Multiple episodes of extensive oceanic anoxia delayed the marine ecosystem recovery from the latest Permian mass extinction.
Abstract
Explaining the ~5-million-year delay in marine biotic recovery following the latest Permian mass extinction, the largest biotic crisis of the Phanerozoic, is a fundamental challenge for both geological and biological sciences. Ocean redox perturbations may have played a critical role in this delayed recovery. However, the lack of quantitative constraints on the details of Early Triassic oceanic anoxia (for example, time, duration, and extent) leaves the links between oceanic conditions and the delayed biotic recovery ambiguous. We report high-resolution U-isotope (δ238U) data from carbonates of the uppermost Permian to lowermost Middle Triassic Zal section (Iran) to characterize the timing and global extent of ocean redox variation during the Early Triassic. Our δ238U record reveals multiple negative shifts during the Early Triassic. Isotope mass-balance modeling suggests that the global area of anoxic seafloor expanded substantially in the Early Triassic, peaking during the latest Permian to mid-Griesbachian, the late Griesbachian to mid-Dienerian, the Smithian-Spathian transition, and the Early/Middle Triassic transition. Comparisons of the U-, C-, and Sr-isotope records with a modeled seawater PO43− concentration curve for the Early Triassic suggest that elevated marine productivity and enhanced oceanic stratification were likely the immediate causes of expanded oceanic anoxia. The patterns of redox variation documented by the U-isotope record show a good first-order correspondence to peaks in ammonoid extinctions during the Early Triassic. Our results indicate that multiple oscillations in oceanic anoxia modulated the recovery of marine ecosystems following the latest Permian mass extinction.
INTRODUCTION
The 252-million-year-old Permian-Triassic boundary (PTB) mass extinction represents the largest biotic crisis in Earth’s history (1), during which ~90% of marine and ~75% of terrestrial species went extinct over ~61(±48) thousand years (ka) (1, 2). The Early Triassic was an interval of protracted marine biotic recovery (1, 3, 4). An initial, aborted recovery occurred soon after the latest Permian mass extinction (LPME) crisis, during the Induan stage of the Early Triassic (1, 5, 6), and a more sustained recovery took place during the late Olenekian stage (Spathian substage) (1, 7–9); however, full marine ecosystem recovery did not occur until the Middle Triassic, 4 to 8 million years (Ma) after the LPME (1). This delay has been attributed to various causes, including the intensity of the PTB extinction event (10), persistently high temperatures (11), productivity crises (12), and/or episodically recurring environmental perturbations (13–16).
Although the mechanisms for the long duration of the post-LPME recovery are debated, marine anoxia has been invoked in many studies (3, 4, 17–21). Ce anomalies and Th/U ratios in conodont apatite were used to reconstruct a 20-Ma redox history from the latest Permian to Late Triassic, revealing anoxic events during the late Changhsingian-Griesbachian, Smithian-Spathian transition, and the mid-Spathian (19). Mo/Al ratios and the pyrite content of mudstones on the continental slope of the eastern Panthalassic margin were used to infer euxinic conditions during the late Changhsingian to mid-Dienerian and mid-Smithian to mid-Spathian intervals (20). Fe speciation was used to demonstrate a dynamic redox history along the Oman margin, with an expanded oxygen-minimum zone during the late Changhsingian to earliest Griesbachian, the Dienerian-Smithian transition, and the Smithian-Spathian transition (21). However, these proxies are inherently local in terms of their paleoredox implications, and high-resolution changes in mean global-ocean redox conditions during the Early Triassic remain poorly constrained despite their likely importance for understanding links between oceanic conditions and the delayed marine biotic recovery.
The present study addresses this gap in knowledge regarding Early Triassic oceanic redox conditions through analysis of a global-ocean redox proxy, the U isotopes in marine carbonates (238U/235U, denoted as δ238U) (18, 22–25). Because of the long residence time of U in the ocean (~500 ka) (26), seawater U is well-mixed and exhibits globally uniform concentrations (3.14 to 3.59 μg/liter) (26) and isotopic compositions [ca. −0.39‰ (per mil)] (27–29). Seawater δ238U will tend to remain well-mixed even when the extent of oceanic oxygenation is significantly lower than today (25). Variations in the U-isotope compositions of primary carbonate precipitates (such as scleractinian corals, calcareous green and red algae, ooids, and mollusks) are thought to track the δ238U of contemporaneous seawater (24).
The U-isotope composition of seawater depends on redox conditions of the global ocean because U isotopes undergo different amounts of isotopic fractionation during incorporation into oxic and anoxic depositional facies. The largest source of U to the ocean is weathering from the upper continental crust and transport of dissolved U(VI) to the oceans via rivers. The δ238U of rivers ranges from −0.18 to −0.38‰, with a mean of −0.26‰ (30), which is slightly higher than the δ238U of seawater (−0.39‰) (29). There are multiple sinks for seawater U, of which biogenic carbonates, sediments in anoxic facies, and sediments in weakly oxygenated facies, represent the bulk of U removal from the ocean, whereas the adsorption to Fe and Mn oxides and the hydrothermal alteration of oceanic crust represent smaller sinks (29, 31). Removal of U(IV) to anoxic sediments favors the 238U isotope and is associated with an average fractionation (Δ238U) of +0.6‰, based on observations from the modern Black Sea, Kyllaren Fjord, and Saanich Inlet (27, 32–34). Removal of U under suboxic conditions (that is, corresponding to the integrated NO3-Fe-Mn reduction zones) favors 238U with a fractionation factor of ca. +0.1‰, based on observations from the Peru Margin, where sediments underlying weakly oxygenated waters have an average δ238U of −0.28 (±0.19)‰ (27). Therefore, seawater is expected to have lower δ238U at times of expanded oceanic anoxia and higher δ238U at times of enhanced oceanic oxygenation (18, 22, 27, 35).
The δ238U of primary marine carbonate precipitates reflects that of the seawater from which they precipitate (24, 27, 28). These natural observations are supported by laboratory experiments that suggest a negligible to small offset (<0.13‰) between primary carbonate and seawater δ238U (36). However, sedimentary carbonates may incorporate U(IV) from sulfidic porewaters, leading to δ238U values that are 0.2 to 0.4‰ higher than that of seawater (discussed further below and in the Supplementary Materials) (24). Thus, provided care is taken to correct for possible diagenetic alteration, U isotopes in ancient marine carbonates can serve as a global-ocean paleoredox proxy.
To date, four published studies have examined U-isotope variation during the Permian-Triassic transition and its aftermath (18, 22, 23, 25). Brennecka et al. (22) analyzed the narrow PTB interval at Dawen, South China, and documented a rapid expansion of oceanic anoxia across the LPME. This pattern was subsequently confirmed by Lau et al. (18) and Elrick et al. (23) in Tethys sections, and by Zhang et al. (25), who confirmed the same trend for a Panthalassic section. Together, these studies confirm the global nature of this event and the overall reliability of contemporaneous carbonate δ238U records from widely spaced sections. Lau et al. (18) provided evidence for widespread oceanic anoxia from the latest Permian until the early Middle Triassic. However, although Lau et al. (18) provided evidence supporting the role of oceanic anoxia in the delayed recovery of marine ecosystems following the LPME, the resolution of this study was insufficient for the recognition of high-frequency redox fluctuations during the first ~2 Ma of the Early Triassic, an interval characterized by large δ13C excursions ranging from −3 to +8‰ (3). Here, we provide a high-resolution U-isotope record spanning the uppermost Permian to the lowermost Middle Triassic at Zal, Iran, to investigate the secular variation in global-ocean redox conditions and its connection to the delayed recovery of marine ecosystems during the Early Triassic.
MATERIALS AND METHODS
We measured U isotopes (δ238U) in well-preserved Permian-Triassic marine carbonates from Zal, Iran, which were deposited on a peri-equatorial carbonate ramp at the margin of a microcontinent in the west-central Tethys Ocean (Fig. 1A). This site accumulated mainly limestones until the late Early Triassic and mainly dolostones thereafter (Fig. 1B; see Supplementary Materials for details of the study section). The Zal section accumulated in a well-aerated deep shelf environment below wave base (15, 37), at estimated water depths of 100 to 200 m during the late Permian and 50 to 100 m during the Griesbachian. Further shallowing yielded a high-energy shelf environment characterized by oolitic and oncoidal facies by the late Early Triassic (15). The Zal study section has a well-developed lithostratigraphic, biostratigraphic, δ13Ccarb, and 87Sr/86Sr chemostratigraphic framework (15, 37, 38).
Fig. 1. Location of Iran at ~252 Ma ago and geochemical profiles for the Zal section.
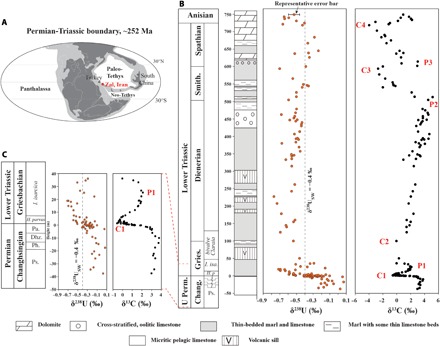
Paleogeographic location of Iran at ~252 Ma [(A) modified after the study of Payne et al. (60)] and geochemical profiles for Zal, Iran (B and C). The 238U/235U ratios are reported in per mil using standard δ-notation, where δ238U = [(238U/235U)sample/(238U/235U)standard(CRM145) − 1] × 1000. δ13C data and stratigraphic column are from Horacek et al. (15) and Richoz et al. (37). With respect to the δ13C profile, C1 to C4 are equivalent to the N1 to N4 negative excursions of Song et al. (19). δ238USW in (B) and (C) denotes δ238U of modern seawater. A representative uncertainty range of 2 SD is shown for the uppermost δ238U data point in (B). (C) Expanded view of the −40- to 40-m interval. Only samples with Mn/Sr < 2.5 are shown. Chang., Changhsingian; Gries., Griesbachian; Smith., Smithian. Following the studies of Clarkson et al. (21) and Martin et al. (44), biozonation of the Zal section is based on ammonoids for the Upper Permian and conodonts for the Lower Triassic. I. isa., Isarcicella isarcica; H. p., Hindeodus parvus; ammonoid zone 3, Pa., Paratirolites; ammonoid zone 2, Dhz., Dhzulfites; ammonoid zone 1, Ph., Phisonites; Ps., Pseudotoceras.
We analyzed a total of 155 carbonate samples for U isotopes. For each sample, ~3 g of powder was dissolved in 1 M hydrochloric acid (HCl). The resulting supernatant was spiked using a double spike containing 236U and 233U, and the spiked U was purified using chromatography methods as described by Zhang et al. (25). The 238U/235U values were determined with a ThermoFinnigan Neptune MC-ICP-MS instrument at Arizona State University (ASU) (W.M. Keck Laboratory for Environmental Biogeochemistry). The U isotopic compositions of samples are reported relative to those of CRM145 standard (whose δ238U value is identical to those of CRM 112a and SRM 950a, other commonly used standards). The analytical precision of this method is better than ±0.08‰ (all uncertainties reported here are 2 SD; see the Supplementary Materials for additional method details).
RESULTS AND DISCUSSION
Multiple episodes of expanded oceanic anoxia during the Early Triassic
Marine carbonate sediments can faithfully record chemical signatures of seawater provided that postdepositional processes and detrital contamination do not cause significant alteration. These processes likely did not confound the U-isotope pattern across the LPME observed in the Zal section because this pattern agrees with those at Dawen, Dajiang, and Daxiakou, China, which were located on the eastern side of the Paleo-Tethys Ocean (18, 22, 23); at Kamura, which was deposited in the open Panthalassic Ocean (25); and at Taşkent, Turkey, which was located northwest of Zal, on the western margin of the Paleo-Tethys Ocean (18). The fact that these six paleogeographically widely separated PTB sections exhibit a similar negative shift of δ238U at the LPME supports the use of carbonate U isotopes as a paleoredox proxy for the Early Triassic global ocean (see the Supplementary Materials for a compilation of δ238U studies across PTB) (18, 22, 23).
Confidence in this conclusion is enhanced by examining trace element compositions (Al, Mn, and Sr) and elemental ratios (Mn/Sr and Rb/Sr) as indicators of sedimentary diagenesis (fig. S1 and table S1). In most of the study samples, these elemental tracers are consistent with the well-preserved marine carbonate, with little influence from detrital components, and cross-plots of δ238U versus these tracers show no evidence of diagenetic alteration (figs. S2 to S6). Carbonate diagenesis models predict that the δ238U signal is more robust than the 87Sr/86Sr signal with respect to secondary alteration (35). 87Sr/86Sr values from Zal carbonates show good agreement with other records of Early Triassic seawater 87Sr/86Sr (38, 39), which suggests that the primary seawater δ238U signal may also be well-preserved. We adopted Mn/Sr < 2.5 as a diagenetic alteration threshold [after (40)], with 127 out of the 155 study samples meeting this criterion (see the Supplementary Materials for evidence of primary δ238U values). Only those samples with Mn/Sr < 2.5 are plotted in Fig. 1 and used in the discussion below.
The δ238U profile for Zal shows scatter of 0.2 to 0.4‰ through the Permian-Triassic transition interval, similar to that seen in sub-recent Bahamian carbonates. Although Bahamian primary carbonate precipitates appear to directly record seawater δ238U, all shallowly buried sediments have δ238U that is isotopically heavier by 0.2 to 0.4‰ (mean, 0.3‰) than δ238U of modern seawater (24). This is thought to reflect the differential incorporation of 238U-enriched U(IV) from anoxic porewaters during early diagenesis or the variation in porewater U speciation during carbonate recrystallization (36). Porewater data from deep Bahamian drill cores suggest that the potential for alteration following burial may be limited because porewater anoxia renders U essentially immobile (41). On this basis, we have applied a diagenetic correction factor of 0.3‰ to measured δ238U values before U-isotope mass-balance calculations (see the Supplementary Materials for further discussion).
The PTB interval at Zal is characterized by a large (3.5‰) and globally recognized negative δ13C excursion commencing immediately before the LPME horizon (Fig. 1C, labeled “C1”) (37). A negative shift in δ238U of ~0.4‰ commenced shortly below the LPME horizon (fig. S8; see the Supplementary Materials for discussion of timing of onset of oceanic anoxia). The shift toward lower carbonate δ238U values in association with the extinction event is most readily interpreted as an increase in the flux of isotopically heavy U into anoxic facies, suggesting a rapid increase in the global area of anoxic seafloor in conjunction with the LPME event (18, 22, 23).
The high-resolution δ238U data set presented here demonstrates that the Early Triassic ocean was characterized by multiple episodes of expanded anoxia. The negative δ238U shift commencing shortly before the LPME reached a minimum of −0.62 ± 0.15‰ about 15 m above the PTB (Fig. 1B). Low δ238U values persisted through the Griesbachian substage, reaching a second, larger negative peak (−0.73 ± 0.09‰) about 220 to 230 m above the PTB. This late Griesbachian to early Dienerian δ238U minimum suggests that the most reducing conditions in the global ocean may have developed following the LPME crisis. The δ238U values then gradually shift toward higher values, from ~230 to 550 m, recording a reduction under anoxic conditions during the mid-Dienerian to mid-Smithian. A third negative peak may be present at ~600 m based on a sample close to the Smithian-Spathian boundary, with a δ238U of −0.70‰, although more data are needed to confirm the existence of this feature. These values suggest widely reducing oceanic conditions throughout the Griesbachian to Smithian, with periodic fluctuations in areal extent. The top of the Zal section (700 to 752 m) shows a fourth negative δ238U shift (−0.57 ± 0.19‰), recording a major anoxic event during the latest Spathian to Early/Middle Triassic transition that is poorly known to date. Earlier U-isotope studies of Lower Triassic sections (18, 22, 23) did not delineate these multiple negative δ238U shifts because of either insufficient stratigraphic coverage or insufficient temporal resolution. We predict that they will be observed in other coeval sections, and we encourage high-resolution δ238U studies to reproduce these excursions.
Oceanic anoxia linked to climatic warming and marine nutrient levels
The U-isotope data set of the present study was generated using the same set of samples reported in earlier studies of δ13C and 87Sr/86Sr at Zal (Fig. 2, A to C) (15, 38), allowing for direct comparisons among these records. All three isotopic records exhibit a series of four correlatable excursions, during each of which negative excursions in δ238U and δ13C were accompanied by an accelerated rate of increase in 87Sr/86Sr (see the Supplementary Materials for a detailed comparison of LOWESS curves). The C1 event, which is latest Changhsingian to earliest Griesbachian in age, represents the well-documented marine environmental response to the LPME (11, 22, 25, 42, 43). The C2 event, which is latest Griesbachian to earliest Dienerian in age, exhibits U- and C-isotope minima that are similar to those of the C1 event, although the magnitudes of the excursions are smaller due to more negative initial values (Fig. 2). The C3 event, which is mid- to late Smithian in age, is well defined in the C- and Sr-isotope records, but more data are needed to fully test the duration and magnitude of a coeval U-isotope shift. The C4 event, which is latest Spathian to earliest Anisian in age, is characterized by smaller and more protracted δ238U and δ13C excursions than for the earlier events and by no apparent change in 87Sr/86Sr (although the existing Sr-isotope data set is too limited to adequately test for the C4 event) (Fig. 2, A to C). Although the patterns of excursions (for example, amplitude and duration) in the δ238U, δ13C, and 87Sr/86Sr profiles vary from one event to the next, the episodic nature of these events points to repeated global-scale perturbations that must have had significant impacts on global climate, weathering, and ocean redox dynamics.
Fig. 2. LOWESS curves for δ238U, δ13C, and 87Sr/86Sr profiles of Zal, Iran.
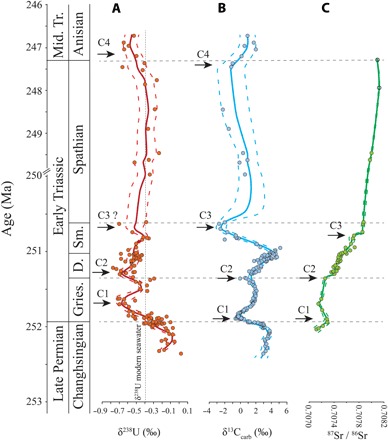
(A) Uranium isotope (δ238U) profile. (B) Carbon isotope (δ13C) profile (15, 37). δ13C of samples without paired δ238U data are not shown in this figure. (C) Strontium isotope (87Sr/86Sr) profile (38). U-C-Sr isotopes were measured from the same suite of samples. Samples with Mn/Sr > 2.5 have been removed from (A) and (B), and samples with Mn/Sr > 2.5 are indicated with open circles in (C). U. Perm., Upper Permian; Mid. Tr., Middle Triassic; Gries., Griesbachian; Di., Dienerian; Sm., Smithian. Note the change in time scale at 250 Ma.
The relationships between the δ238U, δ13C, and 87Sr/86Sr profiles for the Zal section were investigated through cross-correlation analysis of LOWESS-smoothed curves for each record (Fig. 2, A to C) (see the Supplementary Materials for a detailed discussion of cross-correlation methods). This analysis indicates that the negative shifts in δ238U lagged behind the negative δ13C excursions and the stepwise increases in the 87Sr/86Sr profile by 125 to 150 ka and 175 to 200 ka, respectively, and that the negative shifts in δ13C lagged behind the stepwise increases in the 87Sr/86Sr profile by 0 to 50 ka. These lags are presumed to reflect differences in the residence times (and thus the relative response times) of seawater U, dissolved inorganic carbon, and Sr in Early Triassic oceans. Although each transient stepwise increase in the 87Sr/86Sr profile was significantly shorter than the residence time of ~3 Ma for Sr in the modern ocean, the observed pattern could have been generated by a rapid pulsed injection of old radiogenic (that is, 87Sr-enriched) Sr into the ocean system on time scales much shorter than the residence time of Sr. Similar effects have been reported from glacial-interglacial (~100 ka) cycles of the Quaternary (44, 45).
We hypothesize that these isotopic records were linked via a combination of enhanced volcanism and climatic warming, leading to increased crustal weathering and seawater PO43− concentrations. Concurrent rapid warming and increases in seawater nutrient inventories would have led to oceanic anoxia as a result of increased marine primary productivity, reduced oxygen solubility, and reduced vertical mixing due to steeper thermal gradients in the oceanic thermocline.
Sr-isotope paleoweathering and O-isotope paleotemperature records suggest a general causal connection between climate warming and elevated weathering rates during the Early Triassic (11, 38). The increase in seawater 87Sr/86Sr from the latest Permian to the Early/Middle Triassic boundary occurred in a series of steps. Because each step is short, these events must represent large transient increases in the delivery of 87Sr-rich weathering products to the ocean (38). Rising seawater 87Sr/86Sr could not have resulted from weathering of fresh basalts from the Siberian Traps (43) but rather, must have included a large contribution from weathering of old continental rocks with high 87Sr/86Sr ratios. 87Sr/86Sr records and sedimentary flux investigations suggest 3 to 7×, 2 to 5×, 5 to 6×, and 1 to 2× increases in weathering fluxes across the C1, C2, C3, and C4 events, respectively (38, 39, 46). Intensified continental weathering would have flushed large amounts of PO43− and other nutrients into the ocean, leading to higher marine productivity, higher organic carbon export from the euphotic zone, increased respiratory oxygen demand at depth, and a decrease in oceanic dissolved oxygen levels.
We propose that perturbations in marine redox chemistry were closely linked to changes in continental weathering fluxes and increased marine PO43− levels. Phosphorus is commonly considered to be the ultimate biolimiting nutrient on marine productivity at geological time scales, and it plays a significant role in controlling the amount and spatial distribution of dissolved O2 in the oceans (47–50). Increased phosphorus input to the ocean would have led to rapid (103 to 104 ka) increases in new production, higher O2 demand, a larger vertical δ13C gradient, and deep-water anoxia (48, 49, 51). We constructed a box model to estimate average seawater [PO43−] during the study interval (which is described in detail in the Supplementary Materials). We calculated riverine phosphorus inputs from 87Sr/86Sr-derived estimates of the continental weathering flux (38, 39), assuming a P/Sr ratio of 0.61 to 0.91 mol/mol (see the Supplementary Materials for justification of the P/Sr ratio). This ratio may have been locally amplified by preferential recycling of PO43− through an anoxic and nonferruginous water column (50). Our calculations suggest that seawater PO43− concentrations increased to ~12× [PO43−]0, ~10× [PO43−]0, ~12× [PO43−]0, and ~9× [PO43−]0 during the C1, C2, C3, and C4 events, where [PO43−]0 is the initial PO43− concentration in the pre-LPME Late Permian ocean (Fig. 3C). Sensitivity studies conducted using spatially resolved General Circulation Models (GCMs) of early Triassic climate and ocean circulation indicate that [PO43−] increases of this magnitude would have led to a significant expansion of anoxic water masses (47, 48). We therefore hypothesize that elevated seawater PO43− levels associated with enhanced weathering fluxes played a key role in driving expansion of anoxia in the Early Triassic ocean.
Fig. 3. Marine U-cycle mass balance model, calculated PO43− concentrations, and ammonoid extinction rate curve.
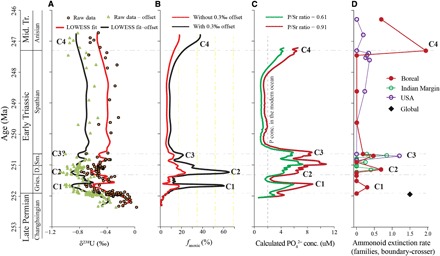
(A) δ238U data with LOWESS smoothing fit; see the top of figure for legend. (B) Model estimates of anoxic seafloor area (fanoxic) during Late Permian through Early/Middle Triassic time. The red and black lines denote modeling output without and with a diagenetic offset of 0.3‰, respectively. We note that the low δ238U data resolution at the C3 event makes its model estimated timing and extent of oceanic anoxia with larger uncertainties compared to the other events. (C) Calculated PO43− concentrations (conc.) in the Early Triassic ocean. The green and red lines denote modeling output assuming a P/Sr ratio of 0.61 and 0.91, respectively (see the Supplementary Materials). (D) Ammonoid extinction rate curve. See Fig. 2 for stage and substage abbreviations.
In addition to enhanced nutrient inputs, intensified thermal stratification and reduced vertical mixing associated with rapid climatic warming may have further contributed to the expansion of oceanic anoxia (11). A previous study (14) argued that large δ13C gradients in the Early Triassic ocean point to evidence of enhanced vertical stratification, yielding δ13C gradients from the ocean surface to the mid-thermocline of ~8.5, ~3.5, ~7.8, and ~2.2‰ during the C1 to C4 intervals, respectively, for sections from the northern Yangtze Platform and Nanpanjiang Basin in South China.
Relationship of marine oceanic and Early Triassic negative δ13C excursions
The relationship between Early Triassic δ13C excursions and marine redox changes has been the subject of lengthy debate (3, 14, 51–53). The negative δ13C excursions have been linked to both higher marine productivity (51) and lower marine productivity (12, 14). In the present study, relationships among the 87Sr/86Sr, δ238U, and δ13C profiles support a relationship of negative δ13C excursions to higher marine productivity and expanded oceanic anoxia. However, this interpretation runs counter to the paradigmatic view of the marine carbon cycle, in which higher productivity and expanded anoxia increase the export flux of organic matter and thus stimulate a positive δ13C excursion. A modeling study (52) suggested that the C1 to C4 negative δ13C excursions might have been triggered by the injection of light carbon associated with eruption of the Siberian Traps or by methane release via contact metamorphism of West Siberian Coal Field deposits. Variations in the proportions of different carbon sources may account for differences in the shape and magnitude of Early Triassic carbon-isotope excursions (52), but determining secular variation in these influences [including volcanic carbon sources (52), marine productivity (51), and soil organic matter (53)] will require further study.
Relationship of oceanic anoxia to the PTB extinction and the protracted recovery of marine ecosystems
There is a growing body of evidence that the evolving redox structure of the oceans has been an important influence on the evolutionary trajectory of animals, including extinctions such as the LPME (22, 25, 54). Given their physiological requirements, oceanic anoxia can rapidly kill animals and potentially trigger restructuring of marine ecosystems. In the modern ocean, continental shelves comprise <7% of the seafloor area but host the majority of marine animal diversity, biomass, and organic carbon and phosphorus burial. A simple U-isotope mass balance model predicts that anoxic seafloor area expanded from an initial value of ~0.2% (assuming that precrisis redox conditions were similar to the modern ocean) to ~17 to 60%, ~23 to 65%, ~12 to 21%, and ~17 to 37% in the latest Changhsingian to earliest Griesbachian, the latest Griesbachian to earliest Dienerian, the mid-to-late Smithian, and the latest Spathian to earliest Anisian (that is, during C1 to C4), respectively (Fig. 3; see the Supplementary Materials for a description of box modeling of fanox). Each expansion of anoxic waters likely covered a large proportion of continental shelves and upper slopes because numerous sections worldwide from such settings show evidence of anoxia (18–21, 55, 56).
The patterns of redox variation documented by the U-isotope record show a good first-order correspondence to ammonoid extinction rates during the Early Triassic (Fig. 3D; see the Supplementary Materials for adescription of ammonoid extinction rates) (1, 5, 6). Intervals of expanded oceanic anoxia approximately coincided with extinction rate peaks in the end-Permian (251.94 Ma), possibly the mid-Griesbachian (251.7 Ma), the mid-Dienerian (251.2 Ma), the late Smithian (250.5 Ma), and the end-Spathian (247.2 Ma) (Fig. 3D). Ammonoid extinctions appear to be synchronous in multiple regions (for example, the Indian margin of the Neo-Tethys Ocean, the western Laurentian margin of the Panthalassic Ocean, and the Boreal ocean; Fig. 3D), suggesting that the crises were global in extent. Global diversity and extinction data for other invertebrate clades (for example, foraminifers, gastropods, bivalves, brachiopods, and ostracods) lack the high (biozone level) resolution of the ammonoid data but suffice to show that biodiversity levels were generally low until the early Middle Triassic, when a rapid recovery ensued (4, 8). Although benthic invertebrate communities may have undergone restructuring during the late Dienerian and late Smithian (57, 58), active swimming organisms such as ammonoids, as well as vertebrates (59), were more dramatically affected because of their active physiology and higher metabolic oxygen demand. Therefore, our results imply that the delayed Early Triassic marine recovery was a function of repeated environmental perturbations rather than the severity of the LPME event itself (18, 20).
Methods (extended description)
Fresh carbonate samples collected from the field were crushed into small fragments, cleaned using Milli-Q water, and dried. The freshest fragments without veins were hand-selected under a binocular microscope and powdered to ~200 mesh using an agate ball mill.
Approximately 3 g of each sample were dissolved in 1 M HCl using a 50-ml trace-metal-clean centrifuge tube and following this protocol: 6 × 5 ml of 1 M HCl was slowly added in the tube with a time gap of 10 min between steps. To continue the reaction, we added 1 ml of 10 M HCl every 25 min (producing 0.3 M HCl) for a total of five times. Finally, 1 M HCl was added to make a final volume of 45 ml. After sitting at room temperature for 24 hours, the samples were centrifuged to separate the supernatant and undissolved residues. This dissolution protocol uses a 1.5× excess of HCl to ensure complete dissolution of the carbonate, thus avoiding U-isotope fractionation from selective leaching of various carbonate phases.
Major, minor, and trace element concentrations were measured on a Thermo iCAP quadrupole inductively coupled plasma mass spectrometer (ICP-MS) at the W. M. Keck Laboratory for Environmental Biogeochemistry at ASU on splits from each supernatant. Typical precision was better than 3 and 5% for major and trace elements, respectively, based on repeated analysis of in-run check standards.
Before U-isotope column chemistry (ion exchange chromatography), appropriate amounts of the IRMM 3636 236U:233U double spike (22, 24, 27) were added to an amount of sample solution corresponding to 400 to 500 ng of U to facilitate correction for instrumental mass fractionation and any U-isotope fractionation during column chemistry. The spike-sample mixtures were evaporated to dryness and taken up in 3 M HNO3. Uranium for isotopic analysis was purified using the Uranium und TEtraValents Actinides (UTEVA) method (22, 24, 27). Following column chemistry, samples were evaporated in a mixture of concentrated nitric acid and 32% hydrogen peroxide repeatedly (3×) to oxidize any organic materials leached from the Eichrom UTEVA resin. All samples have been put through U-isotope column chemistry twice to completely remove matrix ions. Purified U was dissolved in 0.32 M HNO3 and diluted to a U concentration of 50 parts per billion (ppb). Uranium isotopes were measured at ASU on a ThermoFinnigan Neptune multicollector ICP-MS at low mass resolution. When using a nebulizer (100 μl/min), a 50-ppb sample solution yielded 30 to 40 V of 238U signal on a 1011-ohm amplifier. The standard solution CRM145 (50 ppb of U) was analyzed every two samples. Two secondary standards (CRM129a and an in-house Ricca ICP solution) were measured after every 15 sample measurements. Sample δ238U values were normalized by the average of the bracketing CRM145 standards. CRM145 shares its isotopic composition with another common standard CRM112a, from which it was prepared (24, 27, 29). The measured isotopic compositions of standards CRM145, CRM129a, and Ricca are −0.00 ± 0.07‰ (2σ, n = 61), −1.74 ± 0.06‰ (2σ, n = 9), and −0.28 ± 0.08‰ (2σ, n = 7), respectively.
Supplementary Material
Acknowledgments
We thank A. Baud, L. Krystyn, R. Brandner, M. Tayebeh Mohtat, and B. Hamdi for help in the field and G. Gordon for her assistance with the laboratory work. We also thank Dr. Noah Planavsky and the two anonymous reviewers for their constrictive comments on our manuscript. We also thank Dr. Noah Planavsky and the two anonymous reviewers for their constructive comments on our manuscript. Funding: This research is supported by funding from the NASA Exobiology Program (award NNX13AJ71G) and the NSF Frontiers in Earth System Dynamics program (award EAR-1338810). Author contributions: F.Z., S.J.R., T.J.A., and A.D.A. designed research. F.Z. performed research and analyzed data. S.R. and M.H. undertook fieldwork and provided sedimentological framework. F.Z., S.J.R., and K.V.L. contributed to the model calculations. All authors contributed with discussions. F.Z. prepared the manuscript with significant inputs from S.J.R., T.J.A, and A.D.A. Competing interests: The authors declare that they have no conflict of interest. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper can be requested from the authors. Split for many samples is available at the Geological Museum of Lausanne, Switzerland.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/e1602921/DC1
The study section
Evidence for primary seawater δ238U values
Compilation of global carbonate δ238U records for PTB interval
Possible timing of onset of oceanic anoxia
Age-thickness model for Zal section
Cross-correlation analysis
High-resolution intercomparison of Zal δ13C, 87Sr/86Sr, and δ238U records
Estimation of weathering rates and seawater PO43− levels in the Early Triassic ocean
Box model estimates for fanox
Patterns of marine invertebrate clade recovery following the LPME
Ammonoid extinction rates
table S1. Strontium and phosphorus model parameterization.
table S2. Uranium box model parameterization.
fig. S1. Geochemical profile of Zal, Iran.
fig. S2. Diagenetic evaluation cross-plots of δ238U-[Sr], δ238U-Mn/Sr, and δ238U-Mg/Ca (mol/mol) of all samples, samples below 3.5 m, samples between 3.5 and 500 m, and samples above 500 m.
fig. S3. Cross-plots of δ238U-Rb/Sr and δ238U-U/Al ratio [parts per million/weight % (wt %)] for all samples and samples below and above 500 m.
fig. S4. Cross-plots of δ238U-Mn/Sr, δ238U-Mg/Ca, δ238U-Rb/Sr, and δ238U-U/Al (wt %) for anoxic events 1 and 2.
fig. S5. Cross-plots of δ238U-Mn/Sr, δ238U-Mg/Ca, δ238U-Rb/Sr, and δ238U-U/Al (wt %) for anoxic events 3 and 4.
fig. S6. Cross-plots of δ13C-δ18O for the Zal section.
fig. S7. Location of Iran, South China, and Turkey during the Permian-Triassic transition, ~252 Ma (modified after Payne et al. (60).
fig. S8. Comparison of U- and C-isotope profiles for Zal, Dawen, Dajiang, Taşkent, and Kamura.
fig. S9. A LOWESS trend showing inferred timing of onset of latest Permian oceanic anoxia.
fig. S10. Age-depth model for the Zal, Iran study section.
fig. S11. Cross-correlation analysis of LOWESS-smoothed curves for U-C-Sr isotope records.
fig. S12. 87Sr/86Sr-derived estimates of the continental weathering flux and the calculated seawater PO43− concentrations for the Early Triassic ocean.
fig. S13. Interregional ammonoid zonation scheme.
dataset S1. δ238U data with associated geochemical data.
REFERENCES AND NOTES
- 1.Chen Z.-Q., Benton M. J., The timing and pattern of biotic recovery following the end-Permian mass extinction. Nat. Geosci. 5, 375–383 (2012). [Google Scholar]
- 2.Burgess S. D., Bowring S., Shen S.-z., High-precision timeline for Earth’s most severe extinction. Proc. Natl. Acad. Sci. U.S.A. 111, 3316–3321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne J. L., Lehrmann D. J., Wei J., Orchard M. J., Schrag D. P., Knoll A. H., Large perturbations of the carbon cycle during recovery from the end-permian extinction. Science 305, 506–509 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Wei H., Shen J., Schoepfer S. D., Krystyn L., Richoz S., Algeo T. J., Environmental controls on marine ecosystem recovery following mass extinctions, with an example from the Early Triassic. Earth Sci. Rev. 149, 108–135 (2015). [Google Scholar]
- 5.Brayard A., Escarguel G., Bucher H., Monnet C., Brühwiler T., Goudemand N., Galfetti T., Guex J., Good genes and good luck: Ammonoid diversity and the end-Permian mass extinction. Science 325, 1118–1121 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Stanley S. M., Evidence from ammonoids and conodonts for multiple Early Triassic mass extinctions. Proc. Natl. Acad. Sci. U.S.A. 106, 15264–15267 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z.-Q., Tong J., Fraiser M. L., Trace fossil evidence for restoration of marine ecosystems following the end-Permian mass extinction in the Lower Yangtze region, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 299, 449–474 (2011). [Google Scholar]
- 8.Payne J. L., Summers M., Rego B. L., Altiner D., Wei J., Yu M., Lehrmann D. J., Early and Middle Triassic trends in diversity, evenness, and size of foraminifers on a carbonate platform in south China: Implications for tempo and mode of biotic recovery from the end-Permian mass extinction. Paleobiology 37, 409–425 (2011). [Google Scholar]
- 9.Song H. J., Wignall P. B., Chen Z.-Q., Tong J., Bond D. P. G., Lai X., Zhao X., Jiang H., Yan C., Niu Z., Chen J., Yang H., Wang Y., Recovery tempo and pattern of marine ecosystems after the end-Permian mass extinction. Geology 39, 739–742 (2011). [Google Scholar]
- 10.Solé R. V., Montoya J. M., Erwin D. H., Recovery after mass extinction: Evolutionary assembly in large–scale biosphere dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 697–707 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y., Joachimski M. M., Wignall P. B., Yan C., Chen Y., Jiang H., Wang L., Lai X., Lethally hot temperatures during the Early Triassic greenhouse. Science 338, 366–370 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Grasby S. E., Beauchamp B., Knies J., Early Triassic productivity crises delayed recovery from world’s worst mass extinction. Geology 44, 779–782 (2016). [Google Scholar]
- 13.Retallack G. J., Sheldon N. D., Carr P. F., Fanning M., Thompson C. A., Williams M. L., Jones B. G., Multiple Early Triassic greenhouse crises impeded recovery from Late Permian mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 308, 233–251 (2011). [Google Scholar]
- 14.Song H., Tong J., Algeo T. J., Horacek M., Qiu H., Song H., Tian L., Chen Z.-Q., Large vertical δ13CDIC gradients in Early Triassic seas of the South China craton: Implications for oceanographic changes related to Siberian Traps volcanism. Glob. Planet. Change 105, 7–20 (2013). [Google Scholar]
- 15.Horacek M., Richoz S., Brandner R., Krystyn L., Spötl C., Evidence for recurrent changes in Lower Triassic oceanic circulation of the Tethys: The δ13C record from marine sections in Iran. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 355–369 (2007). [Google Scholar]
- 16.Zhang G., Zhang X., Hu D., Li D., Algeo T. J., Farquhar J., Henderson C. M., Qin L., Shen M., Shen D., Schoepfer S. D., Chen K., Shen Y., Redox chemistry changes in the Panthalassic Ocean linked to the end-Permian mass extinction and delayed Early Triassic biotic recovery. Proc. Natl. Acad. Sci. U.S.A. 114, 1806–1810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isozaki Y., Permo-Triassic boundary superanoxia and stratified superocean: Records from lost deep sea. Science 276, 235–238 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Lau K. V., Maher K., Altiner D., Kelley B. M., Kump L. R., Lehrmann D. J., Silva-Tamayo J. C., Weaver K. L., Yu M., Payne J. L., Marine anoxia and delayed Earth system recovery after the end-Permian extinction. Proc. Natl. Acad. Sci. U.S.A. 113, 2360–2365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song H., Wignall P. B., Tong J., Bond D. P. G., Song H., Lai X., Zhang K., Wang H., Chen Y., Geochemical evidence from bio-apatite for multiple oceanic anoxic events during Permian–Triassic transition and the link with end-Permian extinction and recovery. Earth Planet. Sci. Lett. 353–354, 12–21 (2012). [Google Scholar]
- 20.Grasby S. E., Beauchamp B., Embry A., Sanei H., Recurrent Early Triassic ocean anoxia. Geology 41, 175–178 (2013). [Google Scholar]
- 21.Clarkson M. O., Wood R. A., Poulton S. W., Richoz S., Newton R. J., Kasemann S. A., Bowyer F., Krystyn L., Dynamic anoxic ferruginous conditions during the end-Permian mass extinction and recovery. Nat. Commun. 7, 12236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennecka G. A., Herrmann A. D., Algeo T. J., Anbar A. D., Rapid expansion of oceanic anoxia immediately before the end-Permian mass extinction. Proc. Natl. Acad. Sci. U.S.A. 108, 17631–17634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elrick M., Polyak V., Algeo T. J., Romaniello S., Asmerom Y., Herrmann A. D., Anbar A. D., Zhao L., Chen Z.-Q., Global-ocean redox variation during the middle-late Permian through Early Triassic based on uranium isotope and Th/U trends of marine carbonates. Geology 45, 163–166 (2017). [Google Scholar]
- 24.Romaniello S. J., Herrmann A. D., Anbar A. D., Uranium concentrations and 238U/235U isotope ratios in modern carbonates from the Bahamas: Assessing a novel paleoredox proxy. Chem. Geol. 362, 305–316 (2013). [Google Scholar]
- 25.Zhang F., Algeo T. J., Romaniello S. J., Cui Y., Zhao L., Chen Z.-Q., Anbar A. D., Congruent Permian-Triassic δ238U records at Panthalassic and Tethyan sites: Confirmation of global-oceanic anoxia and validation of the U-isotope paleoredox proxy. Geology, in press (2018). [Google Scholar]
- 26.Ku T.-L., Knauss K. G., Mathieu G. G., Uranium in open ocean: Concentration and isotopic composition. Deep Sea Res. 24, 1005–1017 (1977). [Google Scholar]
- 27.Weyer S., Anbar A. D., Gerdes A., Gordon G. W., Algeo T. J., Boyle E. A., Natural fractionation of 238U/235U. Geochim. Cosmochim. Acta 72, 345–359 (2008). [Google Scholar]
- 28.Stirling C. H., Andersen M. B., Potter E.-K., Halliday A. N., Low-temperature isotopic fractionation of uranium. Earth Planet. Sci. Lett. 264, 208–225 (2007). [Google Scholar]
- 29.Tissot F. L. H., Dauphas N., Uranium isotopic compositions of the crust and ocean: Age corrections, U budget and global extent of modern anoxia. Geochim. Cosmochim. Acta 167, 113–143 (2015). [Google Scholar]
- 30.Andersen M. B., Stirling C. H., Weyer S., Uranium isotope fractionation. Rev. Mineral. Geochem. 82, 799–850 (2017). [Google Scholar]
- 31.Morford J. L., Emerson S., The geochemistry of redox sensitive trace metals in sediments. Geochim. Cosmochim. Acta 63, 1735–1750 (1999). [Google Scholar]
- 32.Holmden C., Amini M., Francois R., Uranium isotope fractionation in Saanich Inlet: A modern analog study of a paleoredox tracer. Geochim. Cosmochim. Acta 153, 202–215 (2015). [Google Scholar]
- 33.Rolison J. M., Stirling C. H., Middag R., Rijkenberg M. J. A., Uranium stable isotope fractionation in the Black Sea: Modern calibration of the 238U/235U paleo-redox proxy. Geochim. Cosmochim. Acta 203, 69–88 (2017). [Google Scholar]
- 34.Noordmann J., Weyer S., Montoya-Pino C., Dellwig O., Neubert N., Eckert S., Paetzel M., Böttcher M. E., Uranium and molybdenum isotope systematics in modern euxinic basins: Case studies from the central Baltic Sea and the Kyllaren fjord (Norway). Chem. Geol. 396, 182–195 (2015). [Google Scholar]
- 35.Lau K. V., Macdonald F. A., Maher K., Payne J. L., Uranium isotope evidence for temporary ocean oxygenation in the aftermath of the Sturtian Snowball Earth. Earth Planet. Sci. Lett. 458, 282–292 (2017). [Google Scholar]
- 36.Chen X., Romaniello S. J., Herrmann A. D., Wasylenki L. E., Anbar A. D., Uranium isotope fractionation during coprecipitation with aragonite and calcite. Geochim. Cosmochim. Acta 188, 189–207 (2016). [Google Scholar]
- 37.Richoz S., Krystyn L., Baud A., Brandner Rainer, Horacek Micha, Mohtat-Aghai Parvin, Permian–Triassic boundary interval in the Middle East (Iran and N. Oman): Progressive environmental change from detailed carbonate carbon isotope marine curve and sedimentary evolution. J. Asian Earth Sci. 39, 236–253 (2010). [Google Scholar]
- 38.Sedlacek A. R. C., Saltzman M. R., Algeo T. J., Horacek M., Brandner R., Foland K., Denniston R. F., 87Sr/86Sr stratigraphy from the Early Triassic of Zal, Iran: Linking temperature to weathering rates and the tempo of ecosystem recovery. Geology 42, 779–782 (2014). [Google Scholar]
- 39.Song H., Wignall P. B., Tong J., Song H., Chen J., Chu D., Tian L., Luo M., Zong K., Chen Y., Lai X., Zhang K., Wang H., Integrated Sr isotope variations and global environmental changes through the Late Permian to early Late Triassic. Earth Planet. Sci. Lett. 424, 140–147 (2015). [Google Scholar]
- 40.Kaufman A. J., Knoll A. H., Neoproterozoic variations in the C-isotopic composition of seawater: Stratigraphic and biogeochemical implications. Precambrian Res. 73, 27–49 (1995). [DOI] [PubMed] [Google Scholar]
- 41.Henderson G. M., Slowey N. C., Haddad G. A., Fluid flow through carbonate platforms: Constraints from 234U/238U and Cl− in Bahamas pore-waters. Earth Planet. Sci. Lett. 169, 99–111 (1999). [Google Scholar]
- 42.Shen J., Algeo T. J., Hu Q., Zhang N., Zhou L., Xia W., Xie S., Feng Q., Negative C-isotope excursions at the Permian-Triassic boundary linked to volcanism. Geology 40, 963–966 (2012). [Google Scholar]
- 43.Burgess S. D., Bowring S. A., High-precision geochronology confirms voluminous magmatism before, during, and after Earth’s most severe extinction. Sci. Adv. 1, e1500470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin P. A., Lea D. W., Mashiotta T. A., Papenfuss T., Sarnthein M., Variation of foraminiferal Sr/Ca over Quaternary glacial-interglacial cycles: Evidence for changes in mean ocean Sr/Ca? Geochem. Geophys. Geosyst. 1, 1004 (2000). [Google Scholar]
- 45.Tütken T., Eisenhauer A., Wiegand B., Hansen B. T., Glacial–interglacial cycles in Sr and Nd isotopic composition of Arctic marine sediments triggered by the Svalbard/Barents Sea ice sheet. Mar. Geol. 182, 351–372 (2002). [Google Scholar]
- 46.Algeo T. J., Twitchett R. J., Anomalous Early Triassic sediment fluxes due to elevated weathering rates and their biological consequences. Geology 38, 1023–1026 (2010). [Google Scholar]
- 47.Winguth C., Winguth A. M. E., Simulating Permian–Triassic oceanic anoxia distribution: Implications for species extinction and recovery. Geology 40, 127–130 (2012). [Google Scholar]
- 48.Meyer K. M., Ridgwell A., Payne J. L., The influence of the biological pump on ocean chemistry: Implications for long-term trends in marine redox chemistry, the global carbon cycle, and marine animal ecosystems. Geobiology 14, 207–219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenton T. M., Boyle R. A., Poulton S. W., Shields-Zhou G. A., Butterfield N. J., Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat. Geosci. 7, 257–265 (2014). [Google Scholar]
- 50.Van Cappellen P., Ingall E. D., Benthic phosphorus regeneration, net primary production, and ocean anoxia: A model of the coupled marine biogeochemical cycles of carbon and phosphorus. Paleoceanography 9, 677–692 (1994). [Google Scholar]
- 51.Meyer K. M., Yu M., Jost A. B., Kelley B. M., Payne J. L., δ13C evidence that high primary productivity delayed recovery from end-Permian mass extinction. Earth Planet. Sci. Lett. 302, 378–384 (2011). [Google Scholar]
- 52.Payne J. L., Kump L. R., Evidence for recurrent Early Triassic massive volcanism from quantitative interpretation of carbon isotope fluctuations. Earth Planet. Sci. Lett. 256, 264–277 (2007). [Google Scholar]
- 53.Sephton M. A., Looy C. V., Brinkhuis H., Wignall P. B., de Leeuw J. W., Visscher H., Catastrophic soil erosion during the end-Permian biotic crisis. Geology 33, 941–944 (2005). [Google Scholar]
- 54.Feng Q., Algeo T. J., Evolution of oceanic redox conditions during the Permo-Triassic transition: Evidence from deepwater radiolarian facies. Earth Sci. Rev. 137, 34–51 (2014). [Google Scholar]
- 55.Bond D. P. G., Wignall P. B., Pyrite framboid study of marine Permian–Triassic boundary sections: A complex anoxic event and its relationship to contemporaneous mass extinction. Geol. Soc. Am. Bull. 122, 1265–1279 (2010). [Google Scholar]
- 56.Grice K., Cao C., Love G. D., Böttcher M. E., Twitchett R. J., Grosjean E., Summons R. E., Turgeon S. C., Dunning W., Jin Y., Photic zone euxinia during the Permian-Triassic superanoxic event. Science 307, 706–709 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Hofmann R., Hautmann M., Brayard A., Nützel A., Bylund K. G., Jenks J. F., Vennin E., Olivier N., Bucher H., Recovery of benthic marine communities from the end-Permian mass extinction at the low latitudes of eastern Panthalassa. Palaeontology 57, 547–589 (2014). [Google Scholar]
- 58.Foster W. J., Danise S., Price G. D., Twitchett R. J., Subsequent biotic crises delayed marine recovery following the late Permian mass extinction event in northern Italy. PLOS ONE 12, e0172321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheyer T. M., Romano C., Jenks J., Bucher H., Early Triassic marine biotic recovery: The predators’ perspective. PLOS ONE 9, e88987 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Payne J. L., Lehrmann D. J., Follett D., Seibel M., Kump L. R., Riccardi A., Altiner D., Sano H., Wei J., Erosional truncation of uppermost Permian shallow-marine carbonates and implications for Permian-Triassic boundary events. Geol. Soc. Am. Bull. 119, 771–784 (2007). [Google Scholar]
- 61.Leda L., Korn D., Ghaderi A., Hairapetian V., Struck U., Reimold W. U., Lithostratigraphy and carbonate microfacies across the Permian–Triassic boundary near Julfa (NW Iran) and in the Baghuk Mountains (Central Iran). Facies 60, 295–325 (2014). [Google Scholar]
- 62.Banner J. L., Hanson G. N., Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochim. Cosmochim. Acta 54, 3123–3137 (1990). [Google Scholar]
- 63.J. Veizer, Chemical diagenesis of carbonates: Theory and application of trace element technique, in Stable Isotopes in Sedimentary Geology (SEPM Society for Sedimentary Geology, 1983), vol. 10, Society of Economic Paleontologists and Mineralogists Short Course Notes, pp. III-1–III-100. [Google Scholar]
- 64.Vahrenkamp V. C., Swart P. K., New distribution coefficient for the incorporation of strontium into dolomite and its implications for the formation of ancient dolomites. Geology 18, 387–391 (1990). [Google Scholar]
- 65.Mazzullo S. J., Geochemical and neomorphic alteration of dolomite: A review. Carbonate. Evaporite. 7, 21–37 (1992). [Google Scholar]
- 66.Rimstidt J. D., Balog A., Webb J., Distribution of trace elements between carbonate minerals and aqueous solutions. Geochim. Cosmochim. Acta 62, 1851–1863 (1998). [Google Scholar]
- 67.R. L. Rudnick, S. Gao, Major elements of Earth Crust, in Treatise on Geochemistry, H. D. Holland, K. K. Turekian, Eds. (Elsevier, 2003), vol. 3, pp. 1–64. [Google Scholar]
- 68.Algeo T., Henderson C. M., Ellwood B., Rowe H., Elswick E., Bates S., Lyons T., Hower J. C., Smith C., Maynard B., Hays L. E., Summons R. E., Fulton J., Freeman K. H., Evidence for a diachronous Late Permian marine crisis from the Canadian Arctic region. Geol. Soc. Am. Bull. 124, 1424–1448 (2012). [Google Scholar]
- 69.Algeo T. J., Henderson C. M., Tong J., Feng Q., Yin H., Tyson R. V., Plankton and productivity during the Permian–Triassic boundary crisis: An analysis of organic carbon fluxes. Glob. Planet. Change 105, 52–67 (2013). [Google Scholar]
- 70.Lehrmann D. J., Ramezani J., Bowring S. A., Martin M. W., Montgomery P., Enos P., Payne J. L., Orchard M. J., Hongmei W., Jiayong W., Timing of recovery from the end-Permian extinction: Geochronologic and biostratigraphic constraints from south China. Geology 34, 1053–1056 (2006). [Google Scholar]
- 71.Ovtcharova M., Bucher H., Schaltegger U., Galfetti T., Brayard A., Guex J., New Early to Middle Triassic U–Pb ages from South China: Calibration with ammonoid biochronozones and implications for the timing of the Triassic biotic recovery. Earth Planet. Sci. Lett. 243, 463–475 (2006). [Google Scholar]
- 72.Galfetti T., Bucher H., Ovtcharova M., Schaltegger U., Brayard A., Brühwiler T., Goudemand N., Weissert H., Hochuli P. A., Cordey F., Guodun K., Timing of the Early Triassic carbon cycle perturbations inferred from new U–Pb ages and ammonoid biochronozones. Earth Planet. Sci. Lett. 258, 593–604 (2007). [Google Scholar]
- 73.Guo G., Tong J., Zhang S., Zhang J., Bai L., Cyclostratigraphy of the Induan (Early Triassic) in West Pingdingshan Section, Chaohu, Anhui Province. Sci. China Ser. D 51, 22–29 (2008). [Google Scholar]
- 74.J. C. Davis, R. J. Sampson, Statistics and Data Analysis in Geology (Wiley, 1986). [Google Scholar]
- 75.Dunk R. M., Mills R. A., Jenkins W. J., A reevaluation of the oceanic uranium budget for the Holocene. Chem. Geol. 190, 45–67 (2002). [Google Scholar]
- 76.R. E. Zeebe, D. Wolf-Gladrow, CO2 in Seawater: Equilibrium, Kinetics, Isotopes (Elsevier, 2001), Series in Oceanography, vol. 65, 360 pp. [Google Scholar]
- 77.Algeo T. J., Kuwahara K., Sano H., Bates S., Lyons T., Elswick E., Hinnov L., Ellwood B., Moser J., Maynard J. B., Spatial variation in sediment fluxes, redox conditions, and productivity in the Permian–Triassic Panthalassic Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 308, 65–83 (2011). [Google Scholar]
- 78.Song H. Y., Tong J., Algeo T. J., Song H., Qiu H., Zhu Y., Tian L., Bates S., Lyons T. W., Luo G., Kump L. R., Early Triassic seawater sulfate drawdown. Geochim. Cosmochim. Acta 128, 95–113 (2014). [Google Scholar]
- 79.Richter F. M., Turekian K. K., Simple models for the geochemical response of the ocean to climatic and tectonic forcing. Earth Planet. Sci. Lett. 119, 121–131 (1993). [Google Scholar]
- 80.Goddéris Y., François L. M., The Cenozoic evolution of the strontium and carbon cycles: Relative importance of continental erosion and mantle exchanges. Chem. Geol. 126, 169–190 (1995). [Google Scholar]
- 81.Schaller M. F., Wright J. D., Kent D. V., Atmospheric Pco2 perturbations associated with the Central Atlantic Magmatic Province. Science 331, 1404–1409 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Horton F., Did phosphorus derived from the weathering of large igneous provinces fertilize the Neoproterozoic ocean? Geochem. Geophys. Geosyst. 16, 1723–1738 (2015). [Google Scholar]
- 83.Palmer M. R., Edmond J. M., The strontium isotope budget of the modern ocean. Earth Planet. Sci. Lett. 92, 11–26 (1989). [Google Scholar]
- 84.Paytan A., McLaughlin K., The oceanic phosphorus cycle. Chem. Rev. 107, 563–576 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Baturin G. N., Phosphorus cycle in the ocean. Lithol. Miner. Resour. 38, 101–119 (2003). [Google Scholar]
- 86.Van Cappellen P., Ingall E. D., Redox stabilization of the atmosphere and oceans by phosphorus-limited marine productivity. Science 271, 493–496 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Reinhard C. T., Planavsky N. J., Gill B. C., Ozaki K., Robbins L. J., Lyons T. W., Fischer W. W., Wang C., Cole D. B., Konhauser K. O., Evolution of the global phosphorus cycle. Nature 541, 386–389 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Tyrrell T., The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400, 525–531 (1999). [Google Scholar]
- 89.Froelich P. N., Kinetic control of dissolved phosphate in natural rivers and estuaries: A primer on the phosphate buffer mechanism. Limnol. Oceanogr. 33, 649–668 (1988). [Google Scholar]
- 90.Ingall E., Jahnke R., Influence of water-column anoxia on the elemental fractionation of carbon and phosphorus during sediment diagenesis. Mar. Geol. 139, 219–229 (1997). [Google Scholar]
- 91.Handoh I. C., Lenton T. M., Periodic mid-Cretaceous oceanic anoxic events linked by oscillations of the phosphorus and oxygen biogeochemical cycles. Global Biogeochem. Cycles 17, 1092 (2003). [Google Scholar]
- 92.Mort H. P., Adatte T., Föllmi K. B., Keller G., Steinmann P., Matera V., Berner Z., Stüben D., Phosphorus and the roles of productivity and nutrient recycling during oceanic anoxic event 2. Geology 35, 483–486 (2007). [Google Scholar]
- 93.Albarède F., Michard A., Minster J. F., Michard G., 87Sr/86Sr ratios in hydrothermal waters and deposits from the East Pacific Rise at 21°N. Earth Planet. Sci. Lett. 55, 229–236 (1981). [Google Scholar]
- 94.Richter F. M., Liang Y., The rate and consequences of Sr diagenesis in deep-sea carbonates. Earth Planet. Sci. Lett. 117, 553–565 (1993). [Google Scholar]
- 95.Hodell D. A., Mead G. A., Mueller P. A., Variation in the strontium isotopic composition of seawater (8 Ma to present): Implications for chemical weathering rates and dissolved fluxes to the oceans. Chem. Geol. 80, 291–307 (1990). [Google Scholar]
- 96.Andersen M. B., Romaniello S., Vance D., Little S. H., Herdman R., Lyons T. W., A modern framework for the interpretation of 238U/235U in studies of ancient ocean redox. Earth Planet. Sci. Lett. 400, 184–194 (2014). [Google Scholar]
- 97.Montoya-Pino C., Weyer S., Anbar A. D., Pross J., Oschmann W., van de Schootbrugge B., Arz H. W., Global enhancement of ocean anoxia during Oceanic Anoxic Event 2: A quantitative approach using U isotopes. Geology 38, 315–318 (2010). [Google Scholar]
- 98.Wang X., Planavsky N. J., Reinhard C. T., Hein J. R., Johnson T. M., A Cenozoic seawater redox record derived from 238U/235U in ferromanganese crusts. Am. J. Sci. 316, 64–83 (2016). [Google Scholar]
- 99.Orchard M. J., Conodont diversity and evolution through the latest Permian and Early Triassic upheavals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 93–117 (2007). [Google Scholar]
- 100.Fraiser M. L., Bottjer D. J., The non-actualistic early Triassic gastropod fauna: A case study of the lower triassic sinbad limestone member. Palaios 19, 259–275 (2004). [Google Scholar]
- 101.Payne J. L., Evolutionary dynamics of gastropod size across the end-Permian extinction and through the Triassic recovery interval. Paleobiology 31, 269–290 (2005). [Google Scholar]
- 102.Twitchett R. J., The Lilliput effect in the aftermath of the end-Permian extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 132–144 (2007). [Google Scholar]
- 103.Pruss S. B., Bottjer D. J., Early Triassic trace fossils of the western United States and their implications for prolonged environmental stress from the end-permian mass extinction. Palaios 19, 551–564 (2004). [Google Scholar]
- 104.Payne J. L., Lehrmann D. J., Wei J., Knoll A. H., The pattern and timing of biotic recovery from the end-Permian extinction on the great bank of guizhou, guizhou province, china. Palaios 21, 63–85 (2006). [Google Scholar]
- 105.Ware D., Bucher H., Brayard A., Schneebeli-Hermann E., Brühwiler T., High-resolution biochronology and diversity dynamics of the Early Triassic ammonoid recovery: The Dienerian faunas of the Northern Indian Margin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 440, 363–373 (2015). [Google Scholar]
- 106.Alroy J., Accurate and precise estimates of origination and extinction rates. Paleobiology 40, 374–397 (2014). [Google Scholar]
- 107.Brayard A., Bylund K. G., Jenks J. F., Stephen D. A., Olivier N., Escarguel G., Fara E., Vennin E., Smithian ammonoid faunas from Utah: Implications for Early Triassic biostratigraphy, correlation and basinal paleogeography. Swiss J. Palaeontol. 132, 141–219 (2013). [Google Scholar]
- 108.Tozer E. T., Canadian Triassic ammonoid faunas. Geol. Surv. Canada Bull. 467, 1–663 (1994). [Google Scholar]
- 109.S. P. Ermakova, Zonal’nyi Standart Boreal’nogo Nizhnego Trias (Nauka, 2002). [Google Scholar]
- 110.Zakharov Y. D., Mousavi Abnavi N., The ammonoid recovery after the end-Permian mass extinction: Evidence from the Iran-Transcaucasia area, Siberia, Primorye, and Kazakhstan. Acta Palaeontol. Pol. 58, 127–147 (2013). [Google Scholar]
- 111.Brühwiler T., Bucher H., Brayard A., Goudemand N., High-resolution biochronology and diversity dynamics of the Early Triassic ammonoid recovery: The Smithian faunas of the Northern Indian Margin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 297, 491–501 (2010). [Google Scholar]
- 112.J. Guex, A. Hungerbühler, J. F. Jenks, L. O’Dogherty, V. Atudorei, D. G. Taylor, H. Bucher, A. Bartolini, Spathian (Lower Triassic) ammonoids from western USA (Idaho, California, Utah and Nevada), in Mémoires de Géologie (Lausanne), (Université de Lausanne, 2010), vol. 49, pp. 1–82. [Google Scholar]
- 113.Bucher H., Lower Anisian ammonoids from the northern Humboldt Range (northwestern Nevada, USA) and their bearing upon the Lower-Middle Triassic boundary. Eclogae Geol. Helv. 82, 945–1002 (1989). [Google Scholar]
- 114.Chen J., Beatty T. W., Henderson C. M., Rowe H., Conodont biostratigraphy across the Permian–Triassic boundary at the Dawen section, Great Bank of Guizhou, Guizhou Province, South China: Implications for the Late Permian extinction and correlation with Meishan. J. Asian Earth Sci. 36, 442–458 (2009). [Google Scholar]
- 115.Zhang L., Orchard M. J., Algeo T. J., Chen Z.-Q., Lyu Z., Zhao L., Kaiho K., Ma B., Liu S., An intercalibrated Triassic conodont succession and carbonate carbon isotope profile, Kamura, Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol., in press (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/e1602921/DC1
The study section
Evidence for primary seawater δ238U values
Compilation of global carbonate δ238U records for PTB interval
Possible timing of onset of oceanic anoxia
Age-thickness model for Zal section
Cross-correlation analysis
High-resolution intercomparison of Zal δ13C, 87Sr/86Sr, and δ238U records
Estimation of weathering rates and seawater PO43− levels in the Early Triassic ocean
Box model estimates for fanox
Patterns of marine invertebrate clade recovery following the LPME
Ammonoid extinction rates
table S1. Strontium and phosphorus model parameterization.
table S2. Uranium box model parameterization.
fig. S1. Geochemical profile of Zal, Iran.
fig. S2. Diagenetic evaluation cross-plots of δ238U-[Sr], δ238U-Mn/Sr, and δ238U-Mg/Ca (mol/mol) of all samples, samples below 3.5 m, samples between 3.5 and 500 m, and samples above 500 m.
fig. S3. Cross-plots of δ238U-Rb/Sr and δ238U-U/Al ratio [parts per million/weight % (wt %)] for all samples and samples below and above 500 m.
fig. S4. Cross-plots of δ238U-Mn/Sr, δ238U-Mg/Ca, δ238U-Rb/Sr, and δ238U-U/Al (wt %) for anoxic events 1 and 2.
fig. S5. Cross-plots of δ238U-Mn/Sr, δ238U-Mg/Ca, δ238U-Rb/Sr, and δ238U-U/Al (wt %) for anoxic events 3 and 4.
fig. S6. Cross-plots of δ13C-δ18O for the Zal section.
fig. S7. Location of Iran, South China, and Turkey during the Permian-Triassic transition, ~252 Ma (modified after Payne et al. (60).
fig. S8. Comparison of U- and C-isotope profiles for Zal, Dawen, Dajiang, Taşkent, and Kamura.
fig. S9. A LOWESS trend showing inferred timing of onset of latest Permian oceanic anoxia.
fig. S10. Age-depth model for the Zal, Iran study section.
fig. S11. Cross-correlation analysis of LOWESS-smoothed curves for U-C-Sr isotope records.
fig. S12. 87Sr/86Sr-derived estimates of the continental weathering flux and the calculated seawater PO43− concentrations for the Early Triassic ocean.
fig. S13. Interregional ammonoid zonation scheme.
dataset S1. δ238U data with associated geochemical data.


