Supplemental Digital Content is available in the text
Keywords: liposomal bupivacaine, meta-analysis, total knee arthroplasty
Abstract
Background:
Adequate pain control after total knee arthroplasty (TKA) enables quicker recovery and reduces readmissions and treatment costs. The aim of this study was to determine the effect of liposomal bupivacaine (LB) for postoperative pain control in patients prepared for TKA.
Methods:
We searched for the reports that evaluating the effect of liposomal bupivacaine for postoperative pain control in patients prepared for TKA between March 1983 and May 2017 in the electronic database Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, Web of Science, and Ovid. The main outcomes were visual analog scale (VAS) at 24, 48, and 72 hours. The secondary outcomes were total morphine consumption, the length of hospital stay, range of motion, and the occurrence of nausea.
Results:
Seven randomized controlled trials (RCTs) enrolling 825 patients, with 413 in the LB group and 412 in the control group, were included in this meta-analysis. Our results suggested that administration LB was associated with a reduction of VAS by 4.22 points at 72 hours after TKA (WMD = −4.22, 95% CI −7.47, −0.97, P = .011) on a 100-point VAS. What's more, LB can decrease the occurrence of nausea when compared with traditional bupivacaine by 18.3% (risk ratio = 0.70, 95% confidence interval 0.55, 0.89, P = .003). LB was associated with an increase of the range of motion than traditional bupivacaine (P < .05). There was no significant difference between the VAS at 24, 48 hours, total morphine consumption and the length of hospital stay.
Conclusions:
Administration with LB was associated with pain-relieving effects and reduces the morphine-related complications (nausea). Due the limited number of the included RCTs, large number and high quality RCTs are still need to identify the effects of LB for pain control after TKA.
1. Introduction
Control of pain following total knee arthroplasty (TKA) continues to be critical for a successful outcome.[1] Adequate pain control after TKA will lead to quicker recovery and reduces readmissions and treatment costs.[2] The utilization of multimodal pain management, has positive influence on the quality of the postoperative care, pain reduction, decreases opioid consumption, and the subsequent dose related opioid complications.[3,4] Administration with opioid was associated with oppressive complications, such as nausea, vomiting, and respiratory depression.[5,6] Local infiltration anesthesia with multimodal drugs have been introduced to minimize postoperative pain while posing minimal systemic or local risks to the patient; however, the benefits of most local injections are limited to the immediate postoperative period.
Recently, bupivacaine has been manufactured into a multivesicular liposome, which allows for its extended release into the surrounding tissues.[7,8] Liposomal bupivacaine (LB) allows delivery of bupivacaine for 96 hours with a single local administration, with a similar wound-healing profile as regular bupivacaine. Several randomized controlled trials (RCT) have compared LB with traditional bupivacaine for pain control after TKA. Many of these trials contained relatively small cohorts, and demonstrated inconsistent outcomes.[9–11]
Two meta-analyses were published on this topic recently. Kuang et al[12] reported that LB shows no superiority than traditional bupivacaine regarding pain control. However, Wang et al[13] demonstrated that LB preserved greater pain control and less complications than traditional bupivacaine. Above 2 meta-analyses have following shortcomings: both of the 2 meta-analyses included non-RCTs and thus large selection bias were existed in the meta-analysis; and both of the 2 meta-analysis did not perform the dose-response relationship between the dose of LB and pain intensity. Thus, we undertook a further meta-analysis to evaluate whether LB is superior to traditional bupivacaine with respect to: pain score at 24, 48, and 72 hours; opioid intake; length of hospital stay; range of motion; and complications. We hypothesized that LB results in less pain intensity and morphine consumption, and provide less complications.
2. Materials and methods
This systematic review was reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.[14] Ethical approval was unnecessary in this study because it was a meta-analysis analyzing existing articles and did not need to handle individual patient data.
2.1. Search strategies
The following databases were searched in September 2016 without restriction of regions or publication types: PubMed (1950–May 2017), EMBASE (1974–May 2017), Web of Science (1950–May 2017), and Cochrane Library (May 2017 Issue 3). The Mesh terms and their combinations used in the search were as follows: “Arthroplasty, Replacement, Knee” [Mesh]” OR “total knee replacement” OR “total knee arthroplasty” OR “TKR” OR “TKA” AND “liposomal bupivacaine” OR “LB.” The reference lists of related reviews and original articles were searched for any relevant studies, including randomized controlled trials (RCTs) involving adult humans. Only articles originally written in English or translated into English were considered. When multiple reports describing the same sample were published, the most recent or complete report was used.
2.2. Inclusion criteria and study selection
Patients: adult human subjects (age >18 years) prepared for unilateral TKA; intervention: local infiltration LB as an intervention group; comparison: local infiltration traditional bupivacaine as a comparison group; outcomes: visual analogue scale (VAS) at 24, 48, and 72 hours, total morphine consumption, length of hospital stay, range of motion and the occurrence of nausea; and study design: RCTs. Two independent reviewers screened the title and abstracts of the identified studies after removing the duplicates of the search results. Any disagreements about the inclusion or exclusion of a study were solved by discussion or consultation with an expert. The reliability of the study selection was determined by Cohen's kappa test, and the acceptable threshold value was set at 0.61.[15,16]
2.3. Data abstraction
A specific extraction was conducted to collect the following data from the included trials: patients’ number, dose of LB, control group, outcomes, study type, and follow-ups. Outcomes such as VAS at 24, 48, and 72 hours, the occurrence of nausea were abstracted and recorded in a sheet. Postoperative pain intensity was measured by a 100-point VAS. When the numerical rating scale (NRS) was reported, it was converted to a VAS. Additionally, a 10-point VAS was converted to a 100-point VAS.[17] Data in other forms (i.e., median, interquartile range, and mean ± 95% confidence interval [CI]) were converted to the mean ± standard deviation (SD) according to the Cochrane Handbook.[18] If the data were not reported numerically, we extracted these data using “GetData Graph Digitizer” software from the published figures. All the data were extracted by 2 independent reviewers and disagreements were resolved by consulted from original author or discussion with senior expert.
2.4. Quality assessment
The methodological quality of all included trials was independently assessed by 2 reviewers on the basis of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (http://www.cochrane-handbook.org/).
2.5. Outcome measures and statistical analysis
Continuous outcomes (VAS at 24, 48, and 72 hours, total morphine consumption, range of motion, and the length of hospital stay) were expressed as the weighted mean differences (WMD) and respective 95% CI. Dichotomous outcomes (the occurrence of nausea) were expressed as the risk ratio (RR) with 95% CI. Statistical significance was set at P < .05 to summarize the findings across the trials. The meta-analysis was calculated by Stata software, version 12.0 (Stata Corp., College Station, TX). Statistical heterogeneity was tested using the χ2 test and I2 statistic. When there was no statistical evidence of heterogeneity (I2 < 50%, P > .1), a fixed-effects model was adopted; otherwise, a random-effect model was chosen. Subgroup analysis was conducted according to the dose of LB (<266 mg/d or ≥266 mg/d). We considered there to be no publication bias if the funnel plot was symmetrical and the P > .05. The relationship between LB dosage and the VAS at 24, 48, and 72 hours was explored using SPSS software (SPSS Inc., Chicago, IL). The correlation coefficient (r) was used to evaluate the relationship between the dosage of LB and the VAS at 24, 48, and 72 hours.
3. Results
3.1. Search results
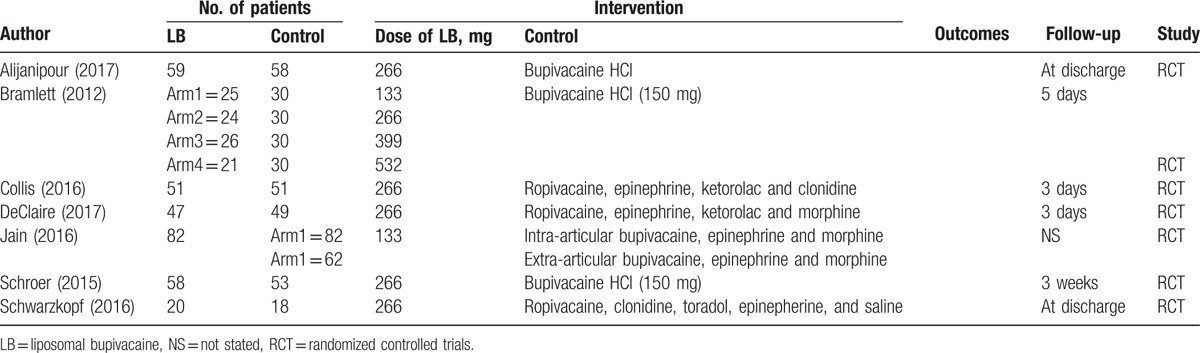
In the initial search, a total of 401 papers were identified from the electronic databases (PubMed = 146, Embase = 39, Web of Science = 131, and Cochrane Library = 85). The number of articles after duplicates had been removed by Endnote X7 software was 305. After screened the abstracts and title of these 305 studies, 298 papers were excluded because they were irrelevant or did not meet the criteria. Finally, we included 7 RCTs for this meta-analysis.[9–11,19–22] PRISMA flowchart for the included studies can be seen in Supplement S1. The general characteristic of the included RCTs can be seen in Table 1. The total dose of LB ranged from 133 to 532 mg. The sample ranged from 18 to 82 TKAs.
Table 1.
The general characteristic of the included studies.
3.2. Quality assessment
Figure 1 outlines the details of the risk of bias summary. And risk of bias graph was summarized in Supplement S2. All of the studies were considered to be at low risk of bias. Randomized sequence generation was implemented adequately in 4 studies; the rest 3 studies mentioned randomized study but did not describe the detailed method of random sequence generation. Allocation concealment was implemented adequately in 6 studies. All the studies reported blinding of the participants and personnel. Only one study did not blind to the outcome assessment.
Figure 1.
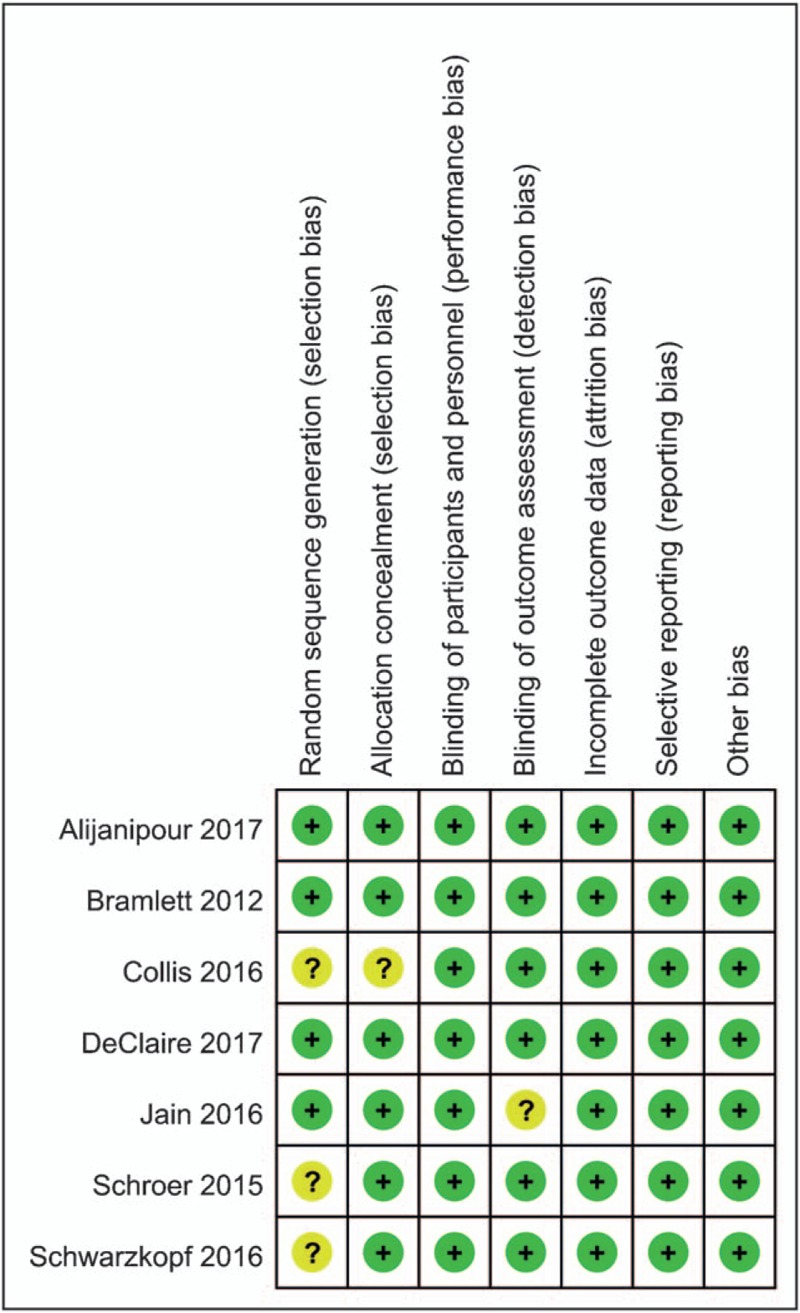
The risk of bias summary for the included studies.
4. Results of meta-analysis
4.1. VAS at 24, 48, and 72 hours
Postoperative VAS scores at 24 hours in the included studies had a large heterogeneity (I2 = 55.6%, P = .016), which required a random-effects model that was performed to analyze the data. The meta-analysis results indicated that there was no significant difference between the LB group and traditional bupivacaine group in terms of the VAS at 24 hours (WMD = 0.92, 95% CI –2.05, 3.88, P = .544, Supplement S3 A). Postoperative VAS scores at 48 hours had a large heterogeneity (I2 = 57.0%, P = .017), which required a random-effects model that was performed to analyze the data. The meta-analysis results indicated that there was no significant difference between the LB group and traditional bupivacaine group in terms of the VAS scores at 48 hours (WMD = −2.69, 95% CI −7.71, 1.79, P = .239, Supplement S3 B). Postoperative VAS scores at 72 hours had no heterogeneity (I2 = 0.0%, P = .514), which required a fixed-effects model that was performed to analyze the data. The meta-analysis results indicated that LB can decrease VAS scores at 72 hours when compared with traditional bupivacaine (WMD = −4.22, 95% CI −7.47, −0.97, P = .011, Supplement S3 C).
4.2. Total morphine consumption
Total morphine consumption in the included studies had little heterogeneity (I2 = 4.8%, P = .379), which required a fixed-effects model that was performed to analyze the data. The meta-analysis results indicated that there was no significant difference between the LB group and traditional bupivacaine group in terms of total morphine consumption (WMD = −0.49, 95% CI −10.19, 9.20, P = .920, Supplement S4).
4.3. Length of hospital stay
Length of hospital stay in the included studies had no heterogeneity (I2 = 0.0%, P = .596), which required a fixed-effects model that was performed to analyze the data. The meta-analysis results indicated that there was no significant difference between the LB group and traditional bupivacaine group in terms of the length of hospital stay (WMD = 0.15, 95% CI −0.02, 0.31, P = .082, Supplement S5).
4.4. Range of motion
Range of motion in the included studies had no heterogeneity (I2 = 0.0%, P = .483), which required a fixed-effects model that was performed to analyze the data. The meta-analysis results indicated that there use of LB group was associates with an increase of the range of motion than traditional bupivacaine group (WMD = 3.28, 95% CI 1.11, 5.45, P = .003, Supplement S6).
4.5. The occurrence of nausea
The occurrence of nausea in the included studies had a large heterogeneity (I2 = 66.0%, P = .019), which required a random-effects model that was performed to analyze the data. The meta-analysis results indicated that LB can decrease the occurrence of nausea when compared with traditional bupivacaine by 18.3% (risk ratio, RR = 0.70, 95% CI 0.55, 0.89, P = .003, Fig. 2).
Figure 2.
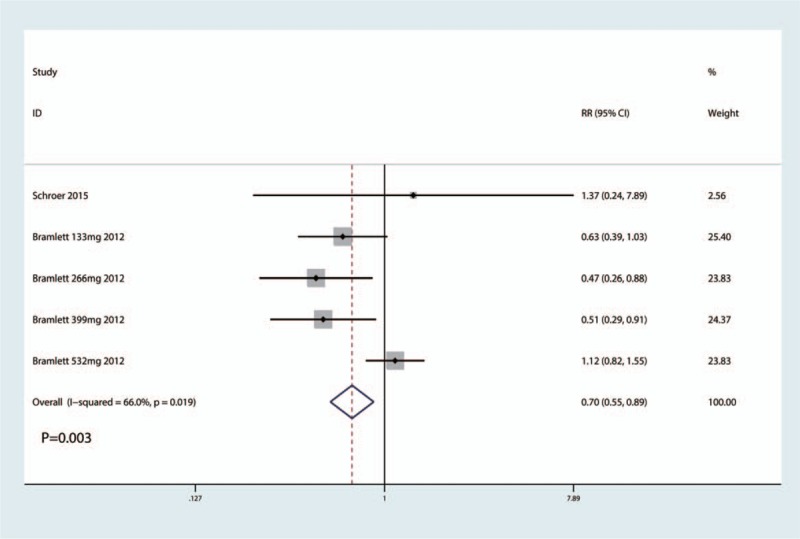
Forest plots comparing the occurrence of nausea between the 2 groups.
4.6. Subgroup analysis, sensitivity analysis and dose–response relationship
Subgroup analysis was conducted according to the dose of LB. The sensitivity analyses, which involved omitting each study, did not alter the outcomes. Supplement S7, displays the details of the sensitivity analyses for the VAS at 24, 48, and 72 hours, total morphine consumption, the length of hospital stay, and the occurrence of nausea. We plotted the LB dose on the abscissa, with the corresponding VAS at 24, 48, and 72 hours as the ordinate, to generate a scatterplot. In addition, the linear correlation coefficient (r) was also calculated. There was a significantly negative correlation between the dosage of LB and the VAS at 24 hours (r = −0.302, P = .023, Fig. 3 A) and 72 hours (r = −0.464, P = .011, Fig. 3 C). The VAS at 24 and 72 hours tended to decrease as the LB dose increased. There was no correlation between the dosage of LB and the VAS at 48 hours (r = 0.104, P = .789, Fig. 3 B).
Figure 3.
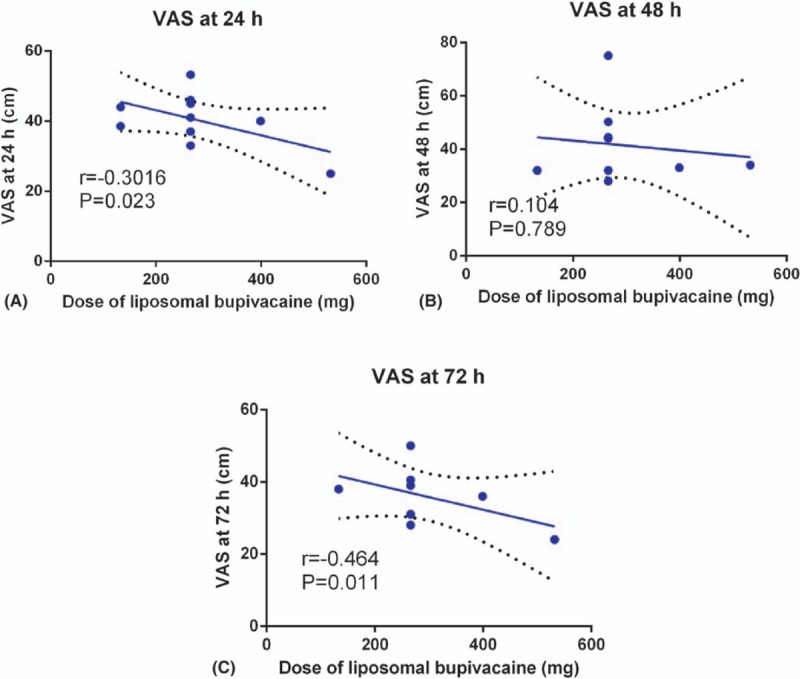
Scatter plot of the VAS at 24 hours (A), 48 hours (B), and 72 hours (C) and the dose of LB.
5. Discussion
This meta-analysis indicated that administration with LB can decrease the pain scores at 72 hours and the occurrence of nausea. There was no significant difference between the pain scores at 24 and 48 hours, total morphine consumption and the length of hospital stay. This meta-analysis included seven relevant RCTs and all of them with high quality.
High dose of LB was more effective than low dose of LB for reducing pain intensity after TKA. Bramlett et al[10] administration different dose of LB (133, 266, 399, and 532 mg) for pain control after TKA. Treatment with LB at 532 mg was associated with statistically significantly greater analgesia while patients were at rest after surgery compared with traditional bupivacaine. Mean and maximum plasma bupivacaine concentrations increased in a dose-related manner. Hamilton et al[23] reported that LB at the surgical site does not demonstrate superiority to bupivacaine hydrochloride. That finding was not consistent with our research, that study was intended to investigate the efficacy and safety of LB for patients with all type of surgeries. Wu et al[24] demonstrated that LB, as a novel anesthetic formulation, demonstrated better pain control than traditional bupivacaine following TKA. The half-lives of bupivacaine was 3.5 hours and this short half-lives cannot provide sustained pain relief after TKA.[25,26] LB use of the liposomes as drug carriers that are lipid-based molecules varying in size, offering sustained release of drugs over an extended period of time.
Nausea was common side effects that are frequently associated with oral or intravenous morphine. Current meta-analysis indicated that administration with LB was associated with a reduction of the occurrence of nausea (RR = 0.70, 95% CI 0.55, 0.89, P = .003).
Singh et al[27] revealed that administration with LB was associated with a reduction of the length of hospital stay by less than 1/4 days. However, the magnitude of the effect was actually quite small and probably with no clinically importance. Current meta-analysis indicated that there was no significant difference between the LB group and standard bupivacaine group in terms of the length of hospital stay. Another concern of administration LB was the economic costs for the patients. Jain et al[20] reported the cost for LB group (402.9 ± 8.78 USD) was significantly higher than the cost for standard bupivacaine (15.99 ± 5.01 USD).
Khlopas et al[28] reported that there is a learning curve associated with liposomal bupivacaine use, and incorporating an appropriate technique can markedly affect post-operative outcomes. We tried to contact the authors to identify whether they pass the learning curve, however, they did not reply to us. Thus, further studies should focus on the effectiveness of LB for pain control after TKA when passed the learning curve.
There were several limitations in this meta-analysis: only 7 RCTs were included, and the sample sizes in each trial were small, which would affect the final results; the duration of follow-up in some studies was unclear, and long-term follow-up was needed to test the complications for this analysis; the publication bias that existed in the meta-analysis also influenced the results; and the dose of LB and postoperative pain protocol were all different, which also likely had an effect on the final results.
6. Conclusions
In conclusion, the results of our meta-analysis indicate local infiltration LB is more effective at 72 hours compared to traditional bupivacaine. Another finding was LB can decrease the morphine-related complication (nausea). For future research, optimal drug dosages should be rigorously defined, and the method of local infiltration anesthesia should also be clarified. Even more importantly, well-designed trials with larger sample sizes are needed to further provide reliable evidence on the safety of LB for pain management after TKA. High-quality RCTs and well-designed trials are still required to detect differences in post-TKA function.
Author contributions
Data curation: L-L. Yao, Z-X. Yu, Z-Z. Yang.
Software: Z-Z. Yang.
Supervision: Z-Z. Yang.
Writing – original draft: L-L. Yao.
Writing – review & editing: Z-X. Yu.
Supplementary Material
Footnotes
Abbreviations: CENTRAL = Cochrane Central Register of Controlled Trials, CI = confidence interval, LB = liposomal bupivacaine, NRS = numerical rating scale, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCTs = randomized controlled trials, RR = risk ratio, SD = standard deviation, TKA = total knee arthroplasty, VAS = visual analog scale, WMD = weighted mean differences.
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Li F, Ma J, Kuang M, et al. The efficacy of pregabalin for the management of postoperative pain in primary total knee and hip arthroplasty: a meta-analysis. J Orthop Surg Res 2017;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rutherford RW, Jennings JM, Dennis DA. Enhancing recovery after total knee arthroplasty. Orthop Clin North Am 2017;48:391–400. [DOI] [PubMed] [Google Scholar]
- [3].Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology 2017;126:923–37. [DOI] [PubMed] [Google Scholar]
- [4].Karlsen AP, Wetterslev M, Hansen SE, et al. Postoperative pain treatment after total knee arthroplasty: a systematic review. PLoS One 2017;12:e0173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wheeler M, Oderda GM, Ashburn MA, et al. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain 2002;3:159–80. [DOI] [PubMed] [Google Scholar]
- [6].Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother 2007;41:400–6. [DOI] [PubMed] [Google Scholar]
- [7].Branney J, Izadpanah M. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Nurs Stand 2017;31:42–3. [DOI] [PubMed] [Google Scholar]
- [8].Asche CV, Ren J, Kim M, et al. Local infiltration for postsurgical analgesia following total hip arthroplasty: a comparison of liposomal bupivacaine to traditional bupivacaine. Curr Med Res Opin 2017;1–8. [DOI] [PubMed] [Google Scholar]
- [9].Alijanipour P, Tan TL, Matthews CN, et al. Periarticular injection of liposomal bupivacaine offers no benefit over standard bupivacaine in total knee arthroplasty: a prospective, randomized controlled trial. J Arthroplasty 2017;32:628–34. [DOI] [PubMed] [Google Scholar]
- [10].Bramlett K, Onel E, Viscusi ER, et al. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee 2012;19:530–6. [DOI] [PubMed] [Google Scholar]
- [11].Collis PN, Hunter AM, Vaughn MD, et al. Periarticular injection after total knee arthroplasty using liposomal bupivacaine vs a modified ranawat suspension: a prospective, randomized study. J Arthroplasty 2016;31:633–6. [DOI] [PubMed] [Google Scholar]
- [12].Kuang MJ, Du Y, Ma JX, et al. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2017;32:1395–402. [DOI] [PubMed] [Google Scholar]
- [13].Wang X, Xiao L, Wang Z, et al. Comparison of peri-articular liposomal bupivacaine and standard bupivacaine for postsurgical analgesia in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg 2017;39:238–48. [DOI] [PubMed] [Google Scholar]
- [14].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363–74. [PubMed] [Google Scholar]
- [16].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [17].Wang C, Cai X-Z, Yan S-G. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015;30:1281–6. [DOI] [PubMed] [Google Scholar]
- [18].GS HJ. Cochrane handbook for systematic reviews of interventions version 5.1.0[updated March 2011]. 2011. [Google Scholar]
- [19].DeClaire JH, Aiello PM, Warritay OK, et al. Effectiveness of bupivacaine liposome injectable suspension for postoperative pain control in total knee arthroplasty: a prospective, randomized, double blind, controlled study. J Arthroplasty 2017;32:S268–71. [DOI] [PubMed] [Google Scholar]
- [20].Jain RK, Porat MD, Klingenstein GG, et al. The AAHKS clinical research award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty 2016;31:22–5. [DOI] [PubMed] [Google Scholar]
- [21].Schroer WC, Diesfeld PG, LeMarr AR, et al. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty 2015;30:64–7. [DOI] [PubMed] [Google Scholar]
- [22].Schwarzkopf R, Drexler M, Ma MW, et al. Is there a benefit for liposomal bupivacaine compared to a traditional periarticular injection in total knee arthroplasty patients with a history of chronic opioid use? J Arthroplasty 2016;31:1702–5. [DOI] [PubMed] [Google Scholar]
- [23].Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev 2017;2:CD011419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu ZQ, Min JK, Wang D, et al. Liposome bupivacaine for pain control after total knee arthroplasty: a meta-analysis. J Orthop Surg Res 2016;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ong JC, Chin PL, Fook-Chong SM, et al. Continuous infiltration of local anaesthetic following total knee arthroplasty. J Orthop Surg (Hong Kong) 2010;18:203–7. [DOI] [PubMed] [Google Scholar]
- [26].Sun XL, Zhao ZH, Ma JX, et al. Continuous local infiltration analgesia for pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh PM, Borle A, Trikha A, et al. Role of periarticular liposomal bupivacaine infiltration in patients undergoing total knee arthroplasty: a meta-analysis of comparative trials. J Arthroplasty 2017;32:675.e1–88.e1. [DOI] [PubMed] [Google Scholar]
- [28].Khlopas A, Elmallah RK, Chughtai M, et al. The learning curve associated with the administration of intra-articular liposomal bupivacaine for total knee arthroplasty: a pilot study. Surg Technol Int 2017;30:314–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


