Supplemental Digital Content is available in the text
Keywords: antibody, antigen, brain, diagnosis, histoplasma, histoplasmosis, meningitis, outcome, treatment
Abstract
Central nervous system (CNS) involvement occurs in 5 to 10% of individuals with disseminated histoplasmosis. Most experience has been derived from small single center case series, or case report literature reviews. Therefore, a larger study of central nervous system (CNS) histoplasmosis is needed in order to guide the approach to diagnosis, and treatment.
A convenience sample of 77 patients with histoplasmosis infection of the CNS was evaluated. Data was collected that focused on recognition of infection, diagnostic techniques, and outcomes of treatment.
Twenty nine percent of patients were not immunosuppressed. Histoplasma antigen, or anti-Histoplasma antibodies were detected in the cerebrospinal fluid (CSF) in 75% of patients. One year survival was 75% among patients treated initially with amphotericin B, and was highest with liposomal, or deoxycholate formulations. Mortality was higher in immunocompromised patients, and patients 54 years of age, or older. Six percent of patients relapsed, all of whom had the acquired immunodeficiency syndrome (AIDS), and were poorly adherent with treatment.
While CNS histoplasmosis occurred most often in immunocompromised individuals, a significant proportion of patients were previously, healthy. The diagnosis can be established by antigen, and antibody testing of the CSF, and serum, and antigen testing of the urine in most patients. Treatment with liposomal amphotericin B (AMB-L) for at least 1 month; followed by itraconazole for at least 1 year, results in survival among the majority of individuals. Patients should be followed for relapse for at least 1 year, after stopping therapy.
1. Introduction
CNS infection occurs in 5 to 10% of patients with disseminated histoplasmosis.[1] The recognition, diagnosis, and treatment of CNS histoplasmosis is not well-characterized. The clinical features, appropriate diagnostic testing, and treatment regimens recommended for the evaluation, and management of CNS histoplasmosis are based on case reports, and small case series of patients.[1–3] These studies have demonstrated that the most common clinical features of CNS histoplasmosis consist of chronic meningitis,[4] focal brain, or spinal cord lesions, stroke syndromes, encephalitis, and hydrocephalus.[5] Over one third of cases reported, have occurred in immunocompetent individuals.[1,2]
Morbidity, and mortality appear to be high in patients with CNS histoplasmosis with high rates of relapse.[1] Despite prolonged courses of treatment, the case fatality was 39% with half of the survivors relapsing in a prior study.[1] Among AIDS patients with CNS histoplasmosis, 3 of 5 patients died, and 1 relapsed despite treatment with deoxycholate amphotericin B (AMB-D) followed by fluconazole, or itraconazole.[3] Although adherence appears to have been suboptimal in that study,[3] this experience shows that the outcome may be poor in some patients.
The diagnosis is often delayed because of failure to consider histoplasmosis in cases of chronic meningitis in which routine cultures are negative. Unfamiliarity with the tests used for diagnosis of histoplasmosis in this setting may also contribute to delayed diagnosis. Histoplasma can be isolated from fungal culture of Cerebrospinal fluid (CSF) in only 1 quarter of patients, and visualization of yeast is rare.[1,2] An example of the difficulty in diagnosis is provided by a case in which CSF cultures were negative on 2 occasions 5 years apart but positive 2 years later, when 30 ml of CSF was cultured.[6]
The most sensitive method for diagnosis of CNS histoplasmosis, based on evidence from the largest single institution series,[1] includes use of tests for detection of antibody, and antigen in the CSF.[5] Anti-Histoplasma antibodies were detected in the CSF by immunodiffusion (ID) or complement fixation (CF) in 50% of patients in one series,[1] and as many as 90% of patients in another study.[2]
The Infectious Disease Society of America (IDSA)[7] guideline for treatment of CNS histoplasmosis recommends an initial course of AMB-L followed by itraconazole for at least 1 year.[7] That recommendation was based upon improved survival, and clinical response in AIDS patients with disseminated histoplasmosis treated with AMB-L rather than with AMB-D.[8] These findings, coupled with poor outcome of CNS histoplasmosis[1] formed the basis for the IDSA guideline recommendation.
The objectives of this study were to expand our knowledge of the clinical manifestations, approach to diagnosis, and treatment, and outcome of CNS histoplasmosis, based on a retrospective multicenter review of the largest number of cases of CNS histoplasmosis studied to date, which were mostly, managed after publication of the IDSA guidelines.
2. Methods
Study design: This is a retrospective multicenter study of cases occurring mostly, between 1997 and 2010 (74 of 77 patients) in which physicians were surveyed for cases of CNS histoplasmosis. Patients were sought from investigators who participated in recent studies,[9–12] or who corresponded with one of the authors (L.J.W.) for advice about diagnosis, or management. This group of patients was obtained from a convenience sample which may lead to selection bias. A standardized case report form was used for data collection, which included information about underlying conditions, clinical manifestations, diagnostic testing, and outcome of therapy. The study was approved by the institutional review board at the institutions of each of the collaborating investigators. Disease severity was classified as mild if the patient was managed without hospitalization, moderate if the patient was hospitalized, and severe if the patient required critical care unit admission. Functional impairment was classified as none, mild (no limitation activity), moderate (slight limitation activity), and severe (unable to work, attend school, or manage household).
A proven diagnosis was achieved by isolation of Histoplasma from fungal culture, or visualization of appropriate morphologic fungal forms in the CSF, brain, or spinal cord tissue. A probable diagnosis required a positive antigen, or antibody test from CSF. A possible diagnosis required laboratory confirmation of pulmonary, or disseminated histoplasmosis without another cause for CNS disease, based upon clinical findings including symptoms, and examination, in the absence of laboratory criteria for proven, or probable CNS histoplasmosis.
2.1. Statistical analysis
Chi-squared analysis was used to compare subgroups using MedCalc software (Acacialaan 22,8400 Ostend, Belgium). P-values < .05 were considered significant. A multivariable logistic regression model for the outcome of death within 12 months was determined by stepwise selection, with P-value entry, and stay criteria of P < .2. Variables noted to be associated with mortality within 12 months with P < .2 in univariable analysis were included in the final model. To control for the effect of age, and disease severity on the outcome, those variables were also incorporated for the final model. Akaike Information Criteria, likelihood ratio test, and area under the receiver operating characteristic curve were used to select the models with the most optimal fitness. Missing data were excluded from the analysis.
Mortality at 12 months was compared across underlying conditions, baseline severity, and between amphotericin B treatment types using Fisher's exact tests. Additionally, time to death was assessed between amphotericin B treatment types using Kaplan-Meier product limit estimates and the log-rank test. Analyses were performed using R (version 3.3.2) (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Demographics
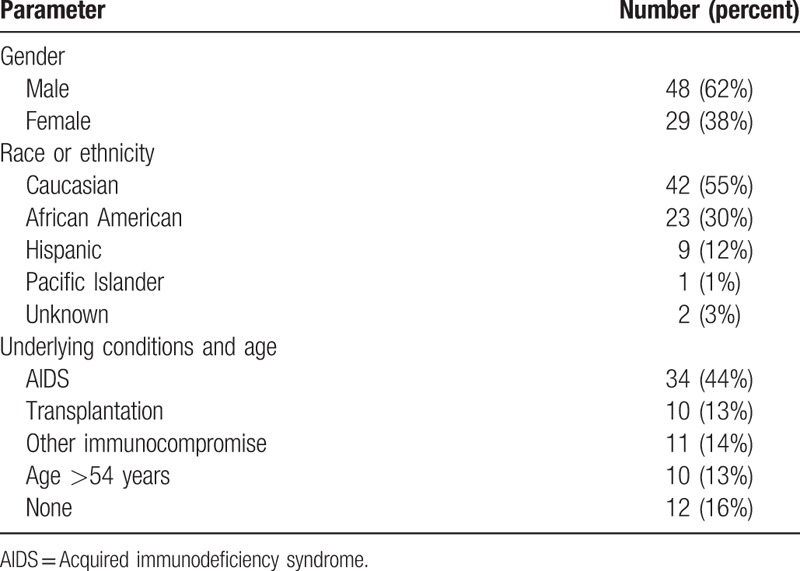
The demographic characteristics are presented in Table 1. A total of 77 patients were identified by the authors at their respective institutions. Seventy one percent of patients were immunocompromised, and 13% were over 54 years of age. Sixteen percent of infections occurred in otherwise healthy individuals less than 55 years old.
Table 1.
Assessment of demographic characteristics, and underlying conditions, or age in 77 patients.
3.2. Clinical findings
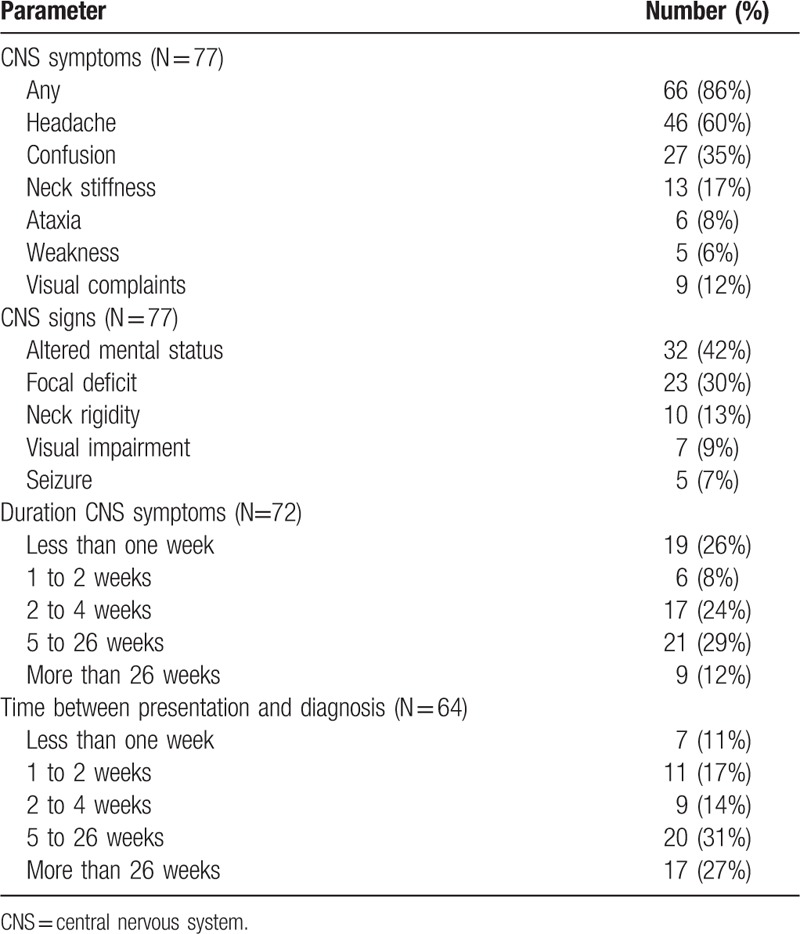
Clinical features are presented in Table 2. CNS disease was the initial manifestation of histoplasmosis in 84% of patients, and a relapse of disseminated histoplasmosis occurred in 16%. Presentation as a relapse occurred more often in AIDS patients (9 relapses among 34 patients with AIDS, 26%) than in others (4 relapses among 43 patients with conditions other than AIDS, 9%), P = .09. Symptoms had been present for 2 weeks or less in 35% of patients, and more than 26 weeks in 12%.
Table 2.
Clinical findings, duration of CNS symptoms, and time interval between initial presentation, and diagnosis of CNS histoplasmosis.
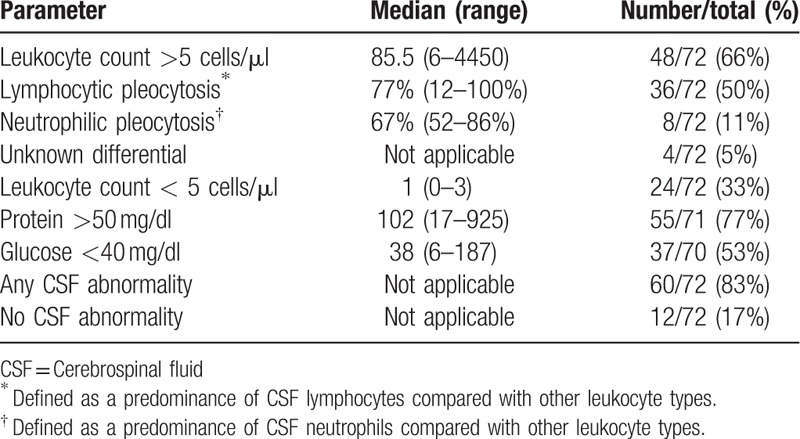
3.3. CSF findings
CSF findings are summarized in Table 3 and were available from 72 out of the 77 patients identified. Most patients (50%) were found to have a lymphocytic pleocytosis, 11% presented with a neutrophilic pleocytosis, and one third of patients had less than 5 cells/μL. No CSF abnormality was noted in 12 patients. Brain, or spinal cord imaging that was performed in 11 of these 12 patients exhibited changes consistent with CNS histoplasmosis in 9, leaving 2 with no CSF, or imaging abnormalities by computerized tomography (CT) scan. In these 2 patients, the clues to diagnosis were based on CNS symptoms, and examination supported by positive antigen, or culture of the CSF. Confusion, and impaired mentation were prominent in an AIDS patient in whom the CSF antigen was 5.3 ng/ml in 1 of these 2 patients, and headache was a major complaint in the other, a renal transplant patient in whom the CSF culture was positive.
Table 3.
Summary of CSF findings.
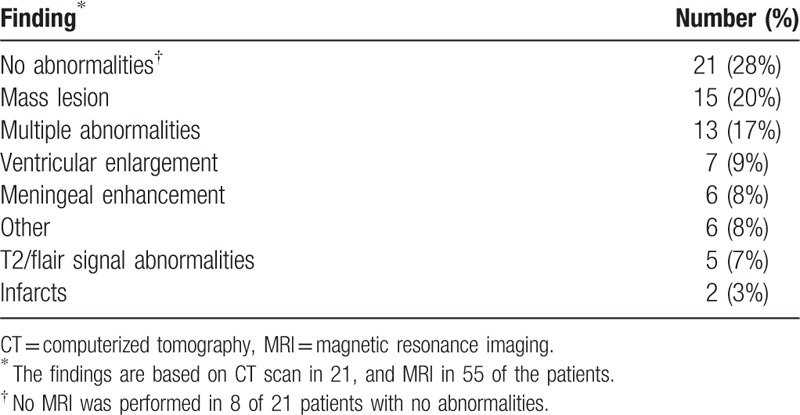
3.4. Imaging findings
Brain imaging was abnormal in 72% of patients (n = 76) (Table 4). Magnetic resonance imaging (MRI) detected lesions that were not observed on CT in 4 patients (5%). Focal mass lesions were the most commonly observed abnormality seen in 20% of patients. Less common lesions identified in 1 patient each included diffuse white matter changes, areas of restricted diffusion, low density lesions in the cerebellum, subdural hematoma, and focal mass lesions. Spinal cord lesions included enhancement in 3, non-mass lesions in 3, and disc protrusion with cord compression, and bone lesions in 1 patient each.
Table 4.
CT or MRI of the brain in 76 patients.
3.5. Diagnostic findings
Diagnosis was established within 2 weeks of presentation in 28% of patients, and more than 26 weeks after presentation in 27% (Table 2). The time to diagnosis was more than 26 weeks in 50% of non-immunocompromised patients, and 12% of immunocompromised patients. Failure to suspect histoplasmosis lead to delays in diagnosis in 9 patients (12%). Four patients were erroneously treated for presumed tuberculosis before histoplasmosis was diagnosed. Other misdiagnoses included neurosarcoidosis in 2 patients, toxoplasmosis, multiple sclerosis, and Behcet's syndrome in 1 patient each. Four of these five patients were treated with corticosteroids. Another patient who had a history of pulmonary histoplasmosis was misdiagnosed as having CNS lymphoma. However, H. capsulatum was isolated from CSF following the patient's death.
Results of diagnostic tests are summarized in Table 5. Detection of antigen in the CSF was the most common method for diagnosis. Antigen (n = 53) was detected more often in immunocompromised than in non-immunocompromised patients, and in those with severe disease. Antibody detection by ID, or CF (n = 32) was the next most common method for diagnosis, with similar findings in immunocompromised, and non-immunocompromised patients, and with severe, and non-severe disease. The antibody test was positive more often than the antigen test in non-immunocompromised patients. The highest diagnostic yield was achieved by testing the CSF for antigen, and anti-Histoplasma antibody (n = 57). At least 1 of these 2 tests was positive in 3 quarters of patients overall, including 85% of immunocompromised, and 56% of non-immunocompromised patients. Among all patients, antigen was detected in the urine in 44 of 60 (73%) patients, and in the serum in 14 of 28 (50%) that were tested. Anti-Histoplasma antibodies were detected in the serum in 22 of 37 patients (59%), including 14 of 32 (44%) by ID, and 22 of 35 (63%) by CF.
Table 5.
Correlation of the sensitivity of diagnostic tests performed on CSF with immunosuppression, and disease severity.
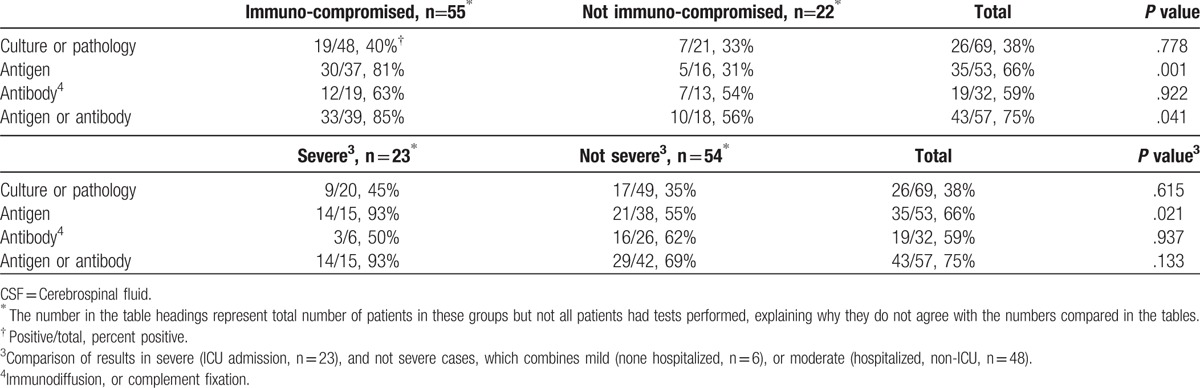
Histoplasma was isolated from culture of CSF, or yeast resembling H. capsulatum were observed in brain tissue histopathology, or in CSF in 38% of patients (n = 69) (Table 5). The initial CSF culture was negative but a subsequent culture was positive in 5 patients. Culture of CSF was negative with a positive culture of brain tissue in 2 patients. Histopathology of brain tissue was positive but culture of CSF was negative in 2 patients. Culture, or histopathology of the brain was positive in 3 patients with negative cultures from CSF.
The etiology of CNS disease was not definitively established by pathology, culture, or serology in 22% of patients (17 of 77 patients). These were classified as casesof “possible” CNS histoplasmosis. They were typically characterized as patients with pulmonary, or disseminated histoplasmosis who had meningitis, or CNS signs, or symptoms with brain imaging abnormalities without another etiology identified. Pathology, and/or culture of CSF, or tissue were negative in 16 of these patients. Tests for antigen, or antibody in the CSF were negative in 9, and not performed in 8 patients. Detection of antigen, or antibody in the urine, or serum provided laboratory basis for diagnosis of histoplasmosis in 16 of the possible cases. Disseminated histoplasmosis was previously, diagnosed in the 17th patient.
3.6. Outcome of treatment
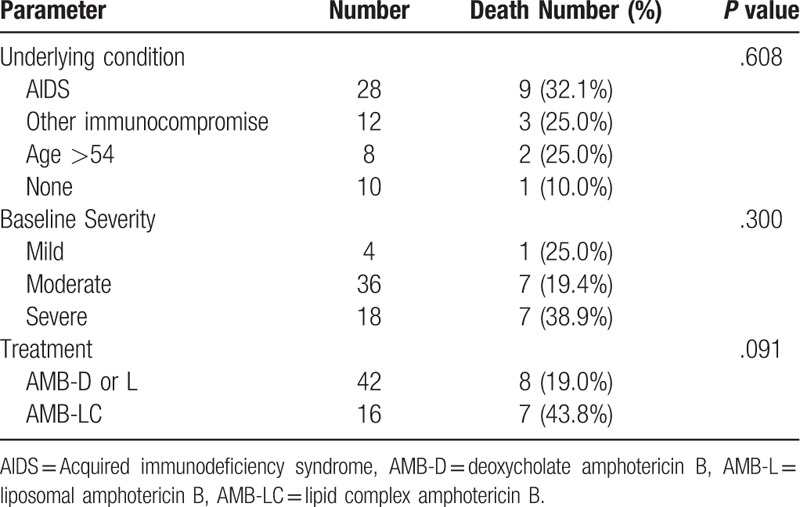
Patient characteristics associated with survival for at least 12 months are summarized in Table 6. The risk for death increased with age, management in an intensive care unit (ICU), and duration of amphotericin B treatment, but these differences were not statistically, significant. Survival was assessed in 58 patients who were treated initially, with an amphotericin B formulation, and followed for at least 1 year. The median follow-up in patients who survived at least 1 year was 26 months. Survival was not impacted by underlying condition, or the severity of illness (Table 7).
Table 6.
Factors associated with death within 12 months following diagnosis.
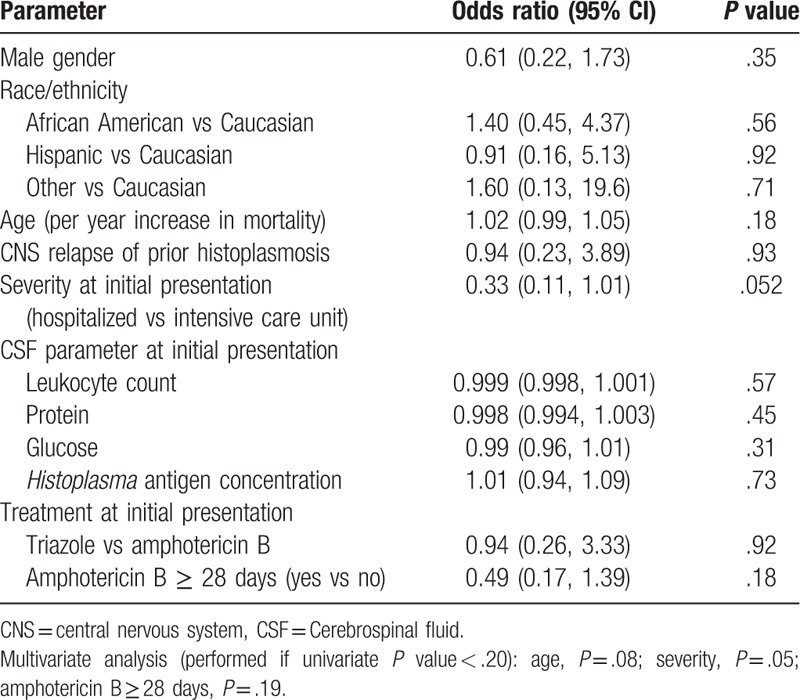
Table 7.
Factors associated with death in patients treated with amphotericin B.
One year survival was higher in patients treated with deoxycholate (13 of 16, 81.2%), or AMB-L (21 of 26, 80.8%) than with amphotericin B lipid complex (9 of 16, 56.2%), P = .20. There was a trend for improved survival when patients treated with deoxycholate, or liposomal formulations of amphotericin B were grouped together, and compared with those treated with amphotericin B lipid complex (Table 7), P = .09. The mean survival time during the 1st year of follow-up was longer in patients treated with deoxycholate, or AMB-L than in patients treated with amphotericin B lipid complex, P = .040 (Fig. 1). Serial CT, or MRI imaging, or the brain cord was performed in 22 patients, and the findings are described in a supplemental table.
Figure 1.
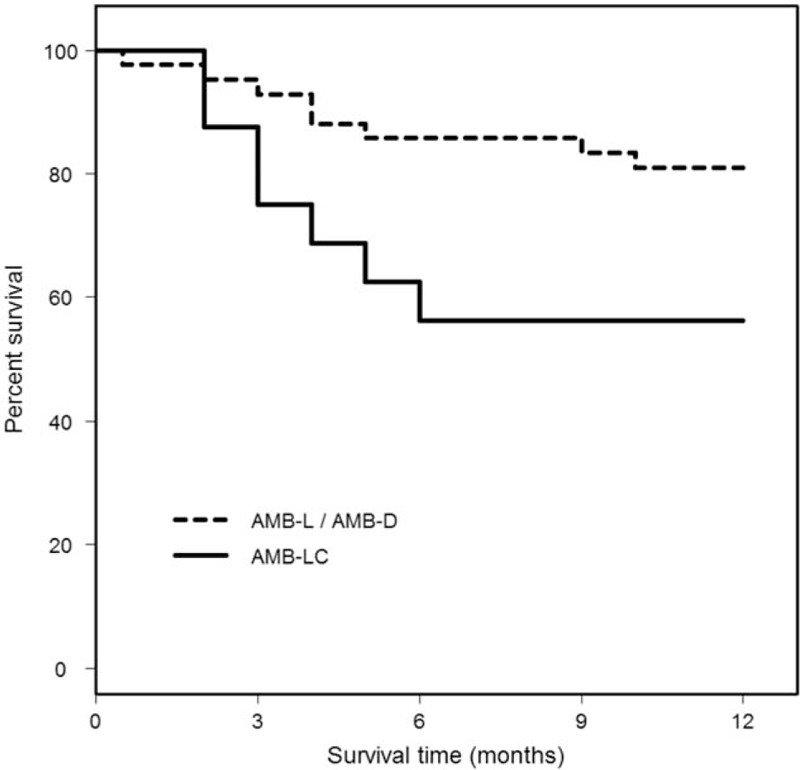
Twelve month survival in patients treated with AMB-D, or AMB-L compared to AMB-LC. The mean survival time (standard error) during the 1 year of followup was 10.6 months (0.5 months) in patients treated with AMB-D, or AMB-L, and 8.3 months (1.1 months) in patients treated with amphotericin B lipid complex, P = .040. Data are calculated as the restricted mean with an upper limit of 12 months. This is the expected number of months, out of the first 12, that would be experienced by each group. AMB-D = deoxycholate amphotericin B, AMB-L = liposomal amphotericin B, AMB-LC = lipid complex amphotericin B.
Eleven patients were treated initially with a triazole (itraconazole, 6; voriconazole, 5), 4 of whom died during the first month of therapy (itraconazole, 2; voriconazole, 2). Mortality was similar in patients treated initially, with a triazole, and with any amphotericin B formulation (Table 6).
Nine patients died more than 1 year after diagnosis. Two were attributed to histoplasmosis, occurring at 16 and 18 months after diagnosis. Both were patients with AIDS who were poorly, adherent to antifungal therapy.
Relapse occurred in 3 of 49 (6%) patients with follow-up of at least 1 year (median, 21 months). The 3 relapses occurred among 21 patients with CNS histoplasmosis who had AIDS, at 18, 29, and 57 months of follow-up, during continued therapy with itraconazole, or fluconazole. These patients were considered to have been non-adherent to therapy.
Functional status was determined in 44 patients who survived for more than 1 year (median, 26 months). Fifty-four percent exhibited no functional impairment, 14% were mildly impaired, 18% moderately impaired, and 14% were severely impaired. Severe impairment occurred in 5 of 29 (17%) immunocompromised, and 1 of 15 (6%) non-immunocompromised patients, P = .58. Functional status at initial presentation was not recorded, precluding assessment of change in functional status.
4. Discussion
This is the largest study of CNS histoplasmosis yet performed, expanding our knowledge regarding clinical presentation, diagnosis, and outcomes, complementing prior case series.[1–3] Some important, but frequently overlooked findings include the fact that CNS histoplasmosis is not restricted to immunocompromised individuals, often presents as an acute illness, and rarely, is rapidly, progressive. Diagnosis remains difficult, and often delayed, especially in non-immunocompromised patients, largely, because of failure to consider, and test for histoplasmosis, and lack of awareness of the importance of antigen, and antibody testing. Treatment according to IDSA guidelines[7] is highly effective, but mortality, and morbidity remain high in immunocompromised patients, particularly those with AIDS who are poorly, adherent to therapy.
Histoplasmosis should be considered in the evaluation of patients with chronic lymphocytic meningitis, and may be overlooked in patients with acute disease, or predominantly neutrophilic pleocytosis. The absence of immunocompromise should not be a deterrent for testing for histoplasmosis as a cause for chronic meningitis as well as a variety of other CNS syndromes in patients who reside in endemic areas. Rarely, otherwise healthy patients with disseminated histoplasmosis have been found to have specific defects in cellular immunity that may not be apparent on initial evaluation.[13,14] CNS symptoms had been present for less than 1 month in over one half of patients, and for 1 week, or less in one quarter of patients in our case series, findings that are uncharacteristic of chronic meningitis.[4] Some patients had been treated for hydrocephalus for years without consideration of fungal meningitis as the etiology. The clinical findings of CNS histoplasmosis are often subtle, and not necessarily characteristic of an infectious process. Forty-two percent of patients in our series were afebrile. Headache, and confusion were the most common complaints, and altered mental status was the most common physical finding. Further complicating the diagnosis for this infection, the CSF profile was normal in nearly 20% of patients, and imaging of the brain was normal in over 1 quarter. The diagnosis was not established within the first 2 weeks of presentation in nearly, two-thirds of cases. Tuberculosis, and noninfectious, inflammatory disorders, including neurosarcoidosis were thought to be the cause of chronic meningitis thus leading to inappropriate management, and delays in therapy. Histoplasmosis infection of the CNS should be considered in patients who reside in endemic areas with otherwise unexplained acute sub-acute, or chronic neurological illness, including meningitis, mass lesions suggesting malignancy, stroke syndromes suggesting vasculitis, or embolic events, and hydrocephalus.
The diagnosis of CNS histoplasmosis can be established non-invasively, in 1 week or less in most patients by antigen, and anti-Histoplasma antibody testing of CSF, and serum, and antigen detection in urine. Recent studies reported that detection of antibodies in the CSF using a previously reported enzyme immunoassay[15] further improved the sensitivity for diagnosis over that achieved by the older methods used in this study. Nevertheless, antigen detection, antibody detection, culture, and pathology of CSF, or brain tissue may be non-diagnostic in some patients. In 5 patients in whom antigen, or antibody testing was not performed, the diagnosis was established by demonstration of characteristic yeast in brain tissue. Culture of the CSF usually does not provide a rapid diagnosis, as growth requires more than 2 weeks in most patients, and up to 6 weeks in some. Brain biopsy for histopathology, and culture may be required in patients in whom delay for serologic testing is unacceptable, or a diagnosis cannot be made via non-invasive means.
Patient outcome appears to be improved in this cohort compared to earlier studies. One year survival in patients treated with any amphotericin B formulation (deoxycholate, liposomal, or lipid complex) was 74% (43 of 58), compared to 55% in a review published in 1990.[1] In a review of more contemporary papers published between 2011 and 2016 reporting outcome in 15 patients, 80% survived.[16–30] Others have reported in smaller studies that 6 of 8 patients were successfully treated with AMB-D followed by itraconazole.[31] In that study 1 patient died, and another relapsed 3 times, but was successfully, treated after shunt replacement.[31] More recently, of 4 AIDS patients treated with amphotericin B lipid complex followed by itraconazole, or fluconazole, 3 died, and 1 relapsed, all of whom were non-adherent to therapy.[3] The IDSA treatment guideline recommends initial treatment with AMB-L for 4 to 6 weeks followed by itraconazole for at least 1 year.[7] Consistent with these guidelines, we observed a trend towards increased survival, and significantly longer survival time in patients treated with liposomal, or AMB-D than with amphotericin B lipid complex. Others have made similar observations in an experimental animal model of Candida albicans infection of the CNS.[32] Deoxycholate, and AMB-L reduced the fungal burden in the brain more completely than amphotericin B lipid complex, or amphotericin B colloidal dispersion. Pharmacodynamic parameters, namely the ratio of maximum plasma concentration, and area under the curve to minimum inhibitory concentration of free amphotericin B, and time during dosing level above the minimum inhibitory concentration, correlated with clearance of the organism from brain tissue.[32] These pharmacodynamic differences were attributed to structural characteristics responsible for a shorter half-life in the plasma, smaller area under the curve, and larger volume of distribution of the lipid complex, and colloidal dispersion formulations. None of the amphotericin B formulations achieved quantifiable concentrations in the CSF but all achieve quantifiable concentrations in the brain tissue. AMB-L achieved the highest concentration in the brain, about 6-fold higher than deoxycholate, or lipid complex amphotericin B (AMB-LC), and 4-fold higher than amphotericin B colloidal dispersion. Because of reduced toxicity,[33] superior pharmacodynamic characteristics, and greater concentration in brain tissue, as well as improved survival demonstrated in our study, AMB-L is the treatment of choice for CNS histoplasmosis.
The best triazole for continued treatment after amphotericin B induction therapy is uncertain. Theoretically, a triazole that achieves therapeutic concentrations in CSF would be best. Studies in an experimental animal model of Histoplasma meningitis demonstrated that fluconazole, which achieves excellent concentrations in the CSF, was inferior to itraconazole, which does not achieve therapeutic levels in CSF.[34] Therefore, it appears that despite inadequate CSF penetration, itraconazole remains the triazole of choice for “step-down” therapy following initial treatment with AMB -L in patients with CNS histoplasmosis infection.
The risk for relapse appears to be lower than in previous studies. Relapse occurred in 6% of surviving patients followed for at least 1 year in this study compared to 50% in the earlier study.[1] The 3 relapses all occurred in patients with AIDS who were not adherent to therapy in the current study, confirming recent reports in which non-adherence was a significant risk factor for failure of treatment.[3,11]
Some insight was gained into functional status of patients who survived following treatment for the infection in this study. Functional status at last follow-up was normal in over half of patients but severely impaired in 14%, occurring predominantly in the immunocompromised group. Whether impaired function was related to a higher burden of disease, or severity of CNS infection, or other factors, most notably underlying immunocompromising condition, or comorbidities is unknown.
The limitations of retrospective studies must be recognized, most notably selection bias, incomplete information, uncontrolled therapy, and inconsistent follow-up. This group of patients was obtained from a convenience sample which may lead to selection bias. The sensitivity for antigen detection in the CSF may have been inflated by including patients for whom the treating physician sought advice about diagnosis, and management from one of the authors (L.J.W.). Use of antigen testing for diagnosis is not readily, available outside of the US, and our treatment recommendations may not be feasible in resource-limited countries.
In conclusion, clinicians should have a low threshold for considering histoplasmosis as a cause for meningitis, brain lesions, vascular events, or hydrocephalus in immunocompromised, and non-immunocompromised patients who reside in endemic areas, and for whom an alternative diagnosis has not been established. A diagnosis of CNS histoplasmosis can be established in a timely manner by detection of antigen, or anti-Histoplasma antibodies in the CSF. The current IDSA guidelines[7] that recommend treatment with AMB-L administered for 4 to 6 weeks followed by itraconazole for at least 1 year are supported with this study. Although not specifically addressed in this study, it is well known that itraconazole levels should be carefully monitored, and maintained above 1 μg/ml when treating systemic fungal infections.[7] Although relapse was less common in this study than in the earlier review,[1] some relapses may have been missed because of the retrospective nature of the study, and inconsistent length of follow-up. Therefore, it remains important to document complete resolution of infection in response to therapy to prevent relapse. This can be accomplished by monitoring imaging, as well as antigen, and antibody levels in serum, urine, and CSF. The risk of relapsed infection is likely to be less when it has been confirmed that CSF antigen tests have become negative at some point during treatment, the CSF formula has normalized, and patients have received at least 12 months of therapy in total.
Acknowledgments
We thank Mr. W. K, MiraVista Diagnostics, Indianapolis, Indiana for assisting with the statistical analysis.
Author contributions
Conceptualization: A.M. Anderson, C.A. Hage.
Data curation: C. Terry, P. Kemmer, Y. Guo.
Formal analysis: A.M. Anderson, C.A. Hage, C. Terry, L. J. Wheat, P. Kemmer.
Funding acquisition: A.M. Anderson.
Investigation: A.M. Anderson, B.A. Pahud, C. Passaretti, C.A. Hage, D. Bamberger, D.M. Allen, E. Esguerra, E. Jenny-Avital, G. Sivasubramanian, J. Johnson, J. Riddell, J.W. Baddley, J.C. Sarria, K. Shehab, K.L. Bowlware, K.O. Cleveland, M.A. Assi, M.M. Azar, P. Vergidis, P.W. Wright, P.T. Ender, R.N. Khairy, S. Mocherla, S.F. Chen, S.S. Huprikar, T. Myint, T. Tsai, V. Douglas, V. Prakash, V.R. Holenarasipur, W.W. Ooi.
Methodology: A.M. Anderson, L. J. Wheat, P. Kemmer, Y. Guo.
Project administration: A.M. Anderson, L. J. Wheat.
Resources: A.M. Anderson.
Supervision: A.M. Anderson, L. J. Wheat.
Validation: L. J. Wheat.
Writing – original draft: A.M. Anderson, C.A. Hage, L. J. Wheat, T. Myint.
Writing – review & editing: A.M. Anderson, B.A. Pahud, C. Passaretti, C.A. Hage, C. Terry, D. Bamberger, E. Esguerra, E. Jenny-Avital, G. Sivasubramanian, J. Johnson, J. Riddell, J.W. Baddley, J.C. Sarria, K. Shehab, K.L. Bowlware, K.O. Cleveland, L. J. Wheat, M.A. Assi, M.M. Azar, P. Vergidis, P.W. Wright, P.T. Ender, P. Kemmer, R.N. Khairy, S. Mocherla, S.F. Chen, S.S. Huprikar, T. Myint, T. Tsai, V. Douglas, V. Prakash, V.R. Holenarasipur, W.W. Ooi, Y. Guo.
Supplementary Material
Footnotes
Abbreviations: AIDS = Acquired immunodeficiency syndrome, CF = Complement fixation, CNS = central nervous system, CSF = Cerebrospinal fluid, CT = computerized tomography, IDSA = Infectious Disease Society of America, MRI = magnetic resonance imaging.
Summary of Study: Histoplasmosis of the central nervous system (CNS) is a rare disease. While most patients are immunocompromised, or of advanced age, some are otherwise healthy. Diagnosis can be established by serologic testing for antigen, or antibody in the majority of patients. Treatment with liposomal amphotericin B (AMB-L) followed by itraconazole is recommended, but mortality remains high in immunocompromised patients, and those of advanced age. Relapse is uncommon in patients who are adherent to therapy.
Funding: Dr A is currently receiving funding through NIH grant K23MH095679
Potential conflicts of interest: L.J.W is a medical director, and part owner of MiraVista Diagnostics, a company that offers the some of the described tests (antigen and antibody testing) commercially. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE form for disclosure of potential conflicts of interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplemental Digital Content is available for this article.
References
- [1].Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system. A clinical review. Medicine (Baltimore) 1990;69:244–60. [DOI] [PubMed] [Google Scholar]
- [2].Schestatsky P, Chedid MF, Amaral OB, et al. Isolated central nervous system histoplasmosis in immunocompetent hosts: a series of 11 cases. Scand J Infect Dis 2006;38:43–8. [DOI] [PubMed] [Google Scholar]
- [3].Nyalakonda H, Albuerne M, Suazo Hernandez LP, et al. Central Nervous System Histoplasmosis in Acquired Immunodeficiency Syndrome. Am J Med Sci 2016;351:177–86. [DOI] [PubMed] [Google Scholar]
- [4].Ellner JJ, Bennett JE. Chronic meningitis. Medicine (Baltimore) 1976;55:341–69. [DOI] [PubMed] [Google Scholar]
- [5].Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005;40:844–52. [DOI] [PubMed] [Google Scholar]
- [6].Gelfand JA, Bennett JE. Active histoplasma meningitis of 22 years’ duration. JAMA 1975;233:1294–5. [PubMed] [Google Scholar]
- [7].Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the infectious diseases society of America. Clin Infect Dis 2007;45:807–25. [DOI] [PubMed] [Google Scholar]
- [8].Johnson PC, Wheat LJ, Cloud GA, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med 2002;137:105–9. [DOI] [PubMed] [Google Scholar]
- [9].Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011;53:448–54. [DOI] [PubMed] [Google Scholar]
- [10].Assi M, Martin S, Wheat LJ, et al. Histoplasmosis after solid organ transplant. Clin Infect Dis 2013;57:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Myint T, Anderson AM, Sanchez A, et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): multicenter study of outcomes and factors associated with relapse. Medicine (Baltimore) 2014;93:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-alpha blocker therapy: a retrospective analysis of 98 Cases. Clin Infect Dis 2015;61:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis 2005;41:e38–41. [DOI] [PubMed] [Google Scholar]
- [14].Lionakis MS, Holland SM. Human invasive mycoses: immunogenetics on the rise. J Infect Dis 2015;211:1205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Richer SM, Smedema ML, Durkin MM, et al. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis 2016;62:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ramireddy S, Wanger A, Ostrosky L. An instructive case of CNS histoplasmosis in an immunocompetent host. Med Mycol Case Rep 2012;1:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Threlkeld ZD, Broughton R, Khan GQ, et al. Isolated histoplasma capsulatum meningoencephalitis in an immunocompetent child. J Child Neurol 2012;27:532–5. [DOI] [PubMed] [Google Scholar]
- [18].Andrade AI, Donato M, Previgliano C, et al. Histoplasmosis brain abscesses in an immunocompetent adult. a case report and literature review. Neuroradiol J 2014;27:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Batra V, Khararjian A, Wheat J, et al. From suspected Creutzfeldt-Jakob disease to confirmed histoplasma meningitis. BMJ Case Rep 20162016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bolen RD, Bushnell CD, Reynolds PS. Acute ischemic strokes from small vessel vasculitis due to disseminated histoplasmosis infection. J Neurol Sci 2015;352:125–6. [DOI] [PubMed] [Google Scholar]
- [21].Estrada-Bellmann I, Camara-Lemarroy CR, Flores-Cantu H, et al. Hemichorea in a patient with HIV-associated central nervous system histoplasmosis. Int J STD AIDS 2016;27:75–7. [DOI] [PubMed] [Google Scholar]
- [22].Hariri OR, Minasian T, Quadri SA, et al. Histoplasmosis with deep CNS involvement: case presentation with discussion and literature review. J Neurol Surg Rep 2015;76:e167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].John AM, Vasanthi N, Khurana A, et al. An unusual cause for rings in the brain. Hong Kong Med J 2012;18:346–7. [PubMed] [Google Scholar]
- [24].Khasawneh FA, Ahmed S, Halloush RA. Progressive disseminated histoplasmosis presenting with cachexia and hypercalcemia. Int J Gen Med 2013;6:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nguyen FN, Kar JK, Zakaria A, et al. Isolated central nervous system histoplasmosis presenting with ischemic pontine stroke and meningitis in an immune-competent patient. JAMA Neurol 2013;70:638–41. [DOI] [PubMed] [Google Scholar]
- [26].Parihar A, Tomar V, Ojha BK, et al. Magnetic resonance imaging findings in a patient with isolated histoplasma brain abscess. Arch Neurol 2011;68:534–5. [DOI] [PubMed] [Google Scholar]
- [27].Rangel-Castilla L, Hwang SW, White AC, et al. Neuroendoscopic diagnosis of central nervous system histoplasmosis with basilar arachnoiditis. World Neurosurg 2012;77:399–413. [DOI] [PubMed] [Google Scholar]
- [28].Schuster JE, Wushensky CA, Di Pentima MC. Chronic primary central nervous system histoplasmosis in a healthy child with intermittent neurological manifestations. Pediatr Infect Dis J 2013;32:794–6. [DOI] [PubMed] [Google Scholar]
- [29].Tyler KL, Johnson EC, Cantu DS, et al. A 20-year-old woman with headache and transient numbness. Neurohospitalist 2013;3:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Veeravagu A, Ludwig C, Camara-Quintana JQ, et al. Fungal infection of a ventriculoperitoneal shunt: histoplasmosis diagnosis and treatment. World Neurosurg 2013;80:222–313. [DOI] [PubMed] [Google Scholar]
- [31].Mata-Essayag S, Colella MT, Rosello A, et al. Histoplasmosis: a atudy of 158 cases in Venezuela, 2000-2005. Medicine (Baltimore) 2008;87:193–202. [DOI] [PubMed] [Google Scholar]
- [32].Groll AH, Giri N, Petraitis V, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis 2000;182:274–82. [DOI] [PubMed] [Google Scholar]
- [33].Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 2013;73:919–34. [DOI] [PubMed] [Google Scholar]
- [34].Haynes RR, Connolly PA, Durkin MM, et al. Antifungal therapy for central nervous system histoplasmosis, using a newly developed intracranial model of infection. J Infect Dis 2002;185:1830–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


