (Sambrook et al., 1989). Induction of the At-PGMp expression was achieved with 1 mm isopropylthio-β-galactoside for 5 h. The bacterial cells were pelleted through centrifugation and stored at −80°C. For enzyme purification, frozen cells were resuspended in 2 mL of buffer containing 20 mm Tris-HCl, pH 7.4, 0.2 m NaCl, 10 mm β-mercaptoethanol, and 1 mm EDTA. The cells were then disrupted by pulsed sonication at 4°C, and cell debris were removed by centrifugation. The purification of the recombinant enzyme was conducted with an amylose column according to the manufacturer's protocol (New England Biolabs). The protein concentration in the purified recombinant protein preparation was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA), where lyophilized bovine plasma γ-globulin was used as a standard.
Enzyme Assays
Activity of the purified recombinant enzyme was measured based on conditions described by Hattenbach and Heineke (1999). The standard reaction mixture contained 20 mm imidazole (pH 7.85), 10 mm MgCl2, 3 mm EDTA, 0.1 mm Glc-1,6-bisP, 0.8 unit torrela yeast Glc-6-P dehydrogenase (Sigma), 0.5 mm NADP+, and a suitable amount of enzyme protein in a total volume of 1 mL. Fru-1,6-bis P (FBP) and Glc-1-P were added, as described in the figures. Enzyme activity was determined by measuring the rate of increase in A340 due to NADPH formation from NADP+ using a spectrophotometer (DU-65, Beckman Instruments, Fullerton, CA). Kinetic constants were determined from the Lineweaver-Burk plot using the Grafit (Leatherbarrow, 1990) program.
Southern and Northern Analysis
Genomic DNA was prepared from young green leaves of Arabidopsis (ecotype Columbia) as described by Dellaporta et al. (1983). Genomic DNA (15 μg) was digested with the restriction enzymes ClaI, ScaI, and SpeI, respectively. A 32P-labeled DNA probe was prepared using a DNA labeling system (Random Primers, Life Technologies/Gibco-BRL, Cleveland). Hybridization and high-stringency washing were carried out at 65°C, as suggested by the manufacturer.
Total RNA was extracted as described by Wilkins and Smart (1996). RNA (20 μg) was run in a 1.2% (w/v) agarose-formaldehyde gel as described previously (Sambrook et al., 1989). rRNA bands, visualized by ethidium bromide staining, were used as a loading control. Gels were blotted onto Zeta-Probe membranes (Bio-Rad), and high-stringency hybridization and washings were performed according to the manufacturer's protocol.
Seed Lipid Analysis
Using intact seeds harvested from wild-type and Arabidopsis pgm-1 mutant plants grown under identical conditions, oil content was determined by gas chromatography of fatty acid methyl esters prepared from a total lipid extract and by 1H-NMR analysis, both described by Zou et al. (1997).
RESULTS
Identification of the At-PGMp cDNA
Through homology searches of the EST databases using the coding sequence of the rabbit muscle PGM (accession no. 548497), we identified several Arabidopsis EST clones with putative reading frames that shared homology with sequences of known PGMs. Upon sequencing, one of the EST clones (clone G3G5T7, accession no. N96760) was founded to represent a nearly full-length cDNA (deposited into GenBank under accession no. AJ242601). The first ATG triplet of this 2,075-bp cDNA was designated as the translation start site because the surrounding sequence (AACAATGACT) agrees well with the general consensus sequence (AACAATGGC) flanking plant initiator codons (Lutcke et al., 1987). Moreover, an in-frame stop codon is found 45 nucleotides upstream of the putative translation initiation codon. The translation of the cDNA predicted an open reading frame encoding a 623-amino acid protein with a calculated Mr of 68,039. The first 65 amino acids of the protein are rich in Ser (23%) and Thr (12%) with an estimated pI of 10.5. These properties are common features of the transit peptides of many chloroplast proteins (Heijne et al., 1989). Although we could not precisely predict the signal peptide cleavage site, amino acid sequence alignment with other PGMs suggested that the mature protein most likely starts at amino acid 66. If this is true, the molecular mass of the mature protein would be 61 kD, very close to the reported size (62 kD) of the tobacco PGMp (Salvucci et al., 1990). These results suggest that the cDNA encodes for a plastidic PGM, and we therefore named it At-PGMp (for Arabidopsis plastidic PGM isoform).
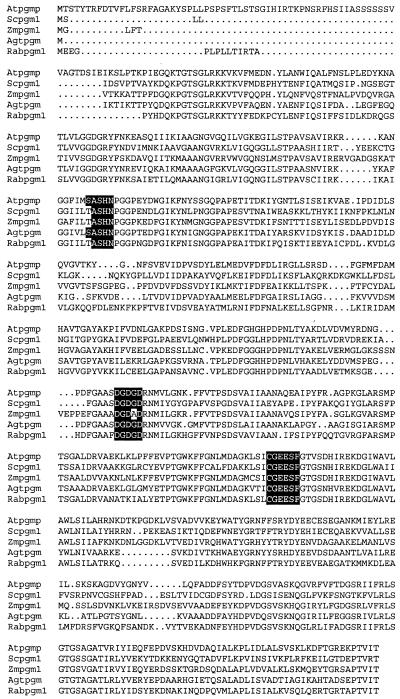
The sequence alignment of At-PGMp with PGMs from other eukaryotes and prokaryotes is shown in Figure 1. At-PGMp also exhibited about 50% sequence identity at the amino acid level to the cytosolic PGM from Arabidopsis (accession no. AJ242650, C. Periappuram and J. Zou, unpublished data). The putative phosphorylation site is most likely to be Ser-181, surrounding which is the catalytic reaction center (Ser-Ala-Ser-181-His-Asn). A metal-binding site (Asp-Gly-Asp-Gly-Asp) and the Glc ring-binding site (Cys-Gly-Glu-Glu-Ser-Phe) are also located in the most conserved regions (Dai et al., 1992) of the sequence alignment.
Figure 1.
Amino acid sequence alignment of AtPGMp with PGMs from other species. Arabidopsis (Atpgm; AJ242601), Zea mays cytosolic PGM (Zpgm1; U89342), Saccharomyces cerevisiae (Scpgm1; P33401), rabbit muscle (Rabpgm1; M97664), and Agrobacterium tumefaciens (Agtpgm; 400331). The most conserved regions, such as the catalytic reaction center (Ser-Ala-Ser-His-Asn), metal-binding site (Asp-Gly-Asp-Gly-Asp), and Glc-ring-binding site (Cys-Gly-Glu-Glu-Ser-Phe), are highlighted.
A spinach cDNA clone identified through complementation of a yeast hexokinase mutant had previously been reported as a plastidic PGM cDNA based on sequence homology with other PGMs and a transit peptide sequence (Penger et al., 1994). However, our sequence comparison revealed very limited sequence identity (13%) between At-PGMp and the putative spinach plastidic PGM, although certain degree of sequence conservation was observed in the putative functional domains (Manjunath et al., 1998).
The Arabidopsis pgm-1 Mutant Contains a Nonsense Mutation in the At-PGMp Gene
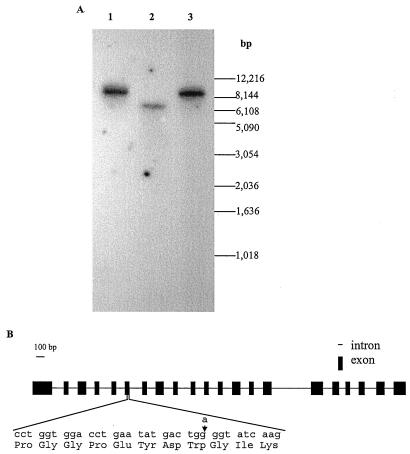
The Arabidopsis mutant pgm-1, which was isolated by Caspar et al. (1985), has a single recessive nuclear mutation mapped at cM 63 on chromosome V (http://aims.cps.msu.edu/aims/menu/catalog.html). Histochemical localization following resolution of PGM isozymes by electrophoresis in starch gels indicated that pgm-1 completely lacked activity of the plastidic isoform (Caspar et al., 1985). This is in accordance with our Southern analysis data, which indicated that At-PGMp is most likely present as a single-copy gene in the Arabidopsis genome (Fig. 2A). Our database search results revealed that a GenBank entry (accession no. AB010074) generated from sequencing of P1 clone MIO24 on chromosome V (Y. Nakamura, Kazusa DNA Research Institute, Laboratory of Gene Structure 2; 1532–3, Yana, Kisarazu, Chiba 292, Japan) contained the At-PGMp gene. Alignment of the cDNA with the genomic sequence revealed that the At-PGMp gene has 21 exons spanning in a region of about 4.5 kb (Fig. 2B). To elucidate the nature of the mutation, we PCR amplified the At-PGMp allele from pgm-1. Upon sequencing of the product, a point mutation was identified in exon 6 of the At-PGMp gene in pgm-1. Sequencing results from several independent PCR reactions, along with wild-type genomic DNA as a control, excluded the possibility that the mutation was a PCR artifact. The point mutation was a transition from TGG (codon 192 encoding for Trp-192) to TGA (stop codon), so that the translation of the transcript is truncated at about one-third of the wild-type open reading frame, thus rendering the polypeptide nonfunctional.
Figure 2.
A, Genomic Southern-blot analysis. Lane 1, ClaI; lane 2, ScaI; and lane 3, SpeI. B, The structure of AtPGMp gene, the point mutation in the Arabidopsis pgm-1 mutant is an A to G substitution at the third nucleotide of codon 179 (TGG) encoding for Trp-192.
Biochemical Characterizations of a Recombinant Enzyme
To biochemically verify that At-PGMp indeed encodes for a PGM, the At-PGMp cDNA omitting the region encoding for the first 65 amino acids predicted as the plastid targeting signal was inserted into an E. coli expression vector, pMALC2 (New England Biolabs). The recombinant protein encoded by this expression vector would have a predicted size of approximately 100 kD with the maltose-binding domain at its N terminus. An E. coli mutant (Adhya and Schwartz, 1971) defective in PGM activity was transformed with the fusion construct to determine its ability to generate PGM activity. After isopropylthio-β-galactoside induction, SDS-PAGE was run to confirm production of the recombinant protein (data not shown). The lysate of the E. coli transformed with the fusion construct exhibited a PGM activity of 19.1 nmol min−1 mg−1 protein when assayed in the presence of 2 mm Glc-1-P and 8 μm Glc-1,6-bisP. The control lysate (E. coli mutant harboring vector alone) did not display significant activity (0.6 nmol min−1 mg−1 protein) over the background.
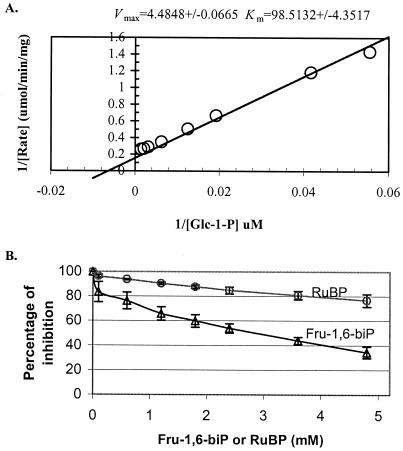
To further characterize the biochemical properties of the recombinant enzyme, the fusion protein was purified to apparent homogeneity through an amylose column (the homogeneity was subsequently confirmed by SDS-PAGE, data not shown). The PGM assay conditions were adopted from the method described by Hattenbach and Heineke (1999) with 0.1 mm Glc-1,6-bisP, an obligate cofactor for PGM. Figure 3A shows the determination of Km and Vmax values for Glc-1-P with the purified recombinant enzyme. An apparent Km value of 98.5 μm and a Vmax of 4.5 μmol min−1 mg−1 protein were found through Lineweaver-Burk plot analysis.
Figure 3.
Activity of the purified recombinant AtPGMp. A, Lineweaver-Burk plot showing the dependence of PGM activity on the Glc-1-P concentration in the presence of 0.1 mm Glc-1,6-bisP. B, Inhibition of PGM activity by Fru-1,6-bisP and RuBP.
In photosynthetic tissues, the activity of the plastidic PGM links Calvin cycle metabolism with starch metabolism. Under conditions where the Calvin cycle activity is restricted, the PGM activity is inhibited by the increased concentration of Calvin cycle intermediates, and thus the carbon exchange between the Calvin cycle and starch turnover is limited (Hattenbach and Heineke, 1999). We therefore measured PGM activity of the recombinant enzyme in the presence of various concentrations of FBP and ribulose-1,5-bisphosphate (RuBP). Consistent with the results assayed with a suspension of isolated chloroplasts from spinach leaves (Hattenbach and Heineke, 1999), the activity of the recombinant PGM was reduced as much as 50% in the presence of 2.4 mm FBP (Fig. 3B). RuBP exerted a similar inhibition effect, albeit to a lesser extent.
Gene Expression of At-PGMp
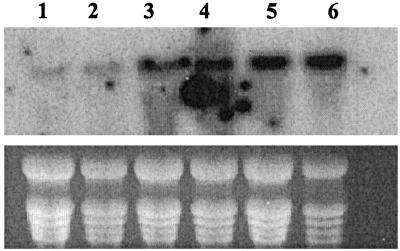
To analyze the tissue distribution of the At-PGMp transcript, a northern blot of total RNA isolated from several tissues was probed with the cDNA clone. A hybridization band corresponding to a transcript of approximately 2 kb was observed in all tissues examined, of which the germinating seeds and young seedlings had the highest level of transcript signal (Fig. 4). The At-PGMp transcript was also abundant in flowers and developing siliques. Interestingly, fully expanded leaves had the lowest level of the steady-state transcript. We observed no effect on the accumulation of the transcript by light; neither was there any diurnal difference in the transcript level (data not shown).
Figure 4.
Northern-blot analysis of At-PGMp gene expression profiles. About 20 μg of total RNA was loaded in each lane. Lane 1, Root; lane 2, leaf; lane 3, flower; lane 4, silique; lane 5, seedling; and lane 6, germinating seeds.
Defective Plastidic PGM Reduced Oil Content in Arabidopsis Seeds
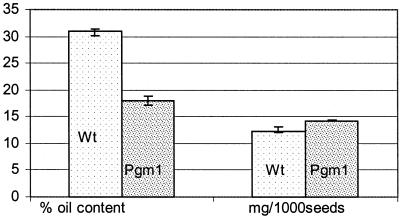
In Brassicaceae, active starch biosynthesis occurs during the early stages of seed development, but the starch is broken down to an undetectable level in mature seeds. It has been proposed that the starch accumulated during early seed development is used to provide a carbon source for subsequent lipid accumulation (Kang and Rawsthorne, 1994). To examine the significance of this transient starch on the accumulation of storage lipids during seed maturation, we conducted analyses of the oil content of the pgm-1 mutant seeds compared with that of wild-type seeds. There was no significant difference in the seed weight between wild type and the pgm-1 mutant growing under identical conditions (Fig. 5). Therefore, the oil content was measured on an equal seed weight basis. As indicated in Figure 5, there is an approximately 40% reduction in the seed oil content in the PGM mutant based on gas chromatography analysis of fatty acid methyl esters prepared from total lipid extracts. Using the non-destructive magic angle spinning spectroscopy NMR technique (Rutar et al., 1989), a similar trend was observed with respect to the decrease of oil content in the PGM mutant seeds (data not shown). Thus, our results indicated that a defective PGM compromises the carbon flux required for storage lipid synthesis, and consequently reduces oil content in oilseed plants, most likely through its essential role in starch accumulation during the early stages of seed development. The non-lipid storage product(s) that compensated for the loss of storage lipid without affecting seed weight was not determined.
Figure 5.
Percentage seed oil content of PGM Arabidopsis mutant (Pgm-1) and Columbia wild type (wt). Comparison of seed weight in milligrams per 1,000 seeds is also shown.
DISSCUSION
We provide molecular, genetic, and biochemical data to identify and characterize the plastidic PGM cDNA from Arabidopsis, which displays high sequence homology to the cytosolic isoform. This was not unexpected, since previous studies have indicated that the two PGM isoforms have many properties in common, and the plant PGM is very similar to those from a variety of non-plant organisms (Muhlbach and Schnarrenberger, 1978). Plastidic PGM is involved in almost every phase of plant growth and development, including coupling Calvin cycle in source tissues, channeling carbohydrate in sink tissues, and contributing to plastid sedimentation in gravitropism responses in roots (Vitha et al., 1998). The ubiquitous expression pattern of the At-PGMp gene signifies its essential role in plant primary metabolism.
In light of the high degree of sequence conservation, our results also question the identity of a spinach cDNA previously reported as encoding for a PGM. Sequence alignment indicated that this putative PGM exhibited a very limited sequence identity with both the AtPGMp (13%) and the cytosolic PGM (19%) (Manjunath et al., 1998). Although sequence alignment itself would not be sufficient to either confirm or rule out the function of a protein, this level of sequence conservation is uncharacteristically low, especially when one considers the high sequence identity observed among PGMs from plant and non-plant species. The conservation of the functional domains, on the other hand, does suggest a related enzyme activity. Our sequence search with the deduced amino acid sequence of the spinach cDNA indicated that it was more closely related to phosphomannomutase (PMM) than to PGM. In fact, the top 20 entries from the BLAST search with the putative spinach PGM were all sequences of PMM. This putative PGM had lower sequence identity to E. coli PGM1 than to PGM2, the latter of which is also believed to code for a PMM. It is therefore more likely that the spinach cDNA encodes for a PMM instead of a PGM. However, the true function of the spinach protein awaits further unequivocal biochemical confirmation.
Enzymes such as PGM, catalyzing readily reversible steps, have generally not been considered to be involved in metabolic control. However, recent studies demonstrate that control of principal pathways is generally distributed throughout a pathway (Haake et al., 1998). The biochemical properties of the recombinant PGM are consistent with previous studies (Hattenbach and Heineke, 1999) on a partially purified enzyme indicating that physiological concentrations of FBP and RuBP, both intermediates of the Calvin cycle, exert control over PGM activity. Our results support the model proposed by Hattenbach and Heineke (1999) that, at least under certain conditions, the plastidic PGM has regulatory properties, suggesting that it could be an important control point in photosynthetic carbon flow.
In sink tissues, in addition to the apparent lack of starch, mutants defective in plastidic PGM also exhibited dramatic changes in the accumulation of other storage products. The importance of the plastidic PGM in seed storage product accumulation was previously demonstrated in a pea mutant that had reduced seed carbohydrate content and altered storage protein profiles (Casey et al., 1998). In a species such as pea, in which mature seeds accumulate significant amounts of both starch and storage lipid, mutants with lower starch content normally have elevated level of storage lipids. This negative correlation between starch and oil accumulation in such species is readily explicable, because starch and fatty acid biosynthesis share common substrates (e.g. Glc-6-P) and are localized in the same plastidic compartment (Harrison et al., 1998).
Studies with intact amyloplasts isolated from cauliflower have also demonstrated that rising rates of starch synthesis are correlated with a significant decrease of fatty acid synthesis (Mohlmann et al., 1994), but the interactions of these two biosynthesis pathways in Brassicaceae seeds are dynamic and developmentally dependent. Active starch biosynthesis has been well documented during the early stages of seed development (Kang and Rawsthorne, 1994), but a negligible amount of starch is present in the mature seeds. In fact, starch accounts for approximately 8% to 10% of the dry weight of developing embryos during early cotyledon filling in Brassica napus (Da Silva et al., 1997). It was proposed that one of the potential functions of the transient starch is to provide a carbon source for lipid synthesis during the embryo maturation process (Da Silva et al., 1997); however, no direct evidence has been reported to confirm this hypothesis.
Our oil content analysis results showed that the Arabidopsis pgm-1 mutant has a significantly lower oil content in its seeds. A plausible explanation for this phenotype would be that, due to its inability to synthesize starch during early seed development, the developing seeds of the mutant have a smaller pool of carbon resources required to sustain lipid synthesis during oil deposition. Alternatively, although Glc-6-P is the preferred hexose phosphate taken up by the plastids for carbohydrate storage product biosynthesis, it is possible that Glc-1-P still enters plastids in the sink tissues of Arabidopsis at a lower level and may contribute carbon resources for lipid synthesis. There is also the possibility that the pgm-1 mutant has reduced assimilate translocation to the developing seed, and thus reduced oil content. Our data suggest that one consequence of blocking the conversion of G-1-P to Glc-6-P in the PGM mutant is a restriction in carbon flow toward fatty acid synthesis, which at least partially results in a reduction in seed oil content. Our study adds another angle to the understanding of the complexity of carbon partitioning in oilseed plants.
ACKNOWLEDGMENTS
We are grateful to the Arabidopsis Biological Resource Center for providing the Arabidopsis EST clones and pgm-1 mutant, and the E. coli Stock Center at Yale University for the E. coli pgm mutant. We thank Barry Panchuk, Don Schwab, and Dr. Larry Pelcher of the Plant Biotechnology Institute DNA Technologies Unit for DNA sequencing and primer synthesis. The authors also thank Brenda Lougheed for technical advice and Drs. W. Keller, Q. Qi, P. Covello, and S. Abrams for helpful discussions during the course of this work.
Footnotes
This is National Research Council of Canada publication no. 43,782.
LITERATURE CITED
- Adhya S, Schwart M. Phosphoglucomutase mutants of Escherichia coli K-12. J Bacteriol. 1971;108:621–626. doi: 10.1128/jb.108.2.621-626.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bettey M, Smith AM. Nature of the effect of the γ-locus on the lipid content of embryos of peas (Pisum sativum L) Planta. 1990;180:420–428. doi: 10.1007/BF00198795. [DOI] [PubMed] [Google Scholar]
- Casey R, Domoney C, Forster C, Hedley C, Hitchin E, Wang T. The effect of modifying carbohydrate metabolism on seed protein gene expression in peas. J Plant Physiol. 1998;152:636–640. [Google Scholar]
- Caspar T, Huber SC, Somerville C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985;79:11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J-B, Liu Y, Ray WJ, Jr, Konno M. The crystal structure of muscle phosphoglucomutase refined at 2.7-angstrom resolution. J Biol Chem. 1992;267:6322–6337. [PubMed] [Google Scholar]
- Da Silva PMFR, Eastmond PJ, Hill LM, Smith AM, Rawsthorne S. Starch metabolism in developing embryos of oilseed rape. Planta. 1997;203:480–487. [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Dietz KJ. Control function of hexosemonophosphate isomerase and phosphoglucomutase in starch synthesis in leaves. In: Biggins J, editor. Proceedings of the 7th International Congress on Photosynthesis. Vol. 3. The Hague, The Netherlands: Martinius/Nijhoff/Dr. Junk Publishers; 1987. pp. 329–332. [Google Scholar]
- Haake V, Zrenner R, Sonnewald U, Stitt M. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and star, and inhibits growth of potato plants. Plant J. 1998;14:147–157. doi: 10.1046/j.1365-313x.1998.00089.x. [DOI] [PubMed] [Google Scholar]
- Hanson KR, McHale NA. A starchless mutant of Nicotiana sylvestris containing a modified plastid phosphoglucomutase. Plant Physiol. 1988;88:838–844. doi: 10.1104/pp.88.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Hedley CL, Wang TL. Evidence that the rug3 locus of pea (Pisum sativum L.) encodes plastidial phosphoglucomutase confirms that the imported substrate for starch synthesis in pea amyloplasts is glucose-6-phosphate. Plant J. 1998;13:753–762. [Google Scholar]
- Hattenbach A, Heineke D. On the role of chloroplastic phosphoglucomutase in the regulation of starch turn over. Planta. 1999;207:527–532. [Google Scholar]
- Heijne GV, Steppuhn J, Herrmann RG. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- Huber SC, Hanson KR. Carbon partitioning and growth of a starchless mutant of Nicotiana sylvestris. Plant Physiol. 1992;99:1449–1454. doi: 10.1104/pp.99.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang F, Rawsthorne S. Starch and fatty acid synthesis in plastids from developing embryos of oilseed rape (Brassica napus L.) Plant J. 1994;6:795–805. [Google Scholar]
- Leatherbarrow . GraFit. Ed 3.0. Staines, UK: Erithacaus Software; 1990. [Google Scholar]
- Lutcke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Scheele GA. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987;6:43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath S, Lee CHK, VanWinkle P, Bailey-Serres J. Molecular and biochemical characterization of cytosolic phosphoglucomutase in maize expression during development and in response to oxygen deprivation. Plant Physiol. 1998;117:997–1006. doi: 10.1104/pp.117.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlmann T, Scheibe R, Neuhaus HE. Interaction between fatty-acid and starch synthesis in isolated amyloplasts from cauliflower floral buds. Planta. 1994;194:492–497. [Google Scholar]
- Muhlbach H, Schnarrenberger C. Properties and intercellular distribution of two phosphoglucomutases from spinach leaves. Planta. 1978;141:65–70. doi: 10.1007/BF00387746. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Murphy DJ, Cummins I. Biosynthesis of seed storage products during embryogenesis in rapeseed, Brassica napus. J Plant Physiol. 1989;135:63–69. [Google Scholar]
- Penger A, Pelzer-Reith B, Schnarrenberger C. cDNA sequence for the plastidic phosphoglucomutase from Spinacia oleracea (L) Plant Physiol. 1994;105:1439–1440. doi: 10.1104/pp.105.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutar V. Magic angle sample spinning NMR spectroscopy of liquids as a non-destructive method for studies of plant seeds. J Agric Food Chem. 1989;37:67–70. [Google Scholar]
- Salvucci ME, Drake RR, Broadbent KP, Haley BE, Hanson KR, McHale NA. Identification of the 64-kilodalton chloroplast stromal phosphoprotein as phosphoglucomutase. Plant Physiol. 1990;93:105–109. doi: 10.1104/pp.93.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sicher RC. Evidence for a light dependent increase of phosphoglucomutase activity in isolated, intact spinach chloroplasts. Plant Physiol. 1989;89:557–563. doi: 10.1104/pp.89.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow IJ, Blissett KJ, Emes MJ. Metabolite pools during starch synthesis and carbohydrate oxidation in amyloplasts isolated from wheat endosperm. Planta. 1998;204:100–108. [Google Scholar]
- Vitha S, Yang M, Kiss JZ, Sack FD. Light promotion of hypocotyl gravitropism of a starch-deficient tobacco mutant correlates with plastid enlargement and sedimentation. Plant Physiol. 1998;116:495–502. doi: 10.1104/pp.116.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T, Smart LB. Isolation of RNA from plant tissue. In: Krieg PA, editor. A Laboratory Guide to RNA, Isolation, Analysis, and Synthesis. New York: Wiley-Liss; 1996. pp. 21–42. [Google Scholar]
- Zou J, Katavic V, Giblin EM, Barton DL, MacKenzie SL, Keller WA, Hu X, Taylor DC. Modification of seed oil content and acyl composition in the Brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell. 1997;9:909–923. doi: 10.1105/tpc.9.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]