Abstract
Prognosis in colon cancer is based on pathological criteria including TNM staging. However, there are deficiencies in this approach, and the lymph node ratio (LNR) has been proposed to improve the prediction of outcomes. LNR is dependent on optimal retrieval of lymph nodes—lymph node count (LNC). Recent studies have suggested that an elevated preoperative systemic inflammatory response (SIR) was associated with a lower LNC and a higher LNR. However, there are a number of potential confounding factors. The aim of the present study was to examine, in detail, these relationships in a large cohort of patients.
A prospectively maintained database of all patients undergoing colon cancer resection in our institution was examined. The SIR was measured by a number of inflammatory markers and their scores: modified Glasgow Prognostic Score (mGPS) (C-reactive protein/albumin), neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), and lymphocyte monocyte ratio (LMR) using standard thresholds. The relationships between LNC and LNR, and clinicopathological characteristics (including the mGPS, NLR, PLR, and LMR) were examined using chi-square test for trend and binary logistic regression analysis, where appropriate.
Of the 896 patients included in the study, 418 (47%) were male, the median LNC was 17 (1–71), and the median LNR in node positive disease was 0.16 (0.03–1). On multivariate analysis, there was a significant independent relationship between an elevated LNC (≥12) and laparoscopic surgery (P < .001), right-sided tumors (P < .001), later date of resection (2007–2016) (P < .001), T stage (P < .001), and venous invasion (P < .001). In those patients with a LNC ≥12 and node-positive disease (n = 272), on multivariate analysis, there was a significant relationship between an elevated LNR (≥0.25), and T stage (P < .01) and differentiation (P < .05). Finally, in patients with node-positive disease who had surgery later (2007–2016), LNR was directly superior to N stage for both cancer-specific survival (LNR: hazard ratio [HR] 2.62, 95% confidence interval [CI] 1.25–5.52, P = .011) and overall survival (LNR: HR 2.02, 95% CI 1.12–3.68, P = .022).
Neither LNC nor LNR was associated with markers of the SIR; however, LNC and LNR were directly associated. In high-quality surgical and pathological practice, LNR had superior prognostic value compared with N stage in patients undergoing surgery for colon cancer.
Keywords: cancer specific survival, inflammation, lymph node count, lymph node ratio, lymphocyte monocyte ratio, modified Glasgow Prognostic Score, neutrophil lymphocyte ratio, overall survival, platelet lymphocyte ratio
1. Introduction
Cancer remains 1 of the leading causes of death worldwide and is responsible for 7.6 million deaths per year.[1–3] In the United Kingdom, it is responsible for at least 50,000 deaths each year in the 35 to 64-year age groups, and in their lifetime, 1 in 3 will develop cancer, and 1 in 4 will die from it.[1] In potentially curative disease, surgical resection is the treatment of choice and prognosis is significantly based on clinicopathological criteria, including the TNM staging system, which divides patients into groups based on tumor invasion, local nodal involvement, and distant metastatic spread.[4]
A high lymph node count (LNC) at resection has been reported to be associated with improved outcomes, regardless of tumor stage, with a LNC of ≥12 been widely accepted as indicative of an oncologically sound surgical resection.[5,6] The prognostic value of LNC would appear to be dependent on node-positive disease, because an increase in the proportion of positive nodes (lymph node ratio [LNR]) within any given resected specimen is strongly associated with poorer outcomes, with the cut-off value of 0.25 (1 in 4 nodes positive) having particular significance.[4,7,8] However, with variable retrieval of lymph nodes from surgical specimens LNR has not been incorporated into routine tumor staging.
Over the past 15 years, it has become clear that disease progression and cancer is not just dependent on local tumor factors, but rather on a complex interaction of both tumor and the host inflammatory responses.[9,10] Local tumor responses in colorectal cancer can be assessed by lymphocytic infiltration with improved survival in those who express a local lymphocytic response.[11,12] Also, the systemic inflammatory response (SIR) can be assessed by combined prognostic scores such as modified Glasgow Prognostic Score (mGPS) (C-reactive protein [CRP] and albumin), neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), and lymphocyte monocyte ratio (LMR).[1,10,13,14]
Therefore, it is of interest that an elevated SIR in patients with colon cancer (n = 303)[4] and in colorectal cancer (n = 501)[15] has been reported to be associated with a lower LNC. Also, an elevated SIR was associated with an increased LNR and poorer survival.[4] These studies would suggest that the SIR may result in a lower LNC with a consequent increase in the LNR with a direct impact on survival.[4]
Therefore, the primary aim of the present study was to examine, in detail, the relationships between LNC and LNR, and clinicopathological characteristics (including markers of SIR), in a large cohort of patients undergoing surgery for colon cancer. The secondary aim of this study was to assess the impact of both LNC and LNR on survival. No previous studies have comprehensively examined combination markers of the SIR in relation to both LNC and LNR in such a large cohort of patients undergoing surgery for colorectal cancer.
2. Patients and methods
Patients were identified from a prospectively collected and maintained database of colon cancer resections undertaken in a single surgical unit at Glasgow Royal Infirmary. In the present study, there were 2 main inclusion criteria, namely patients who, on the basis of preoperative abdominal computed tomography and laparotomy findings, were considered to have undergone potentially curative resection for colonic cancer between January, 1997 and May, 2016, and patients who had preoperative measurement of serum CRP, albumin, and differential blood cell counts within 30 days before surgery. Due to the prospective nature of the database, less than 10% of patients were excluded from the study. In particular, those patients with documented underlying inflammatory conditions, inflammatory bowel disease-related cancer, and, who underwent resection with palliative intent or local resection only, or had not had preoperative measurement of CRP or albumin, were excluded from the analysis. Tumors were staged using the fifth edition of the TNM, with additional data taken from pathological reports issued after resection.[16] The fifth edition of the TNM staging was used as per pathological practice in Glasgow Royal Infirmary. After surgery, all patients were discussed at a multidisciplinary meeting involving surgeons, oncologists, radiologists, and pathologists with special interest in colorectal cancer; patients with stage III or high-risk stage II disease and no significant comorbidities precluding chemotherapy use were offered primarily 5-fluorouracil-based adjuvant chemotherapy on the basis of current guidelines at the time. A LNC ≥12 was used in this study as it has been reported in the literature as been indicative of an adequate and safe surgical resection with improved outcomes.[5,6] A LNR ≥0.25 was used in this study, as it has been reported in the literature and has been associated with a higher tumor load associated with poorer outcomes.[4,7,8]
Preoperative serum CRP, albumin, and differential blood cell counts were recorded prospectively. Patients undergoing resection, serum CRP, albumin, and differential blood cell counts were measured routinely within 30 days before surgery. The mGPS[1] was constructed as previously described (patients with a CRP ≤10 mg/L were allocated a score of 0, a CRP >10 mg/L a score of 1, and a CRP >10 mg/L and albumin <35 g/L a score of 2). NLR, PLR, and LMR were all calculated by directly dividing the former by the latter.
Patients were routinely followed up for 5 years after surgery. Date and cause of death were crosschecked with the cancer registration system and the Registrar General (Scotland). Death records were complete until May 31, 2016, which acted as the censor date. Cancer-specific survival was measured from date of surgery until date of death from recurrent or metastatic colonic cancer, and was expressed as a percentage with an associated standard error with significance been assessed using a log-rank P test. Overall survival (OS) was measured until the date of death from any cause, and was expressed as a percentage with an associated standard error with significance been assessed using a log-rank P test. Lymph node size was not assessed formally in this study, and consultant pathologists assessed all pathological specimens to ensure consistency. The West of Scotland Research Ethics Committee approved the study.
The primary endpoint of examining the relationships between LNC and LNR, and clinicopathological characteristics (including the mGPS, NLR, PLR, and LMR) was examined using chi-square test for trend and binary logistic regression analysis. To adjust for multiple comparisons, a P value of <0.01 was considered significant. All characteristics that were statistically significant on chi-square test were entered into univariate binary logistic regression for both LNC and LNR. Clinicopathological factors associated with the LNC and LNR on univariate analysis that had a P value <0.05 were taken into a multivariate model using a backward conditional model to identify independently significant factors. The secondary endpoint of examining the relationship between LNC and LNR, and survival was examined using a log-rank P test. Statistical analysis was performed using SPSS version 22.0 (IBM Corp, Armonk, NY).
3. Results
Of the 896 patients included in the study, 418 (47%) were male, 478 (53%) female, the median LNC was 17 (1–71), and the median LNR in node-positive disease was 0.16 (0.03–1). The was a significant association between LNC and LNR (r = 0.379, P < .001; Fig. 1)
Figure 1.
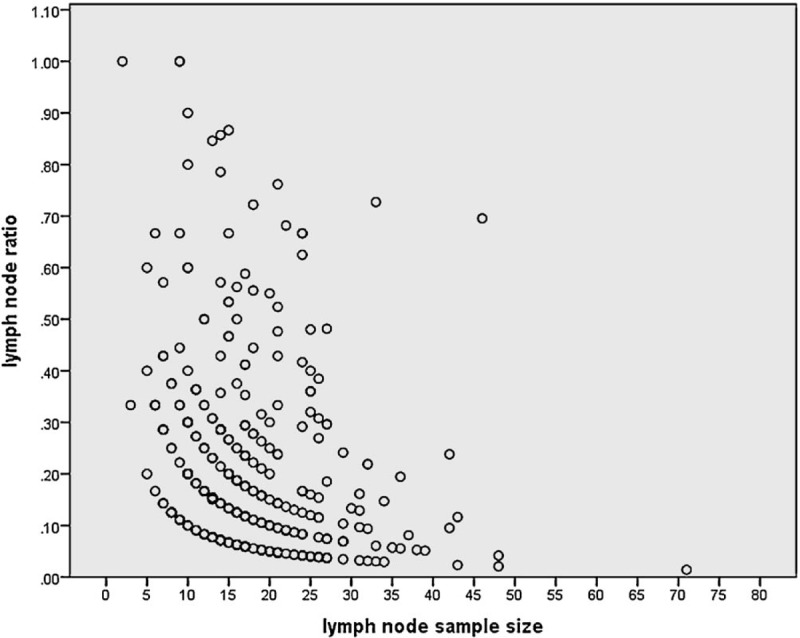
The relationship between LNC and LNR in patients with a positive lymph node in patients undergoing surgery for colon cancer (n = 377, r = 0.379, P < .001). LNC = lymph node count, LNR = lymph node ratio.
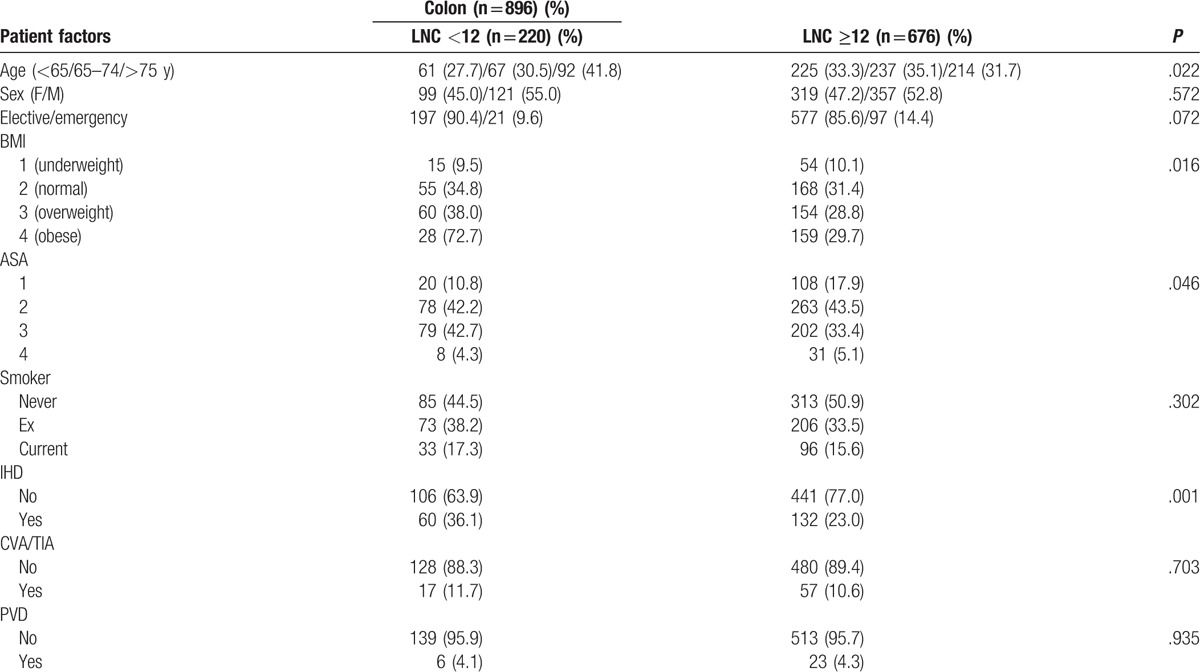
The relationship between the LNC (<12/≥12), clinicopathological characteristics, markers of the SIR, LNR, and survival in patients undergoing surgery for colon cancer is shown in Table 1 . LNC ≥12 (n = 676) was significantly associated with no ischemic heart disease (n = 132, P = .001), laparoscopic surgery (n = 148, P < .001), surgery carried out between 2007 and 2016 (n = 444, P < .001), right-sided tumors (n = 383, P < .001), higher T stage (n = 581, P < .001), and venous invasion (n = 378, P < .001). LNC ≥12 was not significantly associated with any of the markers of the SIR or with improved cancer specific (P = .176), or OS (P = .296). LNC ≥12 was significantly associated with a lower LNR (n = 613, P = .001).
Table 1.
The relationship between the LNC (<12/≥12), clinicopathological characteristics, LNR and survival in patients undergoing surgery for colon cancer (n = 896).
Binary logistic regression analysis was carried out on those variables with a significant association with LNC < 12/≥12 (Table 2). On multivariate analysis, there was a significant independent relationship between an elevated LNC (≥12) and laparoscopic surgery (P < .05), right-sided tumors (P < .01), surgery carried out between 2007 and 2016 (P < .001), T stage (P < .001), and LNR ≥0.25 (P < .05).
Table 1 (Continued).
The relationship between the LNC (<12/≥12), clinicopathological characteristics, LNR and survival in patients undergoing surgery for colon cancer (n = 896).
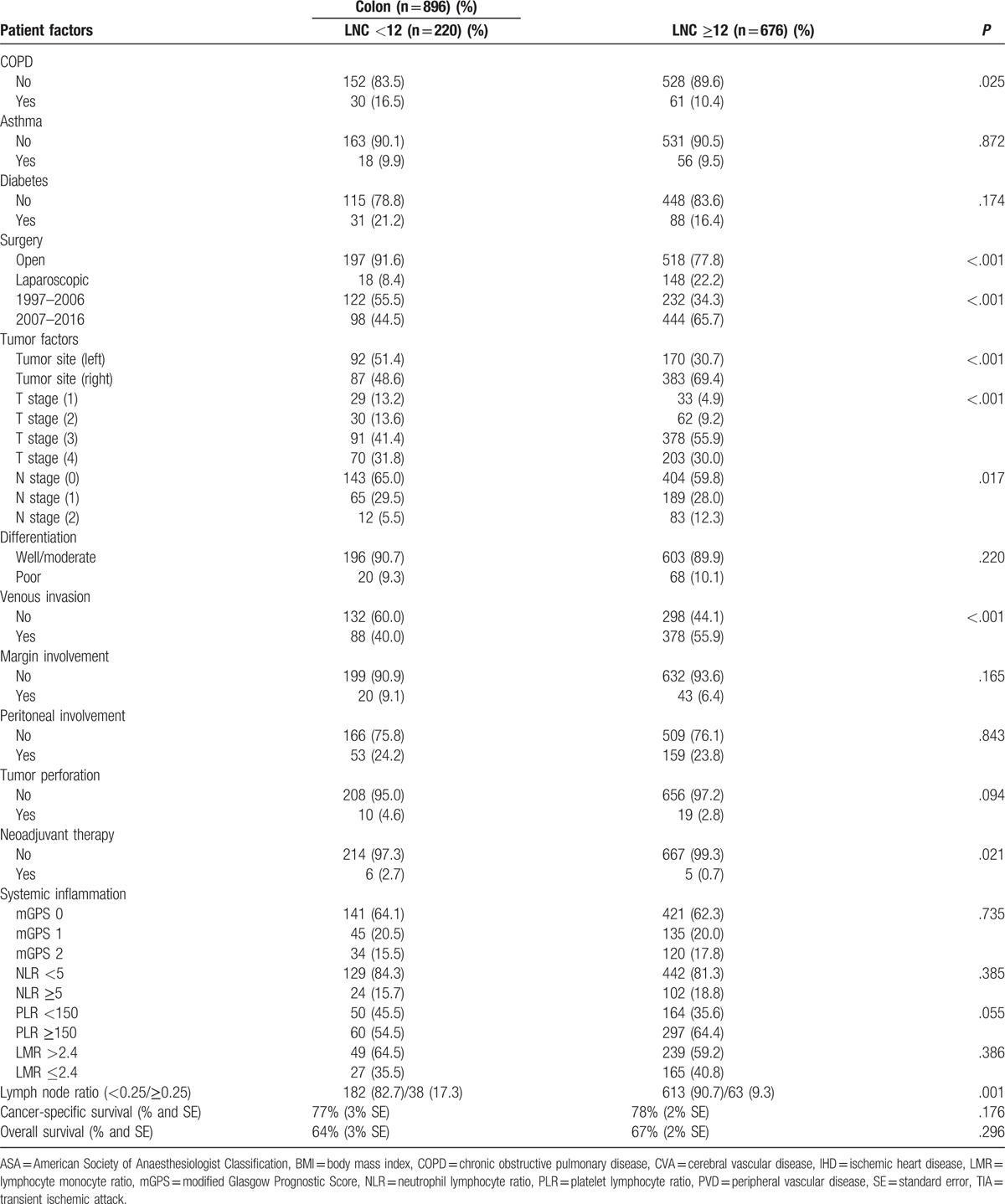
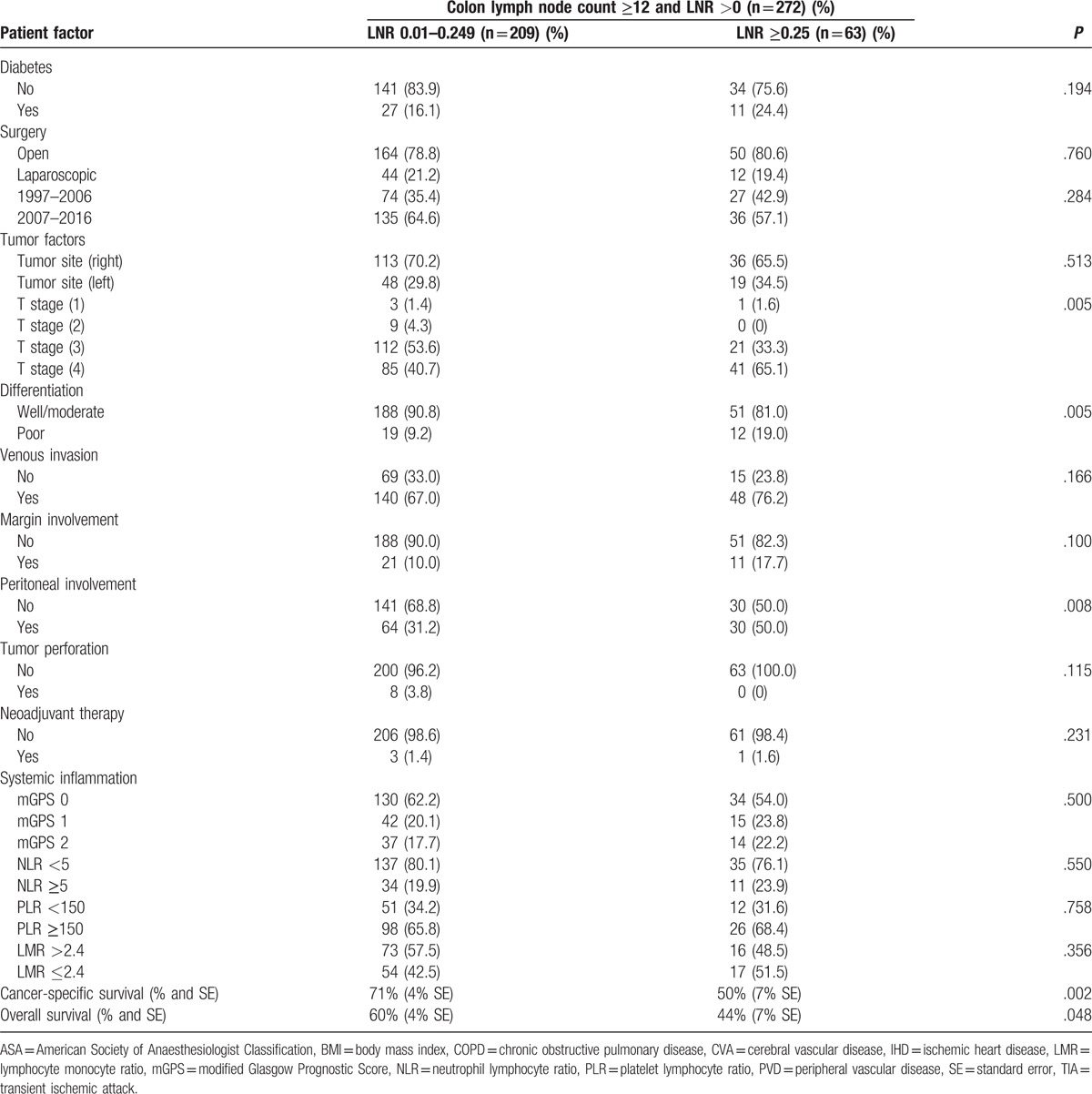
In those patients who had a LNC ≥12 and a LNR >0, the relationship between the LNR (<0.25/≥0.25), and clinicopathological characteristics and survival in patients undergoing surgery for colon cancer is shown in Table 3 . LNR ≥0.25 (n = 63) was significantly associated with higher T stage (n = 62, P < .01), poorer differentiation (n = 12, P < .01), and peritoneal involvement (n = 30, P < .01). LNR ≥0.25 was not significantly associated with any of the markers of the SIR. LNR ≥0.25 was significantly associated with poorer cancer-specific survival (P = .002) and OS (P = .048).
Table 2.
The relationship between the LNC (<12/≥12), clinicopathological characteristics, and LNR in patients undergoing surgery for colon cancer (binary logistic regression analysis).
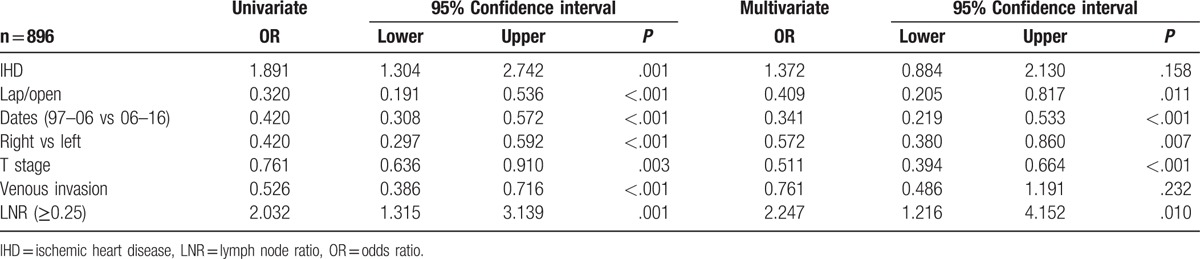
Table 3 (Continued).
The relationship between the LNR (<0.25/≥0.25), clinicopathological characteristics, and survival in patients undergoing surgery for colon cancer and with a resectional lymph node count of ≥12 and a LNR >0 (n = 272).
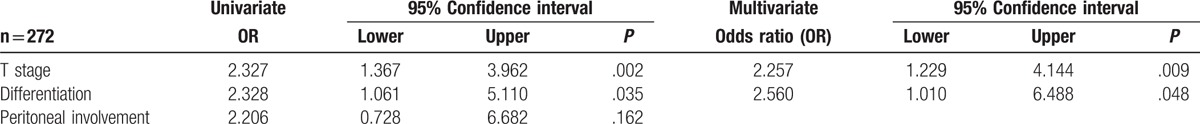
Binary logistic regression analysis was carried out on those variables with a significant association with LNR ≥0.25 (Table 4). On multivariate analysis, there was a significant independent relationship between an elevated LNR (≥0.25) and T stage (P < .01).
Table 3.
The relationship between the LNR (<0.25/≥0.25), clinicopathological characteristics, and survival in patients undergoing surgery for colon cancer and with a resectional lymph node count of ≥12 and a LNR >0 (n = 272).
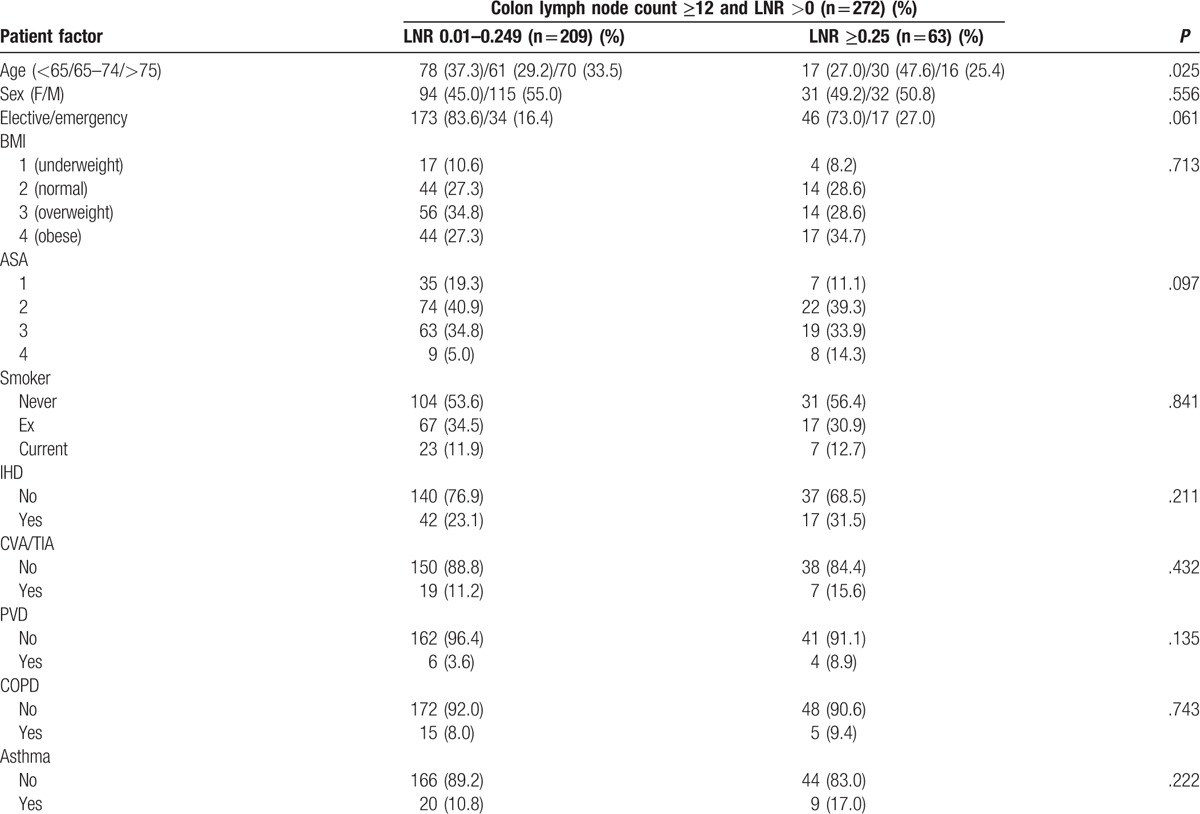
Table 4.
The relationship between the LNR (<0.25/≥0.25) and clinicopathological characteristics in patients undergoing surgery for colon cancer and with a resectional lymph node count of ≥12 and a LNR >0 (n = 272) (binary logistic regression analysis).
4. Discussion
The results of the present study confirm that the LNR is dependent on the LNC and has prognostic value in patients with colon cancer. The present study does not confirm the reported relationship between the LNC and LNR, and the SIR, as measured by mGPS, NLR, PLR, and LMR. Therefore, the present results do not support the proposal that a SIR in colon cancer results in lymph node hypertrophy leading to a lower LNC and a higher LNR.[4,15] In contrast, the present results highlight the importance of T stage, and high-quality surgery and pathology in ensuring optimal assessment of nodal spread in patients with colon cancer.
There is now good evidence that, after resection of colon cancer, a LNC of 12 or greater provides for a better characterization of nodal status.[6] Indeed, consistent with the present results LNCs of below 12 correlate with poor outcomes, and this has been largely explained by variances in the quality of surgical and pathological practice.[17,18] However, there remains doubt whether such a lymph node retrieval benchmark can be achieved in all resected colon cancer specimens.[19,20]
The LNR has repeatedly been reported as an effective stratification factor in patients with node-positive colorectal cancer. However, as shown in the present study, it is critically dependent on the quality of pathology performed, in particular, lymph node retrieval, and this limitation has precluded incorporation into routine clinical staging. In the present study, the quality benchmark of 12 or more nodes retrieved was confirmed to be independently associated with laparoscopic surgery, right-sided tumors, more invasive tumors, and the period of surgery. With reference to the latter, 12 or more nodes retrieved improved from 34% in 1997 to 2006, to 66% in 2007 to 2016, and when this benchmark was achieved, the LNR was similar in the 2 time periods. Given the improvement in meeting this benchmark, it may be that the LNR is now a suitable replacement for N stage in patients with colon cancer. Indeed, in the later period (2007–2016) when the prognostic value of LNR and N stage were directly compared in node-positive disease, LNR had superior prognostic value for both cancer-specific survival (LNR: hazard ratio [HR] 2.62, 95% confidence interval [CI] 1.25–5.52, P = .011; N stage: HR 1.22, 95% CI 0.83–1.80, P = .308) and OS (LNR: HR 2.02, 95% CI 1.12–3.68, P = .022; N stage: HR 1.09, 95% CI 0.82–1.46, P = .552). Therefore, with high-quality surgery and pathology, LNR offers additional prognostic value in patients with colon cancer, and may be a more useful measure to guide adjuvant therapy.
When the benchmark of ≥12 nodes retrieved/examined was met in node-positive disease only T stage was an independent determinant of the LNR. These results confirm the importance of tumor invasiveness in the process of lymph node spread and staging of colon cancer. Therefore, where there is suboptimal retrieval of lymph nodes after surgery for colon cancer, there is a case that patients with T3/T4 stage should be considered at high risk of recurrence.
The main limitation of the present study was that it is a retrospective analysis. However, it was carried out on a prospectively collected dataset, and the cohort size was substantial, with detailed information on the clinicopathological characteristics of the patients included.
5. Conclusions
In summary, the results of the present study confirm that the LNR is dependent on the LNC, and has prognostic value in patients with colon cancer. The present study does not confirm the reported relationship between the LNC and LNR, and the SIR, as measured by mGPS, NLR, PLR, and LMR. In high-quality surgical and pathological practice, LNR has superior prognostic value compared with N stage in patients undergoing surgery for colon cancer.
Author contributions
Conceptualization: R.D. Dolan.
Formal analysis: S.T. McSorley.
Methodology: S.T. McSorley.
Supervision: P.G. Horgan, D.C. McMillan.
Writing – original draft: R.D. Dolan
Writing – review & editing: R.D. Dolan, D.C. McMillan.
Footnotes
Abbreviations: CRP = C-reactive protein, LMR = lymphocyte monocyte ratio, LNC = lymph node count, LNR = lymph node ratio, mGPS = modified Glasgow Prognostic Score, NLR = neutrophil lymphyocyte ratio, OS = overall survival, PLR = platelet lymphocyte ratio, SIR = systemic inflammatory response, TNM = tumor node metastasis.
Funding: This research was funded from the Academic Unit of Surgery, University of Glasgow
The authors report no conflicts of interest.
References
- [1].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [2].Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. [DOI] [PubMed] [Google Scholar]
- [3].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [4].Kennelly RP, Murphy B, Larkin JO, et al. Activated systemic inflammatory response at diagnosis reduces lymph node count in colonic carcinoma. World J Gastrointest Oncol 2016;8:623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Le Voyer TE, Murphy B, Larkin JO, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912–9. [DOI] [PubMed] [Google Scholar]
- [6].McDonald JR, Steele SR, Eberhardt J, et al. Lymph node harvest in colon and rectal cancer: current considerations. World J Gastrointest Surg 2012;4:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen SL, Steele SR, Eberhardt J, et al. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg 2011;253:82–7. [DOI] [PubMed] [Google Scholar]
- [8].Vaccaro CA, Im V, Rossi GL, et al. Lymph node ratio as prognosis factor for colon cancer treated by colorectal surgeons. Dis Colon Rectum 2009;52:1244–50. [DOI] [PubMed] [Google Scholar]
- [9].Park JH, Powell AG, Roxburgh CS, et al. Mismatch repair status in patients with primary operable colorectal cancer: associations with the local and systemic tumour environment. Br J Cancer 2016;114:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63. [DOI] [PubMed] [Google Scholar]
- [11].Park JH, Watt DG, Roxburgh CS, et al. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg 2016;263:326–36. [DOI] [PubMed] [Google Scholar]
- [12].Klintrup K, Mäkinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005;41:2645–54. [DOI] [PubMed] [Google Scholar]
- [13].Roxburgh CS, Salmond JM, Horgan PG, et al. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg 2009;249:788–93. [DOI] [PubMed] [Google Scholar]
- [14].Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:134–146. [DOI] [PubMed] [Google Scholar]
- [15].Zhang ZY, Li C, Gao W, et al. A nomogram to predict adequate lymph node recovery before resection of colorectal cancer. PLoS One 2016;11:e0168156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fleming ID. American Joint Committee on Cancer, American Cancer Society, in AJCC Cancer Staging Manual. Philadelphia: Lippincott Raven; 1997. [Google Scholar]
- [17].Lykke J, Roikjaer O, Jess P. The relation between lymph node status and survival in stage I-III colon cancer: results from a prospective nationwide cohort study. Colorectal Dis 2013;15:559–65. [DOI] [PubMed] [Google Scholar]
- [18].Li Q, Zhuo C, Cai G, et al. Increased number of negative lymph nodes is associated with improved cancer specific survival in pathological IIIB and IIIC rectal cancer treated with preoperative radiotherapy. Oncotarget 2014;5:12459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mathis KL, Green EM, Sargent DJ, et al. Surgical quality surrogates do not predict colon cancer survival in the setting of technical credentialing: a report from the prospective COST trial. Ann Surg 2013;257:102–7. [DOI] [PubMed] [Google Scholar]
- [20].Li Destri G, Di Carlo I, Scilletta R, et al. Colorectal cancer and lymph nodes: the obsession with the number 12. World J Gastroenterol 2014;20:1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


