Abstract
Rationale:
Owing to the unique structure and function of the heart, tumor metastasis in the heart is rare. Accordingly, no unique symptoms have yet been identified for cardiac metastasis.
Patient and concerns:
A patient presented with cardiac metastasis 3 years after surgical resection of alveolar soft tissue sarcomas in their late stage.
Diagnosis:
Ultrasonography showed a middle-high echo clump on the left surface of the mid-upper interventricular septum, which had an unclear boundary with the myocardium. Meanwhile, blood flow was found in the clump, with no blockage of blood flow having been observed.
Lessons:
Although cardiac metastasis in terminal cancer patients always carries a poor prognosis, there is still no effective treatment for cardiac metastasis. In the clinic, it is important to improve the patient's quality of life, reduce symptoms and signs, and extend the duration of survival.
Keywords: cardiac metastasis, case report, soft tissue sarcoma
1. Introduction
Owing to the unique structure and function of the heart, tumor metastasis in the heart is rare. Herein, we report the case of a patient who presented with cardiac metastasis 3 years after surgical resection of alveolar soft tissue sarcomas in their late stage. The study protocol was approved by the ethics review board of Shandong University and the patient provided written informed consent for the publication of this report. All procedures were performed in accordance with the Declaration of Helsinki and relevant policies in China.
2. Case report
A 38-year-old male underwent left forearm lumpectomy on December 4, 2010. The forearm alveolar soft tissue sarcoma was approximately 2 × 1 cm in size. On January 13, 2011, the surgeon underwent an extended local excision of 2 cm, and there was no tumor residue around the incision. No further treatment was performed postoperatively. In June 2014, without any readily discernable reason, the patient presented with symptom of dizziness, headache, and nausea. On June 26, 2014, a computed tomography (CT) scan results showed multiple abnormal densities in both lungs, the brain, and the spleen. In July 2014, the patient underwent a series of treatments, including brain palliative irradiation (cumulative dose was DT 30 Gy/10 f), systemic chemotherapy (DDP 40 mg d1–3 + THP 100 mg d1 + ifosfamide 3 g d1–4), intracranial decompression, acid suppression, antiemetic effect, and some supportive treatments. On August 20, 2014, the patient was hospitalized due to the development of palpitations, chest distress, and shortening breath. Physical examination indicated temperature 36.5°C, pulse 81 beats per minute, respiration 18 breaths per minute, and blood pressure 110/83 mm Hg. Superficial lymphadenectasis, thyromegaly, and jugular vein distention were not observed. Although thoracocyllosis and koilosternia were observed, the trachea was in the midline and the auscultation of the lung was clear. In addition, the heart rate and auscultation of the valve area were normal. The patient had a soft abdomen that exhibited no tenderness or rebound tenderness. In addition, the liver, spleen, and kidney were not palpable. Laboratory tests for serum levels, liver function, and blood coagulation were normal. He had no history of hypertension or coronary heart disease. An electrocardiogram showed a fast sinus heart rate and frequent atrial premature beats. Ultrasonography showed a middle-high echo clump on the left surface of the mid-upper interventricular septum (4.0 × 2.1 cm), which had an unclear boundary with the myocardium. Meanwhile, blood flow was observed in the clump, but there was no readily discernable block in the blood flow (Figs. 1 and 2). On August 23, 2014, the patient underwent chemotherapy for 1 week. He was unable to tolerate the treatment, and so it was discontinued. In its place, we continued treatment with cardiotonics, diuretics, and vasodilators. Finally, he died of multiple organ failure.
Figure 1.
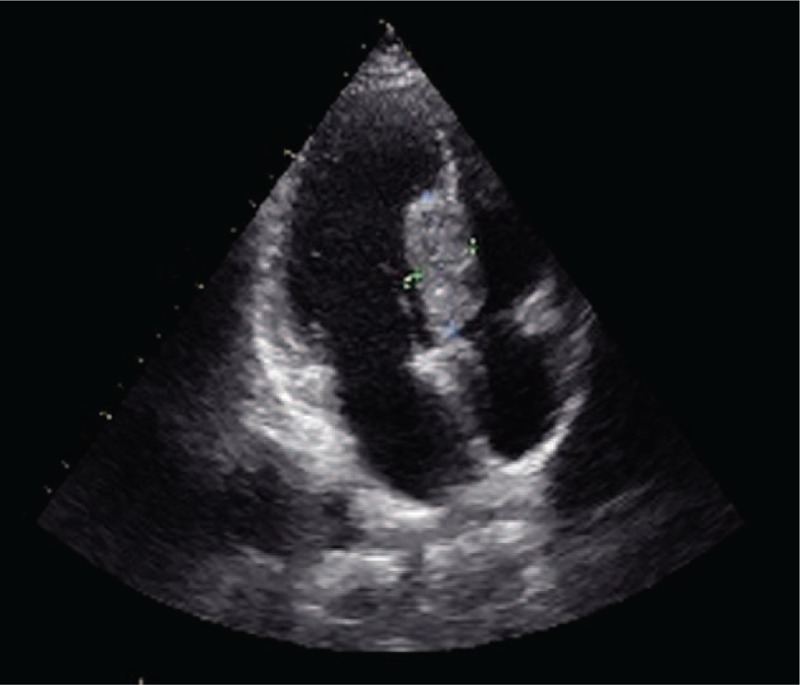
Ultrasonography showed a middle-high echo clump in left surface of mid-upper interventricular septum (4.0 × 2.1 cm), which has an unclear boundary with myocardium.
Figure 2.

Blood flow was found in the clump. Block of blood flow is not obvious.
3. Discussion
Cardiac metastases of sarcoma are uncommon and only a few cases have been described to date. The major primary malignancies associated with cardiac metastases include cancers of the lung, breast, stomach, liver, lymphoma, leukemia, and melanoma.[1] Its incidence ranges from 0.001% to 0.03%, which is approximately 20 to 1000 times lower than that of the primary tumor.[2] In 2016, a research group in the United Kingdom described a case of uterine leiomyosarcoma with cardiac metastasis.[3] Osteosarcoma (OS) is the most common type of bone malignancy, and is frequently highly malignant. A previous study demonstrated that OS rarely metastasizes to the heart. However, that case was unusual in that OS metastasized to the intracaval, right atrial, and right ventricular regions of the heart, which were misdiagnosed as a venous thrombus.[4] Sinus tachycardia, low limb lead voltage, heart rate, ST-T change, and electrocardiogram were traditionally used for tentative diagnosis. The cardiac ultrasound was considered to be the most effective method of achieving a diagnosis at an early stage, and its diagnostic rate in cardiac tumors was 91%.[5] However, ultrasound has a low resolution in tissues and a high technical dependence.[6] Chest CT, magnetic resonance, and positron emission tomography-CT are more accurate for diagnosis. Although cardiac metastasis in terminal cancer patients always carries a poor prognosis, there is still no effective treatment for it. In the clinic, it is important to improve the patient's quality of life, reduce symptoms and signs, and extend the duration of survival. In this study, the patient underwent chemotherapy for 1 week after confirming cardiac metastasis. Unfortunately, disease progression was not controlled effectively and the patient died of multiple organ failure.
Author contributions
Conceptualization: C. Wang, J. Song, Y. Sun.
Data curation: C. Wang, H. Liu, J. Song.
Funding acquisition: C. Wang.
Investigation: C. Wang, H. Liu, J. Xiao.
Methodology: C. Wang, H. Liu, J. Song, J. Xiao.
Project administration: C. Wang, C. Wang, J. Xiao, Y. Sun.
Software: J. Xiao.
Supervision: C. Wang.
Validation: C. Wang.
Writing – original draft: J. Xiao.
Writing – review & editing: J. Xiao.
Footnotes
Abbreviation: OS = osteosarcoma.
This study was supported by the National Natural Science Foundation of China [No. 81473483], the Natural Science Foundation of Shandong Province [BS2013YY048, ZR2014HM106], and the China Postdoctoral Science Foundation [2015M572056].
The authors have no funding and conflicts of interest to disclose.
References
- [1].Wang ZJ, Reddy GP, Gotway MB, et al. CT and MR imaging of pericardial disease. Radiographics 2003;23:167–80. [DOI] [PubMed] [Google Scholar]
- [2].Díaz ML, Villanueva A, Bastarrika G, et al. Non-electrocardiogram-gated multidetector-row computed tomography findings of cardiac pathology in oncologic patients. Curr Probl Diagn Radiol 2009;38:206–17. [DOI] [PubMed] [Google Scholar]
- [3].Artioli G, Borgato L, Calamelli S, et al. Unusual cardiac metastasis of uterine leiomyosarcoma: case report and literature review. Tumori 2016;102(Suppl. 2): [DOI] [PubMed] [Google Scholar]
- [4].Gül M, Babat N, Uçar FM, et al. Massive pulmonary embolism and a cardiac mass: thrombus or metastasis? Turk Kardiyol Dern Ars 2016;44:597–9. [DOI] [PubMed] [Google Scholar]
- [5].Haverkamp MC, Scholte AJ, Holman ER, et al. Contrast echocardiography as a useful additional diagnostic tool in evaluating a primary cardiac tumor. Eur J Echocardiogr 2005;6:388–91. [DOI] [PubMed] [Google Scholar]
- [6].Espinola Zavaleta N, Morales GH, Vargas-Barrón J, et al. Three dimensional transesophageat echocardiography in tumors of the heart. Am Soc Echocardiogr 2002;15:972–9. [DOI] [PubMed] [Google Scholar]


