Abstract
To evaluate the risk of myocardial infarction (MI) after receiving intravitreal bevacizumab (IVB) injection. We retrospectively reviewed the charts of patients who had received IVB injection in 2016, and grouped them according to whether they received the injection for age-related macular degeneration (AMD), diabetes-related complications, or retinal vein occlusion (RVO). We then investigated the prevalence of MI within 2 months after IVB injection and analyzed the possible association of IVB with MI. During 2016, 724 patients were enrolled and received a total of 1870 IVB injections. Seven patients were diagnosed with MI within 2 months after receiving an IVB injection. Of 274 patients with AMD, 2 were diagnosed with MI; of 311 patients with diabetes-related complications, 3 were diagnosed with MI; and of 139 patients with RVO, 2 were diagnosed with MI (P = 0.785). All MIs occurred between 3 days and 3 weeks after IVB injection (mean = 14.00 ± 6.45 days). The MIs after receiving IVB were associated with previous history of MI or cerebrovascular infarction in multivariate logistic regression analysis (P = 0.005). There was no significant difference in MI prevalence after IVB injection according to the reason for receiving the injection. However, care should be taken when administering IVB injections, especially to patients with risk factors such as history of MI or cerebrovascular infarction.
Keywords: age-related macular degeneration, bevacizumab, intravitreal injection, myocardial infarction, retinal vein occlusion
1. Introduction
Anti-vascular endothelial growth factor (VEGF) has become the main treatment to reduce vascular leakage and to regress neovascularization for patients with age-related macular degeneration (AMD). In addition, its use has been expanded to treat diabetic complications, such as proliferative diabetic retinopathy (PDR) with vitreous hemorrhage and diabetic macular edema (DME), retinal vein occlusion (RVO), and retinopathy of prematurity (ROP).[1–6]
Bevacizumab is an anti-VEGF agent that has been approved by the Food and Drug Administration (FDA) to treat colorectal cancer and glioblastoma,[7,8] but not as an intravitreal injection.[9] Ranibizumab was approved by the FDA in 2006 for the treatment of AMD, in 2010 for the treatment of RVO, and in 2012 for the treatment of DME.[9,10] Aflibercept was approved by the FDA in 2011 for the treatment of AMD, and 2014 for the treatment of DME.[9,11]
Despite use being off label, intravitreal bevacizumab (IVB) is often used because of its cost-effectiveness, and is also inevitably used without FDA approval because of its effectiveness for PDR vitreous hemorrhage with or without vitrectomy, neovascular glaucoma, and ROP.[6,9,12–14] However, IVB injection has side effects, such as worsening of the epiretinal membrane, subretinal fibrosis or tractional retinal detachment, and an increased risk of thromboembolism.[15–20] There has also been controversy regarding the possible association between bevacizumab and thromboembolic events. Some studies reported no association between bevacizumab injection and myocardial infarction (MI),[21–25] while others did report a relationship.[18–20] In the present study, patients were grouped according to their reasons for receiving IVB injections, and the possible association between IVB injection and MI prevalence within 2 months was analyzed.
2. Methods
The medical records of all patients treated with IVB injections during 2016, at St Vincent Hospital, Suwon, Republic of Korea, were reviewed retrospectively. This study was performed according to the tenets of the Declaration of Helsinki, and the study protocol was approved by the institutional review/ethics board of the Catholic University of Korea, St Vincent's Hospital. Informed consent was not obtained because this study involved the review of patient records.
All patients underwent a full ophthalmic examination that included a dilated fundus examination and optical coherence tomography (OCT) (Cirrus High Definition-OCT; Carl Zeiss Meditec, Dublin, CA). The MI was diagnosed using serum cardiac biomarkers and an electrocardiogram, and all patients with MI were treated with percutaneous coronary intervention after the MI diagnoses.
Inclusion criteria included receiving IVB injection because of AMD, diabetes-related complications such as PDR with vitreous hemorrhage or center-involved DME, and macular edema (ME) because of RVO. Patients were injected with 1.25 mg of bevacizumab, irrespective of the type of disease,[26,27] and were diagnosed with MI within 2 months after the IVB injection. We checked all the patients twice at 1 and 2 months after IVB. We excluded patients who were not followed-up until 2 months after the injection, or had incomplete data about medical history.
The Kruskal–Wallis test was used to compare age and the number of injections among the 3 groups. Tukey's post hoc analysis was used to compensate for multiple statistical analyses and comparisons. The chi-square test and Fisher's extract test were used to compare the distribution of sex and the prevalence of MI among the groups. And the logistic regression was used to find out the association of risk factors and MIs after IVBs.
Statistical analyses were performed using R software (ver. 3.2.3; [2015-12-10; platform, x86_64-redhat-linux-gnu, R Core Team, 2015]). The R software was a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria; URL https://www.R-project.org/). The statistical significance level was set at P < 0.05.
3. Results
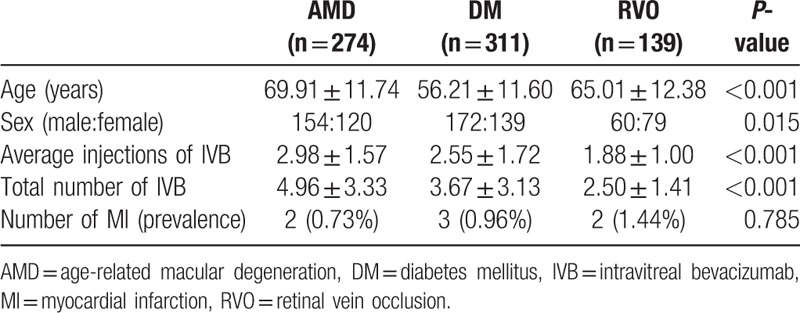
After excluding 37 patients with incomplete records within 2 months after the IVB injection, we enrolled 724 patients, who received a total of 1870 IVB injections. The average age was 62.09 ± 13.39 years. The average age of the diabetes mellitus (DM) group was 56.21 ± 11.60 years, which was significantly younger than the AMD group (69.91 ± 11.74 years; P < 0.001) and the RVO group (65.01 ± 12.38 years; P < 0.001). There was also significant difference in age between the AMD and RVO groups (P < 0.001). There were 386 males and 338 females, and there was a significant difference in sex distribution among the 3 groups (P = 0.015); post hoc analyses showed significant difference between the AMD and RVO groups (P = 0.013). The average number of injections was 2.58 ± 1.60. The number of IVB injections was significantly different among the 3 groups (P < 0.001). The post hoc analyses showed that the average number of IVB injections in patients with AMD (2.98 ± 1.57) was significantly higher than in patients with DM (2.55 ± 1.72; P < 0.001) and patients with RVO (1.88 ± 1.00, P < 0.001). There was also significant difference in the average number of IVB injections between patients with DM and RVO (P = 0.001).
Seven patients were diagnosed with MI within 2 months after receiving an IVB injection. Of 274 patients with AMD, 2 (0.73%) were diagnosed with MI; of 311 patients with diabetes-related complications, 3 (0.96%) were diagnosed with MI; and of 139 patients with RVO, 2 (1.44%) were diagnosed with MI. There was no significant difference in MI prevalence after IVB injection according to the reason for receiving the injection (P = 0.785; Table 1).
Table 1.
Demographic and baseline clinical characteristics of the patients.
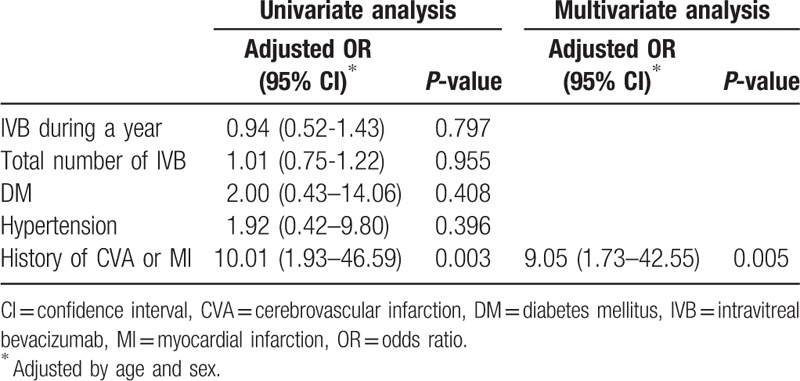
The average age of patients diagnosed with MI after the IVB injection was 64.42 ± 13.22 years (6 males and 1 female). All patients experienced chest pain between 3 and 21 days after the IVB injections. The average time of onset of these pains was 14.00 ± 6.45 days. Four patients were treated with hypertension medications and 5 were treated for DM. Five patients were diagnosed with MI after receiving the first injection and the other 2 patients were diagnosed after the third injection. Two of these patients had a history of MI and 1 had a history of cerebrovascular infarction. In logistic regression analysis, MIs after receiving IVB were associated with previous history of MI or cerebrovascular infarction (P = 0.005; Table 2).
Table 2.
Variables associated with MI to IVB upon logistic regression analysis.
4. Discussion
As previously mentioned, there has been controversy concerning the possible association between IVB injections and thromboembolic accidents. Some studies reported no association between IVB injections and cerebrovascular accidents (CVAs) or MIs,[21–25] but others reported that IVB injections were associated with an increased risk of CVAs or MIs.[18–20,28,29] These studies reported that the cause of the MIs was the use of anti-VEGF treatments that increased the risk of systemic VEGF suppression.[28,30] Some studies have also reported a significant decrease in plasma VEGF levels after bevacizumab or aflibercept intravitreal injections,[31,32] and the presence of ranibizumab in the systemic circulation after intravitreal injections.[33] The VEGF is necessary for normal functioning of the endothelium, where it promotes vascular integrity and endothelial cell survival,[34,35] but in 1 study systemic anti-VEGF agents caused vascular endothelial cell dysfunction, which induced a coagulation cascade.[36] Another study showed that IVB injection resulted in increased serum D-dimer levels and risk of thromboembolism.[37] Some VEGF isoforms play a positive role in cardiovascular function, but nonselective inhibition of VEGF may be involved in the induction of MI.[38] Most of these studies investigated patients with AMD[18,21,22,27,39]; the present study is the first to compare differences in vascular complication according to the type of disease treated with anti-VEGF.
We could not determine the significance of group differences in prevalence of MI, because of the small sample size. The prevalence of MI was 0.73% in patients with AMD, 0.96% in patients with DM, and 1.44% in patients with RVO. Of the 3 groups, the average number of injections was lowest in the patients with RVO, and the average age of the patients with RVO was lower than that of the patients with AMD. In addition, the patients with RVO included a higher proportion of females compared with the other groups. Previous studies have reported a higher prevalence of MI among males than among females in the Korean population.[40,41] Despite these demographic characteristics of the RVO group, the difference in MI prevalence between the AMD and RVO groups was 0.71%, with a 1.97-fold higher rate of MI development in the RVO group compared with the AMD group, although this difference was not significant (P = 0.605). Although the small sample size precluded significance, there was a larger difference in prevalence than we expected. This may indicate that there is no association between IVB injection and the development of MI, not only because there was no significant difference but also because more MIs occurred in patients with DM and RVO, suggesting that the MI was caused by systemic vascular disorders and not by IVB injections. However, it is also possible that DM- or RVO-induced damage to the blood-retinal barrier (BRB) could have affected the systemic circulation and induced vascular damage. A previous study reported that plasma VEGF levels among patients with DME were decreased compared with those in patients with AMD after IVB injections, and that this decrease was maintained for 1 month.[31] The study did not suggest why plasma VEGF levels among patients with DME were lower than those of patients with AMD, but based on the results of our study, we suggest that damage to the BRB may have played a role. It is well known that retinal leakage after breakdown of the BRB and subsequent ME are caused by diabetic retinopathy, AMD, RVO, and uveitis.[42] Although there has been a report of AMD associated with BRB damage,[43] there is a lack of evidence.[44] However, BRB damage associated with DM or ischemic retinal diseases such as RVO is well established.[42,45–49] Furthermore, the patients with RVO with MI were all diagnosed with ischemic central RVO, which is a more severe ischemic retinal condition. Damage to the BRB in patients with DM and RVO could induce a decrease in VEGF levels, which could in turn induce MI. This possibility should be investigated using a prospective design that determines systemic levels of VEGF and anti-VEGF in patients with AMD, RVO, and DM.
A previous study of the prevalence of MI among patients with DM reported 54.62 cases/10,000 per year among the Korean population in 2012 to 2013.[41] This differed from the present study, which showed a prevalence of 106.76/10,000, although the study period and the status of the enrolled patients were not the same. In general, DM retinopathy is associated with a long disease duration, poor glycemic control, and comorbidities such as hypertension and nephropathy.[50–52] In addition, one of the patients with DME already had a history of MI, so the comparison was not valid.
Previous studies reported only the prevalence of MI, but we investigated the interval between IVB injections and the development of MI. All patients had chest pains between 3 and 21 days after the IVB injection. Previous animal pharmacokinetic studies after IVB injection reported that bevacizumab reached maximal levels in the retina at 7 days after injection, then decreased over 30 days.[53] One study reported that after the first IVB injection, the median time to reach maximum systemic levels was 7.0 days, and when comparing bevacizumab, ranibizumab, and aflibercept, bevacizumab showed the highest systemic levels in patients with AMD.[54] Another study reported that VEGF levels were significantly reduced until 28 days after IVB injection in exudative patients with AMD, suggesting a possible systemic safety issue.[55] Overall, these studies showed that the systemic effect of IVB injection reached a maximum at 1 week after injection, then decreased over 1 month. All of the MIs in our patients occurred between 3 and 21 days after the IVB injection, and most occurred between 11 and 21 days, with no occurrences between 1 and 2 months after the injection. This analysis included previous studies that reported the presence of systemic bevacizumab, suggesting a systemic effect of MI caused by IVB injection.
This study had some limitations. First, the sample size was not large enough to obtain definitive results. Second, investigation of the loss of patients to follow-up should have been performed, because these losses may have been associated with thromboembolism. Prospective studies with larger sample populations would therefore be of benefit.
Although most previous studies characterized patients with AMD, characterization of systemic VEGF and anti-VEGF levels in patients with DM and RVO could help to determine the extent of BRB damage, and identify the possible association between MI and IVB injections.
In conclusion, there was no significant difference in MI prevalence according to the type of disease that required IVB injections. The MIs after receiving IVB were associated with previous history of MI or cerebrovascular infarction. Additionally, considering that all MIs developed within 3 weeks after the IVB injections, careful consideration by clinicians is necessary before administering IVB injections, especially to patients who have MI risk factors.
Author contributions
Conceptualization: J-W. Kwon.
Data curation: D. Jee, J-W. Kwon.
Formal analysis: J-W. Kwon.
Funding acquisition: D. Jee.
Investigation: J-W. Kwon.
Software: J-W. Kwon.
Supervision: T.Y. La.
Writing – original draft: J-W. Kwon.
Writing – review & editing: D. Jee, J-W. Kwon.
Footnotes
Abbreviations: AMD = age-related macular degeneration, BRB = blood-retinal barrier, CVA = cerebrovascular accident, DM = diabetes mellitus, DME = diabetic macular edema, IVB = intravitreal bevacizumab, ME = macular edema, MI = myocardial infarction, OCT = optical coherence tomography, PDR = proliferative diabetic retinopathy, ROP = retinopathy of prematurity, RVO = retinal vein occlusion, VEGF = vascular endothelial growth factor.
Funding/support: The authors acknowledge the financial support of the National Research Foundation of Korea Grant funded by the Korean government (MSIP) (No NRF-2016R1D1A1B03932606).
The authors have no conflicts of interest to disclose.
References
- [1].Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 2016;51:156–86. [DOI] [PubMed] [Google Scholar]
- [2].Shin YW, Lee YJ, Lee BR, et al. Effects of an intravitreal bevacizumab injection combined with panretinal photocoagulation on high-risk proliferative diabetic retinopathy. Korean J Ophthalmol 2009;23:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the lucentis compared to avastin study treat-and-extend protocol: two-year results. Ophthalmology 2016;123:51–9. [DOI] [PubMed] [Google Scholar]
- [4].Yoon YH, Kim HK, Yoon HS, et al. Improved visual outcome with early treatment in macular edema secondary to retinal vein occlusions: 6-month results of a Korean RVO study. Jpn J Ophthalmol 2014;58:146–54. [DOI] [PubMed] [Google Scholar]
- [5].Kriechbaum K, Prager F, Geitzenauer W, et al. Association of retinal sensitivity and morphology during antiangiogenic treatment of retinal vein occlusion over one year. Ophthalmology 2009;116:2415–21. [DOI] [PubMed] [Google Scholar]
- [6].Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heinemann V, Hoff PM. Bevacizumab plus irinotecan-based regimens in the treatment of metastatic colorectal cancer. Oncology 2010;79:118–28. [DOI] [PubMed] [Google Scholar]
- [8].Johnson DR, Leeper HE, Uhm JH. Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis. Cancer 2013;119:3489–95. [DOI] [PubMed] [Google Scholar]
- [9].Holfinger S, Miller AG, Rao LJ, et al. Effect of regulatory requirement for patient-specific prescriptions for off-label medications on the use of intravitreal bevacizumab. JAMA Ophthalmol 2016;134:45–8. [DOI] [PubMed] [Google Scholar]
- [10].Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. [DOI] [PubMed] [Google Scholar]
- [11].Verner-Cole EA, Davis SJ, Lauer AK. Aflibercept for the treatment of neovascular age-related macular degeneration. Drugs Today (Barc) 2012;48:317–29. [DOI] [PubMed] [Google Scholar]
- [12].Parikh RN, Traband A, Kolomeyer AM, et al. Intravitreal bevacizumab for the treatment of vitreous hemorrhage due to proliferative diabetic retinopathy. Am J Ophthalmol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berk Ergun S, Toklu Y, Cakmak HB, et al. The effect of intravitreal bevacizumab as a pretreatment of vitrectomy for diabetic vitreous hemorrhage on recurrent hemorrhage. Semin Ophthalmol 2015;30:177–80. [DOI] [PubMed] [Google Scholar]
- [14].Hwang HB, Han JW, Yim HB, et al. Beneficial effects of adjuvant intravitreal bevacizumab injection on outcomes of Ahmed glaucoma valve implantation in patients with neovascular glaucoma: systematic literature review. J Ocul Pharmacol Ther 2015;31:198–203. [DOI] [PubMed] [Google Scholar]
- [15].Karaca EE, Kepez Yldz B, Cubuk MO, et al. Epiretinal membranes in neovascular age-related macular degeneration: effect on outcomes of anti-vascular endothelial growth factor therapy. Retina 2015;35:1540–6. [DOI] [PubMed] [Google Scholar]
- [16].Batman C, Ozdamar Y. The relation between bevacizumab injection and the formation of subretinal fibrosis in diabetic patients with panretinal photocoagulation. Ophthalmic Surg Lasers Imaging 2010;41:190–5. [DOI] [PubMed] [Google Scholar]
- [17].Arevalo JF, Maia M, Flynn HW, Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008;92:213–6. [DOI] [PubMed] [Google Scholar]
- [18].Carneiro AM, Barthelmes D, Falcao MS, et al. Arterial thromboembolic events in patients with exudative age-related macular degeneration treated with intravitreal bevacizumab or ranibizumab. Ophthalmologica 2011;225:211–21. [DOI] [PubMed] [Google Scholar]
- [19].Kemp A, Preen DB, Morlet N, et al. Myocardial infarction after intravitreal vascular endothelial growth factor inhibitors: a whole population study. Retina 2013;33:920–7. [DOI] [PubMed] [Google Scholar]
- [20].Sharma S, Johnson D, Abouammoh M, et al. Rate of serious adverse effects in a series of bevacizumab and ranibizumab injections. Can J Ophthalmol 2012;47:275–9. [DOI] [PubMed] [Google Scholar]
- [21].Etminan M, Maberley DA, Babiuk DW, et al. Risk of myocardial infarction and stroke with single or repeated doses of intravitreal bevacizumab in age-related macular degeneration. Am J Ophthalmol 2016;163:53–8. [DOI] [PubMed] [Google Scholar]
- [22].Curtis LH, Hammill BG, Schulman KA, et al. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol 2010;128:1273–9. [DOI] [PubMed] [Google Scholar]
- [23].Campbell RJ, Gill SS, Bronskill SE, et al. Adverse events with intravitreal injection of vascular endothelial growth factor inhibitors: nested case-control study. BMJ 2012;345:e4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hwang DJ, Kim YW, Woo SJ, et al. Comparison of systemic adverse events associated with intravitreal anti-VEGF injection: ranibizumab versus bevacizumab. J Korean Med Sci 2012;27:1580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thulliez M, Angoulvant D, Le Lez ML, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol 2014;132:1317–26. [DOI] [PubMed] [Google Scholar]
- [26].Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006;113:1695.e1-15. [DOI] [PubMed] [Google Scholar]
- [27].Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006;113:363–72. e5. [DOI] [PubMed] [Google Scholar]
- [28].Ikram MK, Mitchell P, Klein R, et al. Age-related macular degeneration and long-term risk of stroke subtypes. Stroke 2012;43:1681–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schmid MK, Bachmann LM, Fas L, et al. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age-related macular degeneration: a trade-off analysis. Br J Ophthalmol 2015;99:141–6. [DOI] [PubMed] [Google Scholar]
- [30].Wong TY, Tikellis G, Sun C, et al. Age-related macular degeneration and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Ophthalmology 2007;114:86–91. [DOI] [PubMed] [Google Scholar]
- [31].Zehetner C, Kirchmair R, Huber S, et al. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol 2013;97:454–9. [DOI] [PubMed] [Google Scholar]
- [32].Zehetner C, Kralinger MT, Modi YS, et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol 2015;93:e154–9. [DOI] [PubMed] [Google Scholar]
- [33].Boyer DS, Heier JS, Brown DM, et al. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology 2009;116:1731–9. [DOI] [PubMed] [Google Scholar]
- [34].Domigan CK, Warren CM, Antanesian V, et al. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J Cell Sci 2015;128:2236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zechariah A, ElAli A, Doeppner TR, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke 2013;44:1690–7. [DOI] [PubMed] [Google Scholar]
- [36].Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007;99:1232–9. [DOI] [PubMed] [Google Scholar]
- [37].Jee D, Zako M, La TY. Serum D-dimer levels to evaluate the risk for arterial thromboembolism after intravitreal injection of bevacizumab and ranibizumab. J Ocul Pharmacol Ther 2015;31:32–6. [DOI] [PubMed] [Google Scholar]
- [38].Semeraro F, Morescalchi F, Parmeggiani F, et al. Systemic adverse drug reactions secondary to anti-VEGF intravitreal injection in patients with neovascular age-related macular degeneration. Curr Vasc Pharmacol 2011;9:629–46. [DOI] [PubMed] [Google Scholar]
- [39].Mikacic I, Bosnar D. Intravitreal bevacizumab and cardiovascular risk in patients with age-related macular degeneration: systematic review and meta-analysis of randomized controlled trials and observational studies. Drug Saf 2016;39:517–41. [DOI] [PubMed] [Google Scholar]
- [40].Hong J-S, Kang H-C. Regional differences in treatment frequency and case-fatality rates in Korean patients with acute myocardial infarction using the Korea National Health Insurance Claims Database: findings of a large retrospective cohort study. Medicine 2014;93:e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jung CH, Chung JO, Han K, et al. Improved trends in cardiovascular complications among subjects with type 2 diabetes in Korea: a nationwide study (2006-2013). Cardiovasc Diabetol 2017;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013;34:19–48. [DOI] [PubMed] [Google Scholar]
- [43].Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2004;242:91–101. [DOI] [PubMed] [Google Scholar]
- [44].Muthusamy A, Lin CM, Shanmugam S, et al. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J Cereb Blood Flow Metab 2014;34:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Silva PS, Sun JK, Aiello LP. Role of steroids in the management of diabetic macular edema and proliferative diabetic retinopathy. Semin Ophthalmol 2009;24:93–9. [DOI] [PubMed] [Google Scholar]
- [46].Vinores SA, McGehee R, Lee A, et al. Ultrastructural localization of blood-retinal barrier breakdown in diabetic and galactosemic rats. J Histochem Cytochem 1990;38:1341–52. [DOI] [PubMed] [Google Scholar]
- [47].Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia 2001;44:791–804. [DOI] [PubMed] [Google Scholar]
- [48].Tong N, Zhang Z, Zhang W, et al. Diosmin alleviates retinal edema by protecting the blood-retinal barrier and reducing retinal vascular permeability during ischemia/reperfusion injury. PLoS One 2013;8:e61794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ju WK, Gwon JS, Kim KY, et al. Up-regulated eNOS protects blood-retinal barrier in the L-arginine treated ischemic rat retina. Neuroreport 2001;12:2405–9. [DOI] [PubMed] [Google Scholar]
- [50].Penman A, Hancock H, Papavasileiou E, et al. Risk factors for proliferative diabetic retinopathy in African Americans with type 2 diabetes. Ophthalmic Epidemiol 2016;23:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cleland CR, Burton MJ, Hall C, et al. Diabetic retinopathy in Tanzania: prevalence and risk factors at entry into a regional screening programme. Trop Med Int Health 2016;21:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Al-Rubeaan K, Abu El-Asrar AM, Youssef AM, et al. Diabetic retinopathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. Acta Ophthalmol 2015;93:e140–7. [DOI] [PubMed] [Google Scholar]
- [53].Olsen TW, Feng X, Wabner K, et al. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci 2011;52:4749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014;98:1636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Carneiro AM, Costa R, Falcao MS, et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol 2012;90:e25–30. [DOI] [PubMed] [Google Scholar]


