Mesozoic lepidopteran wing scales shed light on the early evolution of moths and structural colors.
Abstract
Lepidopteran scales exhibit remarkably complex ultrastructures, many of which produce structural colors that are the basis for diverse communication strategies. Little is known, however, about the early evolution of lepidopteran scales and their photonic structures. We report scale architectures from Jurassic Lepidoptera from the United Kingdom, Germany, Kazakhstan, and China and from Tarachoptera (a stem group of Amphiesmenoptera) from mid-Cretaceous Burmese amber. The Jurassic lepidopterans exhibit a type 1 bilayer scale vestiture: an upper layer of large fused cover scales and a lower layer of small fused ground scales. This scale arrangement, plus preserved herringbone ornamentation on the cover scale surface, is almost identical to those of some extant Micropterigidae. Critically, the fossil scale ultrastructures have periodicities measuring from 140 to 2000 nm and are therefore capable of scattering visible light, providing the earliest evidence of structural colors in the insect fossil record. Optical modeling confirms that diffraction-related scattering mechanisms dominate the photonic properties of the fossil cover scales, which would have displayed broadband metallic hues as in numerous extant Micropterigidae. The fossil tarachopteran scales exhibit a unique suite of characteristics, including small size, elongate-spatulate shape, ridged ornamentation, and irregular arrangement, providing novel insight into the early evolution of lepidopteran scales. Combined, our results provide the earliest evidence for structural coloration in fossil lepidopterans and support the hypothesis that fused wing scales and the type 1 bilayer covering are groundplan features of the group. Wing scales likely had deep origins in earlier amphiesmenopteran lineages before the appearance of the Lepidoptera.
INTRODUCTION
Structural colors have evolved in a myriad of animals and plants and result from the wavelength-selective scattering of incident light by structures with periodicities measuring from tens to hundreds of nanometers (1–3). Such colors are typically more vibrant and visually arresting than those produced via pigmentation (4) and are often multifunctional, playing important roles in intraspecific sexual signaling, aposematism, and crypsis (5–7). Lepidoptera exhibit in their scales some of the most diverse structural colors produced by insects, with this diversity undoubtedly having contributed to the evolutionary success of the order (4, 8–11). Four types of wing scale covering are present in modern lepidopterans (12), among which, the type 1 bilayer scale covering (in which a layer of large fused cover scales overlies a layer of smaller fused ground scales) is widespread in the most basal extant lepidopterans (12). The color of lepidopteran wings is typically attributed to cover scales [but see the studies of Vukusic et al. (13) and Stavenga et al. (14)]; ground scales in many modern lepidopterans contain pigment that absorbs excess light transmitted by the cover scales, thus enhancing the spectral purity of the structural color (14, 15). Unlike in other types of scale vestiture, that is, those featuring hollow scales, photonic structures in the type 1 bilayer arrangement are restricted to the abwing (upward-facing) surface of cover scales.
Despite sustained interest in the structure, development, and photonic and other biomimetic properties of lepidopteran scales in neontological studies (13–22), and recent research into structural colors in fossil beetles (23, 24) and feathers (25, 26), the deep evolutionary history of scales and structural colors in lepidopterans is poorly understood. Fossil wing scales have been reported from Mesozoic and Cenozoic compression fossils and amber, but the ultrastructure of most of these fossil scales has not been characterized (27–32). The only photonic nanostructures reported in fossil insect scales are from fossil lepidopterans from the Eocene Messel oil shale, Germany (9), and a single weevil from the Pleistocene of Gold Run, Canada (33). The fossil record has the potential to preserve photonic structures in scales; the record of lepidopteran scales is now known to extend to the latest Triassic (34), confirming the feasibility of reconstructing the evolution of photonic structures in this group. Specific questions about the evolution of Lepidoptera are as follows: Were photonic structures present in the earliest lepidopterans, and are they part of the groundplan of lepidopteran scales?
Here, we report scale architectures preserved both in diverse Jurassic lepidopterans from Europe and Asia and in mid-Cretaceous Tarachoptera from Burmese amber [99 million years (Ma) ago] and compare them to those of extant primitive lepidopterans. We use optical microscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and confocal laser scanning microscopy (CLSM) to reveal the gross morphology and ultrastructure of the scales. Using the ultrastructural parameters identified in Jurassic specimens, we demonstrate the use of optical modeling to describe the theoretical optical properties of the type 1 bilayer scale arrangement, thus providing the earliest evidence of structural colors in the insect fossil record.
RESULTS
Wing scales of Jurassic Lepidoptera
We characterized the arrangement, shape, and fine ultrastructure of scales of six fossils from the United Kingdom, Germany, Kazakhstan, and China (representing a substantial portion of known Jurassic lepidopterans) using optical microscopy and SEM (Fig. 1). Before the recent discovery of fossilized lepidopteran scales in latest Triassic sediments from northern Germany (34), Archaeolepis mane (Archaeolepidae) from the Lower Jurassic of Dorset in England was the oldest known lepidopteran (35). This lepidopteran exhibits dense scale coverage on the 5.5-mm-long forewing (Fig. 1, A to C, and fig. S1, A to E). Two types of scale occur: cover scales 100 to 110 μm long and ground scales 76 to 82 μm long (Table 1). The surface of each cover scale exhibits 20 to 25 longitudinal parallel ridges 1.8 to 2.0 μm apart (Fig. 1C). SEM and optical microscopy (under ultraviolet illumination; fig. S1C) show that the wing scales are elongate-spatulate with a rounded apical margin (Fig. 1C); this geometry is identical to that of certain extant Micropterigidae (a family of primitive moths; Fig. 1K) and does not occur in other extant lepidopterans. Similarly, four specimens (possibly Eolepidopterigidae), one from the Upper Jurassic of Kazakhstan (PIN2239/607) (36) and three from the Lower Jurassic of Germany (LGA968, LG1500, and LGA2150) (37, 38), show dense spatulate scales on the forewings (figs. S2 and S3, E to H). Specimen PIN2239/607 has cover and ground scales 100 to 110 μm and 48 to 53 μm long, respectively (Table 1); the cover scales exhibit 23 to 28 longitudinal parallel ridges that extend slightly beyond the apical margin of the scale (Fig. 1F). The microstructure between the parallel ridges of these scales is not preserved clearly in these compression fossils. In addition, we investigated 16 well-preserved lepidopterans from the Middle Jurassic of Daohugou, China. Although fossil insects from Daohugou are famous for their excellent macroscopic preservation (39, 40), the Daohugou lepidopterans (Mesokristenseniidae) preserve only faint traces of wing scales (fig. S1, F and G).
Fig. 1. Wings and scales of Jurassic Lepidoptera and extant Micropterigidae.
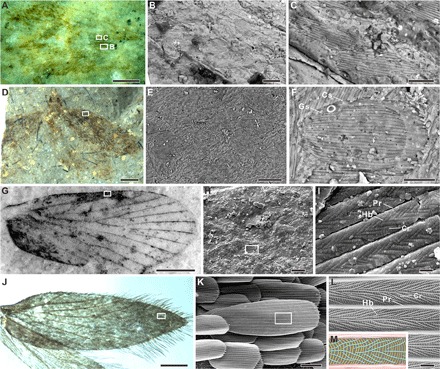
(A to C) A. mane from the Lower Jurassic of Dorset in England, NHMUK In. 59397. (B and C) SEM images of wing scales. (D to F) Lepidopteran specimen from the Upper Jurassic of Karatau in Kazakhstan, PIN2239/607. (E) Enlargement of forewing in (D), SEM image. (F) Cover scale overlapping ground scale, SEM image. (G to I) Lepidopteran specimen from the Lower Jurassic of Grimmen in Germany, LGA1500. (H) Enlargement of forewing in (G), SEM image. (I) Enlargement of forewing scale in (H), SEM image; note that it is an impression fossil and represents an imprint of the original structure. (J to L) Forewing of extant micropterigid moth (M. aruncella). (K) Enlargement of forewing in (J), SEM image. (L) Enlargement of forewing scale in (K), SEM image. (M) Interpretative sketch of herringbone ultrastructure; the regions between the parallel ridges (red) display a striking herringbone pattern formed by oblique-longitudinal parallel crests (green) superimposed on closely spaced parallel crossribs (yellow). Cr, crossrib; Cs, cover scale; Gs, ground scale; Hb, herringbone crest; Pr, parallel ridge. Scale bars, 1 mm (A, D, and G), 0.5 mm (J), 50 μm (E), 20 μm (B, C, F, H, and K), and 1 μm (I and L). Photo credit: B.W., Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. Used with permission.
Table 1. Measurements of Mesozoic forewing scales.
FL, forewing length; CSL, cover scale length; CSW, cover scale width; GSL, ground scale length; GSW, ground scale width.
| Specimen | Taxonomy | FL (mm) | CSL (μm) | CSW (μm) | GSL (μm) | GSW (μm) | Age and locality |
| Kinitocelis brevicostata | Tarachoptera | 4.5 | 30−50 | 15−20 | None | None | Mid-Cretaceous Burmese amber |
| A. mane | Archaeolepidae | 5.5 | 100−110 | 36−42 | 76−82 | 26−33 | Early Jurassic; United Kingdom |
| LGA968 | ? Eolepidopterigidae | 4.7 | 200−230 | 70−80 | 140−160 | 60−70 | Early Jurassic; Germany |
| LGA2150 | ? Eolepidopterigidae | 4.7 | 200−230 | 70−80 | 140−160 | 60−70 | Early Jurassic; Germany |
| LGA1500 | ? Eolepidopterigidae | 4.9 | 200−230 | 65–80 | Unknown | Unknown | Early Jurassic; Germany |
| Mesokristensenia sinica | Mesokristenseniidae | 6.1 | 95−110 | 35–43 | Unknown | Unknown | Middle Jurassic; China |
| PIN2239/607 | ? Eolepidopterigidae | 5.2 | 100−110 | 48–53 | 66−72 | 35−43 | Late Jurassic; Kazakhstan |
Specimen LG1500 (possibly Eolepidopterigidae; Lower Jurassic of Germany), preserved as an external mold (impression), reveals the preservation of three-dimensional ultrastructural features on the surface of the scales. These fossil cover scales are approximately 200 to 230 μm long and 65 to 80 μm wide (Table 1); their surface exhibits 30 to 38 longitudinal parallel ridges spaced 1.8 to 2.0 μm apart. Between the parallel ridges lie ultrastructural features with submicrometer periodicities, namely, a striking herringbone pattern formed by oblique-longitudinal parallel crests superimposed on closely spaced (0.14 μm apart) parallel crossribs (Fig. 1I and figs. S3, C and D, and S4). The crossribs connect with, and are perpendicular to, the longitudinal parallel ridges. The herringbone crests are 1.7 to 1.8 μm long and 0.25 to 0.30 μm apart. Herringbone patterning is present only in the three most primitive extant families of Lepidoptera (Micropterigidae, Agathiphagidae, and Heterobathmiidae) (12, 41, 42). The cover scales of the Jurassic lepidopterans studied here are, however, distinctly different from those of Agathiphagidae: The latter have a deep apical notch, and their parallel ridges are more narrowly spaced (distances less than 2 μm) (43). The scales in the Jurassic fossils are broadly similar in morphology to those of extant Heterobathmiidae but lack the interridge plates present in the latter (12). In addition, the arrangement and ultrastructure of these fossil scales differ from those of known examples in extant Trichoptera (caddisflies, which are closely related to Lepidoptera) (44, 45). The scales do, however, closely resemble those of certain extant Micropterigidae, notably Micropterix aruncella and Micropterix calthella (46), which both exhibit broadband, metallic hues.
Optical modeling
To reconstruct the approximate color of the fossil cover scales of Jurassic lepidopterans, we performed optical modeling. Our model comprised a simplified two-dimensional geometry of the fused cover scale ultrastructure, with crossrib and herringbone crest periodicities measured directly from SEM images of specimen LG1500 (Fig. 1I and fig. S3, C and D). The simulations demonstrate that the overall optical response includes strong coherent scattering (Fig. 2A). At normal incidence, zeroth-order diffraction (producing thin-film interference–like effects) from the modeled scale geometry shows broadband coloration, with an intensity of approximately 7 to 8% for wavelengths above 500 nm (Fig. 2B, solid lines). This response is sensitive to the polarization state of the incident light: Fig. 2B shows both the transverse electric (TE) and transverse magnetic (TM) cases, for which the electric field is linearly polarized parallel or perpendicular to the longitudinal ridges, respectively.
Fig. 2. Optical modeling showing the photonic response of a simplified fused cover scale structure.
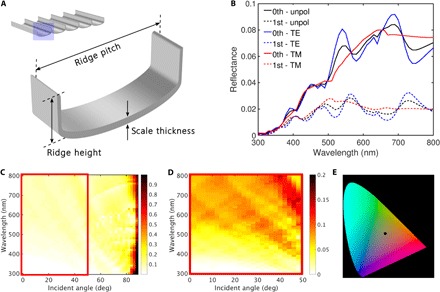
(A) Three-dimensional schematic of the “unit cell” representing the two-dimensional “U-shaped” structure for which simulations were performed, showing the parallel ridges that run longitudinally along the abwing scale surface. Extending the unit cell gives the periodic structure shown at the top left. (B) Reflectance spectra (for excitation at normal incidence) illustrating zeroth-order diffraction (thin-film interference–like; solid lines) and first-order diffraction (dashed lines) for TE (blue lines) and TM (red lines) polarization states. Together, the TE and TM spectra produce the unpolarized response (black lines). (C) The unpolarized zeroth-order response as a function of increasing angle of incidence, with the region enclosed by the red rectangle shown in (D). (E) International Commission on Illumination color chart with the unpolarized zeroth-order spectrum in (B) represented by the black dot illustrating the broadband color produced by the structure in (A).
There are three distinct peaks in the TE response with maxima at about 460, 525, and 690 nm (the last is significantly broader and weighted toward the long-wavelength band edge) (Fig. 2B, solid blue line). The intensity of these peaks increases with increasing wavelength, although the short-wavelength peak (460 nm) is considerably less intense, illustrating the relatively weak reflectance of blue wavelengths. The broad peak at 690 nm exhibits a small, but sharp, dip at approximately 640 nm that corresponds to the onset of diffraction imposed by the periodicity of the longitudinal parallel ridges. Additional minor dips in the thin-film–like response at shorter wavelengths also correspond to peaks in first-order diffraction (Fig. 2B, dashed blue line).
Although the TM response is similar in intensity to that modeled for the TE case, the former exhibits fewer distinct spectral peaks. Specifically, the TM case shows a steady increase in the zeroth-order reflectance above 500 nm, with a maximum at approximately 650 nm (a small dip attributed to diffraction from the parallel ridges from 650 to 670 nm) and a plateau for wavelengths extending beyond 700 nm into the near infrared (Fig. 2B, solid red line). Together, the TE and TM thin-film interference–like contributions produce broadband structural color at normal incidence.
Higher-order diffraction effects, a result of the periodicity imposed by the longitudinal parallel ridges, are less intense. This is shown here for the first-order diffraction term only (Fig. 2B, dashed lines); however, many low-intensity higher-order diffraction effects occur. The higher-order diffracted modes are scattered away from the surface normal and thus increase the angular range over which light is scattered, affecting the directionality of the color. Consequently, diffractive scattering by the parallel ridges maintains the scales’ broadband color appearance with respect to viewing angle: There is minimal blue shift in the spectral features with increasing angle of incidence (Fig. 2, C and D).
We also performed optical simulations to investigate how the photonic properties (scale thickness, ridge height, and ridge pitch) of these Jurassic fused cover scales respond to alterations in several structural parameters (fig. S5, A to C). A strong photonic response is optimized with a scale thickness of 100 to 150 nm coupled with a parallel ridge height exceeding 0.5 μm. Increasing the pitch of the parallel ridges results in a red shift of the spectral features. This shift is clearest for the high-intensity, long-wavelength peak (maximum at 690 nm for default parameters).
In summary, our optical simulations of Jurassic lepidopteran scales demonstrate that the presence of longitudinal parallel ridges alone is sufficient for these scales to produce coherent optical scattering, which, with the appropriate structural parameters, can manifest as broadband, metallic hues comparable to those observed in extant Micropterigidae (Fig. 2E).
Wing scales of Tarachoptera
Tarachoptera is a newly erected stem group of Amphiesmenoptera (which also includes Lepidoptera and Trichoptera) that potentially provides important information on the origin and groundplan of wing scales in Lepidoptera (47). Using optical microscopy, TEM, and CLSM, we examined the wing scales of two newly discovered tarachopteran specimens from mid-Cretaceous Burmese amber. The most notable feature of these newly discovered fossils is the dense arrangement of small scales, 30 to 50 μm long, on both the forewing and hind wing (Fig. 3). In Lepidoptera, the minimum individual scale length reported to date is 40 μm in a nepticulid moth, and the minimum average scale length is 60 μm for an archaic micropterigid moth (48). Thus, despite tarachopterans having both larger wings and bodies than extant micropterigids, the fossil tarachopteran scales are smaller than those in extant lepidopterans. This refutes the suggestion that a lower scale size limit was an important factor preventing the miniaturization of lepidopterans (49).
Fig. 3. Tarachoptera from mid-Cretaceous Burmese amber.
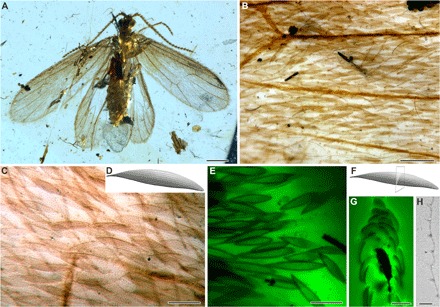
(A to D) K. brevicostata, female, NIGP164785. (B and C) Forewing scales; note the tubercles and setae on wing membrane. (D) Scale reconstruction. (E to H) K. brevicostata, male, NIGP164786. (E) CLSM images of forewing scales detached from the forewing. (F) Interpretative sketch of cross section of scale. (G) CLSM image of cross section of forewing scales. (H) TEM image of cross section of a forewing scale. Scale bars, 0.5 mm (A), 0.1 mm (B), 50 μm (C), 40 μm (E), 20 μm (G), and 2 μm (H). Photo credit: B.W., Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. Used with permission.
The fossil tarachopteran scales consist of a basal pedicel plugged into a socket and an elongate, spatulate main body with an acute apical margin (Fig. 3, D and E). They are distinctly different from other primitive lepidopteran scales, which have rounded or scalloped apical margins (8). The surface of each tarachopteran scale exhibits 8 to 10 longitudinal parallel ridges 2 to 3 μm apart that merge apically into the distal margin; this again contrasts with extant primitive lepidopteran scales, where the ridges form short projections beyond the scale margin (12, 50). The wing scale exhibits no windows or perforations between the parallel ridges (Fig. 3, C and E). Furthermore, the results of CLSM and TEM show that the cross section of the main body of the scales consists of a solid, homogeneous plate (Fig. 3, F to H). All the fossil tarachopteran wing scales are slightly arched whereby the concave side faces the wing membrane, which is densely covered by tubercles (Fig. 3C). Setae are dispersed randomly among the scales on both wings (Fig. 3B), which is a primitive condition retained in most basal lepidopterans (12).
DISCUSSION
Photonic structures and structural colors of Jurassic lepidopteran
Our data confirm the potential of both compression and impression fossils to preserve ultrastructural details of lepidopteran scales. The forewing scales of the Jurassic lepidopterans demonstrate remarkable morphological similarities to those of extant Micropterigidae; both have a type 1 bilayer arrangement, an elongate-spatulate shape, and herringbone crests. Furthermore, these results confirm that Jurassic lepidopterans had photonic nanostructures in their scales and thus exhibited structural colors.
Our optical simulations reveal that the photonic behavior of the fused cover scale architecture in both the extant micropterigids and the Jurassic fossils is driven by the interplay of the spatial periodicities of the longitudinal parallel ridges (mean pitch, 1.9 μm), crossribs (mean pitch, 140 nm), and herringbone crests (mean pitch, 280 nm). Structural periodicity in the plane of the scale surface gives rise to diffractive scattering, whereas periodicity perpendicular to the scale surface leads to thin-film interference. The latter tends to dominate where multiple lamellar components interfere constructively. In the Jurassic fossils studied here, the cover scales comprise a single fused laminar plate with additional ultrastructural elements protruding from the abwing surface. As a result, diffractive scattering contributes significantly to the scales’ photonic properties. The coloration of the bright gold patches on the wings of the extant moth Thysanoplusia orichalcea (Noctuidae: Argyrogrammatini) is similarly attributed to diffraction effects (51). The 140-nm crossrib pitch measured here for specimen LGA1500 corresponds directly to that measured for T. orichalcea (51). Herringbone crests are a distinctive structural characteristic of the cover scales of the lepidopteran fossils described here and, also, gold-colored scales in certain extant Micropterigidae. Given that herringbone crests are absent in gold-colored scales in other lepidopterans, they are most likely not a major determinant of the broadband, metallic hues displayed by species in this group. For example, both higher lepidopterans, such as the aforementioned T. orichalcea, and primitive glossatans that have the type 1 bilayer scale arrangement [for example, Eriocrania semipurpurella (50)] achieve broadband, gold-like structural colors despite lacking herringbone crests. Our modeling illustrates that periodic ultrastructures in Jurassic lepidopteran scales could, nevertheless, produce broadband, metallic colors similar to those in extant Micropterigidae (52, 53), for example, M. aruncella and M. calthella (fig. S6). Before this study, the earliest record of structural color in insects was reported in fossil moths from the Eocene Messel oil shale, Germany (9). Our discovery therefore extends the known geological range of insect structural colors by at least 130 Ma.
Groundplan and early evolution of Lepidoptera
Lepidoptera and Trichoptera are thought to have diverged from a necrotauliid-like ancestor in the latest Triassic (54, 55). This indicates that Tarachoptera with a single-layer scale covering evolved before the Jurassic (Fig. 4). The fossil tarachopteran scales exhibit a unique suite of characteristics, including their small size, elongate-spatulate shape, ridged ornamentation, and irregular arrangement; these features may indicate previously unknown, more archaic conditions (relative to wing scales of extant primitive moths) (Fig. 4A).
Fig. 4. Evolutionary history and paleodistribution of Amphiesmenoptera.
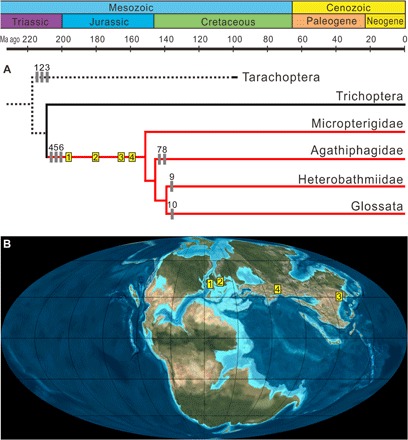
(A) Simplified phylogeny of Amphiesmenoptera (47, 55, 56). (B) Paleogeographic distribution of Jurassic lepidopterans on a Middle Jurassic (approximately 170 Ma ago) geographic map (60). Branches representing Lepidoptera are red. Gray vertical bars represent possible wing vestiture apomorphies: 1, single-layer covering; 2, fused scale with simple microstructure; 3, ridges ending in apical scale margin; 4, fused scale with herringbone crests; 5, type 1 bilayer covering; 6, ridges extending beyond apical scale margin; 7, scale arrangement clustered; 8, hollow scales present; 9, interridge plates present; 10, herringbone crests absent. Jurassic lepidopteran scales in clastic sediments are indicated with yellow vertical bars: 1, Early Jurassic of England (195 Ma ago); 2, Early Jurassic of Grimmen, Germany (181.5 Ma ago); 3, Middle Jurassic of Daohugou, China (165 Ma ago); 4, Late Jurassic of Karatau, Kazakhstan (160 Ma ago).
Except for members of the Agathiphagidae (which have hollow cover scales and fused ground scales) (43, 54, 56), most basal lepidopterans have fused scales that lack perforations and/or windows (50). In more derived lepidopterans, the scales are hollow and have windows and/or perforations on the abwing surface (12). Although most basal lepidopterans have fused wing scales (12), developmental biology studies suggest that the primitive lepidopteran scale should be hollow because it originates from a cylindrical hollow bristle (15, 57). Our data, however, show that tarachopteran scales were fused and that early lepidopterans had a type 1 bilayer scale covering. These observations support the hypotheses that fused wing scales and the type 1 bilayer scale arrangement are groundplan features of Lepidoptera (8).
In summary, our study confirms that the earliest lepidopterans had in their cover scales photonic structures that likely produced metallic broadband colors via a combination of thin-film interference–like and diffraction mechanisms. Our data also demonstrate that the type 1 bilayer scale vestiture and herringbone crossribs (groundplan features of Lepidoptera) found in basal extant lepidopterans had already evolved by the mid-Jurassic (Fig. 4). These findings have broader implications: The widespread occurrence of wing scales in Jurassic lepidopterans and in tarachopterans strongly suggests that wing scales (including some possibly unknown morphotypes) were widespread in stem Amphiesmenoptera before their apogee in the Lepidoptera. Given the presence of structural coloration in these basal fossil lepidopterans, the advent of major lepidopteran clades by the Cretaceous [but see the study of van Eldijk et al. (34)] raises the possibility that this taxonomic radiation may have been accompanied by increased diversity in scale shape, microstructure, and optical effects (Fig. 4). Future studies will characterize the optical response of scale nanostructures in other fossil specimens and will provide evidence for the presence of scale pigments in fossil lepidopterans to inform models of the evolution of structural colors in lepidopterans.
MATERIALS AND METHODS
Materials and depository
The Burmese amber (NIGP164785 and NIGP164786) and Daohugou (NIGP150462) specimens are deposited in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences; the specimen from the United Kingdom (NHMUK In. 59397) is at the Natural History Museum in London, and the archive image (fig. S1C) was taken by P. York at the Natural History Museum; three specimens from Germany (LGA968, LGA1500, and LGA2115) are at the Museum für Naturkunde, Humboldt University; and the specimen from Kazakhstan (PIN2239/607) is at the Paleontological Institute, Russian Academy of Sciences.
Optical microscopy
To reduce the optical distortion caused by the refractive index contrast between amber and air, we sandwiched the amber specimens between two coverslips and filled the space with glycerol. Photographs were taken using a Zeiss Stereo Discovery V16 microscope system at the State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. In most instances, incident and transmitted light were used simultaneously. All images are digitally stacked photomicrographic composites of approximately 50 individual focal planes obtained using the free software CombineZP for better illustration of the three-dimensional structures.
Confocal laser scanning microscopy
Photomicrographs with green background were taken using a CLSM Zeiss LSM710 with ×63 and ×40 objectives and a laser at 488 nm at the State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. To get higher-resolution images, the two amber specimens (NIGP164785 and NIGP164786) were polished to a thickness of 1.1 mm. Fortunately, a cross section of the forewing with scales was exposed in specimen NIGP164786, allowing examination of a transverse section of the main body of the scales.
Scanning electron microscopy
Scanning electron micrographs of the Kazakhstan specimen PIN2239/607 (Fig. 1, E and F, and fig. S2) were obtained by analyzing the uncoated specimen with a LEO1530VP variable pressure scanning electron microscope at an accelerating voltage of 15 kV at the State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. SEM images of wing scales of extant Micropterigidae (Fig. 1, K and L) were obtained using dehydrated, gold-coated tissue samples. SEM images of specimen NHMUK In. 59397 (Fig. 1, B and C, and fig. S1, D and E) were obtained using a LEO 1455VP variable pressure scanning electron microscope at an accelerating voltage of 20 kV at the Natural History Museum, London. SEM images of the Grimmen specimen LGA1500 (Fig. 1, H and I, and figs. S3, B to D, and S4) were obtained using a Zeiss EVO LS10 scanning electron microscope at an accelerating voltage of 10 kV after gold coating at Greifswald University.
Transmission electron microscopy
The cross-sectioned scale was gently isolated from a broken piece of specimen NIGP164786 using a scalpel and then embedded in Epon-812 resin. The embedded specimens were polymerized at 35°C for 24 hours, then at 40°C for 48 hours, and finally at 60°C for 48 hours. The ultrathin section was prepared using a PowerTome XL ultramicrotome and mounted on a carbon-coated copper grid. The section was imaged using a JEM-1400Plus transmission electron microscope (at 80 keV) at the State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences.
Optical modeling
Optical modeling was performed using COMSOL Multiphysics 5.2a (www.comsol.com), a commercial finite element method software package. A simple two-dimensional U-shaped periodic structure representative of the fossil specimen LGA1500 (Fig. 1, G to I) was constructed by repeating the unit cell geometry (Fig. 2A). The pitch of the longitudinal parallel ridges was determined directly from SEM images of the structure (Fig. 1I and fig. S3, C and D). The crossrib pitch is sufficiently small relative to visible wavelengths such that incident light interacts with the abwing surface of the scale (in the interridge areas) as though it were homogenous. As a result, zeroth-order diffraction occurs, which contributes to the specular reflectance as opposed to scattering incident light to higher angles. Furthermore, higher-order diffractive scattering attributable to the crossrib pitch is negligible, thus justifying our approximation of the photonic response via a simple two-dimensional geometry. Details of other structural parameters in the model used data from previous studies: A complex refractive index, n* = 1.56 + 0.06i, was used for the specimen’s chitinous cuticle (13, 58, 59); a default thickness of 100 nm was used for the scale cuticle (8, 51); and the default parallel ridge height was set to 500 nm (8, 51). Considering the fused scale morphology, the refractive index was assumed to remain constant throughout the scale. The simulations presented here correspond to the optical scattering of an unpolarized plane wave at normal incidence unless otherwise indicated. Incident wave polarizations were defined as TE (TM) electric field linearly polarized parallel (perpendicular) to the longitudinal ridges, and the surface normal for the plane of incidence for off-normal excitation conditions was defined as parallel to the longitudinal ridges.
Supplementary Material
Acknowledgments
We are grateful to A. Rasnitysn, A. Ross, and P. Müller for providing specimens; R. Schlüter, Y. Fang, C. Z. Wang, Y. Q. Mao, and L. L. Cong for technical assistance; D. H. Yang for the reconstruction; and J. Clarke and four anonymous reviewers for careful comments that improved this manuscript. Funding: This research was supported by the National Natural Science Foundation of China (41572010, 41622201, and 41688103), Chinese Academy of Sciences (XDPB05), and the Museum für Naturkunde (Berlin). Work by M.E.M. and L.T.M. was supported by a European Research Council Starting Grant H202-2014-ER-StG-637691-ANICOLEVO awarded to M.E.M. B.W. was also supported by Youth Innovation Promotion Association of the Chinese Academy of Sciences (2011224). Author contributions: B.W. designed the project; Q.Z., W.M., J.A., E.A.J., W.W., R.K., X.R., J.C., H.Z., and B.W. performed the comparative and analytical work; Q.Z., J.A., E.A.J., and R.K. collected fossil data and contributed to the discussion; T.A.S. performed the optical modeling and analyzed the results; L.T.M. designed the optical modeling, analyzed the results, and helped write the manuscript; M.E.M. helped write the manuscript; and Q.Z. and B.W. wrote the paper with input from all other authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/e1700988/DC1
fig. S1. Jurassic Lepidoptera from United Kingdom and China.
fig. S2. SEM image of forewing scales on Kazakhstan specimen PIN2239/607.
fig. S3. Jurassic Lepidoptera from Germany.
fig. S4. SEM image of forewing scales on Grimmen specimen LGA1500.
fig. S5. Reflectance color maps.
fig. S6. Structural color of extant Micropterigidae.
REFERENCES AND NOTES
- 1.Vukusic P., Sambles J. R., Photonic structures in biology. Nature 424, 852–855 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Parker A. R., A geological history of reflecting optics. J. R. Soc. Interface 2, 1–17 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinoshita S., Yoshioka S., Miyazaki J., The physics of structural colors. Rep. Prog. Phys. 71, 076401 (2008). [Google Scholar]
- 4.Vukusic P., Structural colour in Lepidoptera. Curr. Biol. 16, R621–R623 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Sweeney A., Jiggins C., Johnsen S., Polarized light as a butterfly mating signal. Nature 423, 31–32 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Prudic K. L., Skemp A. K., Papaj D. R., Aposematic coloration, luminance contrast, and the benefits of conspicuousness. Behav. Ecol. 18, 41–46 (2007). [Google Scholar]
- 7.Kertész K., Bálint Z., Vértesy Z., Márk G. I., Lousse V., Vigneron J. P., Rassart M., Biró L. P., Gleaming and dull surface textures from photonic-crystal-type nanostructures in the butterfly Cyanophrys remus. Phys. Rev. E 74, 021922 (2006). [DOI] [PubMed] [Google Scholar]
- 8.N. P. Kristensen, Lepidoptera, Moths and Butterflies, Volume 2: Morphology, Physiology, and Development (Walter de Gruyter, 2003). [Google Scholar]
- 9.McNamara M. E., Briggs D. E. G., Orr P. J., Wedmann S., Noh H., Cao H., Fossilized biophotonic nanostructures reveal the original colors of 47-million-year-old moths. PLOS Biol. 9, e1001200 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rust J., Fossil record of mass moth migration. Nature 405, 530–531 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Mitter C., Davis D. R., Cummings M. P., Phylogeny and evolution of Lepidoptera. Annu. Rev. Entomol. 62, 265–283 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Simonsen T. J., The wing vestiture of the non-ditrysian Lepidoptera (Insecta). Comparative morphology and phylogenetic implications. Acta Zool. 82, 275–298 (2001). [Google Scholar]
- 13.Vukusic P., Sambles J. R., Lawrence C. R., Wotton R. J., Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. B 266, 1403–1411 (1999). [Google Scholar]
- 14.Stavenga D. G., Leertouwer H. L., Wilts B. D., Coloration principles of nymphaline butterflies—Thin films, melanin, ommochromes and wing scale stacking. J. Exp. Biol. 217, 2171–2180 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Ghiradella H., Insect cuticular surface modifications: Scales and other structural formations. Adv. Insect Physiol. 38, 135–180 (2010). [Google Scholar]
- 16.Ghiradella H., Structure of iridescent lepidopteran scales: Variations on several themes. Ann. Entomol. Soc. Am. 77, 637–645 (1984). [Google Scholar]
- 17.Ghiradella H., Light and color on the wing: Structural colors in butterflies and moths. Appl. Optics 30, 3492–3500 (1991). [DOI] [PubMed] [Google Scholar]
- 18.Ingram A. L., Parker A. R., A review of the diversity and evolution of photonic structures in butterflies, incorporating the work of John Huxley (The Natural History Museum, London from 1961 to 1990). Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2465–2480 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vukusic P., Sambles J. R., Lawrence C. R., Colour mixing in wing scales of a butterfly. Nature 404, 457 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Vukusic P., Hooper I., Directionally controlled fluorescence emission in butterflies. Science 310, 1151 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Zhang D., Zhang W., Gu J., Fan T., Liu Q., Su H., Zhu S., Inspiration from butterfly and moth wing scales: Characterization, modeling, and fabrication. Prog. Mater. Sci. 68, 67–96 (2015). [Google Scholar]
- 22.Potyrailo R. A., Starkey T. A., Vukusic P., Ghiradella H., Vasudev M., Bunning T., Naik R. R., Tang Z., Larsen M., Deng T., Zhong S., Palacios M., Grande J. C., Zorn G., Goddard G., Zalubovsky S., Discovery of the surface polarity gradient on iridescent Morpho butterfly scales reveals a mechanism of their selective vapor response. Proc. Natl. Acad. Sci. U.S.A. 110, 15567–15572 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara M. E., Briggs D. E. G., Orr P. J., Noh H., Cao H., The original colours of fossil beetles. Proc. R. Soc. B 279, 1114–1121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka G., Taniguchi H., Maeda H., Nomura S.-i., Original structural color preserved in an ancient leaf beetle. Geology 38, 127–130 (2010). [Google Scholar]
- 25.Li Q., Gao K.-Q., Vinther J., Shawkey M. D., Clarke J. A., D’Alba L., Meng Q., Briggs D. E. G., Prum R. O., Plumage color patterns of an extinct dinosaur. Science 327, 1369–1372 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Clarke J. A., Ksepka D. T., Salas-Gismondi R., Altamirano A. J., Shawkey M. D., D’Alba L., Vinther J., DeVries T. J., Baby P., Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957 (2010). [DOI] [PubMed] [Google Scholar]
- 27.MacKay M. R., Lepidoptera in Cretaceous amber. Science 167, 379–380 (1970). [DOI] [PubMed] [Google Scholar]
- 28.Schlüter T., Kritisches zum Nachweis von Schmetterlings-schuppen aus einem fossilen Harz der mittleren Kreide Nordwestfrankreichs. Deut. Entomol. Z. 84, 253–256 (1974). [Google Scholar]
- 29.Whalley P., Lower Cretaceous Lepidoptera. Nature 266, 526 (1977).859618 [Google Scholar]
- 30.Whalley P., New taxa of fossil and recent Micropterigidae with a discussion of their evolution and a comment on the evolution of Lepidoptera (Insecta). Ann. Transvaal Mus. 31, 71–86 (1978). [Google Scholar]
- 31.Whalley P., A review of the current fossil evidence of Lepidoptera in the Mesozoic. Biol. J. Linn. Soc. 28, 253–271 (1986). [Google Scholar]
- 32.Sohn J.-C., Labandeira C. C., Davis D. R., The fossil record and taphonomy of butterflies and moths (Insecta, Lepidoptera): Implications for evolutionary diversity and divergence-time estimates. BMC Evol. Biol. 15, 12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNamara M. E., Saranathan V., Locatelli E. R., Noh H., Briggs D. E. G., Orr P. J., Cao H., Cryptic iridescence in a fossil weevil generated by single diamond photonic crystals. J. R. Soc. Interface 11, 20140736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Eldijk T. J. B., Wappler T., Strother P. K., van der Weijst C. M. H., Rajaei H., Visscher H., van de Schootbrugge B., A Triassic-Jurassic window into the evolution of Lepidoptera. Sci. Adv. 4, e1701568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whalley P., The systematics and palaeogeography of the Lower Jurassic insects of Dorset, England. Bull. Br. Mus. 39, 107–189 (1985). [Google Scholar]
- 36.Kozlov M. V., New Lepidoptera (Papilionida) from the Upper Jurassic and Lower Cretaceous. Paleontol. J. 23, 34–39 (1989). [Google Scholar]
- 37.Ansorge J., Revision of the “Trichoptera” described by Geinitz and Handlirsch from the Lower Toarcian of Dobbertin (Germany) based on new material. Nova Suppl. Entomol. 15, 55–74 (2002). [Google Scholar]
- 38.Ansorge J., Insekten aus dem oberen Lias von Grimmen (Vorpommern, Norddeutschland). Neue Paläont. Abhandl. 2, 1–132 (1996). [Google Scholar]
- 39.Zhang W., Shih C., Labandeira C. C., Sohn J.-C., Davis D. R., Santiago-Blay J. A., Flint O., Ren D., New fossil Lepidoptera (Insecta: Amphiesmenoptera) from the Middle Jurassic Jiulongshan Formation of northeastern China. PLOS ONE 8, e79500 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B., Zhang H., Jarzembowski E. A., Fang Y., Zheng D., Taphonomic variability of fossil insects: A biostratinomic study of Palaeontinidae and Tettigarctidae (Insecta: Hemiptera) from the Jurassic Daohugou Lagerstätte. PALAIOS 28, 233–242 (2013). [Google Scholar]
- 41.Wahlberg N., Wheat C. W., Peña C., Timing and patterns in the taxonomic diversification of Lepidoptera (butterflies and moths). PLOS ONE 8, e80875 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regier J. C., Mitter C., Kristensen N. P., Davis D. R., van Nieukerken E. J., Rota J., Simonsen T. J., Mitter K. T., Kawahara A. Y., Yen S.-H., Cummings M. P., Zwick A., A molecular phylogeny for the oldest (nonditrysian) lineages of extant Lepidoptera, with implications for classification, comparative morphology and life-history evolution. Syst. Entomol. 40, 671–704 (2015). [Google Scholar]
- 43.Simonsen T. J., Kristensen N. P., Agathiphaga wing vestiture revisited: Evidence for complex early evolution of lepidopteran scales (Lepidoptera: Agathiphagidae). Insect Syst. Evol. 32, 169–175 (2001). [Google Scholar]
- 44.Huxley J., Barnard P. C., Wing-scales of Pseudoleptocerus chirindensis Kimmins (Trichoptera: Leptoceridae). Zool. J. Linn. Soc. 92, 285–312 (1988). [Google Scholar]
- 45.Robertson D. R., Holzenthal R. W., Two new species and a new record of Protoptila from Bolivia (Trichoptera: Glossosomatidae: Protoptilinae). Ann. Entomol. Soc. Am. 101, 465–473 (2008). [Google Scholar]
- 46.Davis D. R., Landry J.-F., A review of the North American genus Epimartyria (Lepidoptera, Micropterigidae) with a discussion of the larval plastron. Zookeys 2012, 37–83 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mey W., Wichard W., Müller P., Wang B., The blueprint of the Amphiesmenoptera—Tarachoptera, new order of insects from Burmese amber (Insecta, Amphiesmenoptera). Foss. Rec. 20, 129–145 (2017). [Google Scholar]
- 48.Simonsen T. J., Kristensen N. P., Scale length/wing length correlation in Lepidoptera (Insecta). J. Nat. Hist. 37, 673–679 (2003). [Google Scholar]
- 49.Grodnitsky D. L., Kozlov M. V., The structural and functional organisation of wing scale covering of butterflies and moths (Papilionida = Lepidoptera). Prog. Modern Biol. 107, 457–468 (1989). [Google Scholar]
- 50.Kristensen N. P., Morphological observations on the wing scales in some primitive Lepidoptera (Insecta). J. Ultrastruct. Res. 30, 402–410 (1970). [DOI] [PubMed] [Google Scholar]
- 51.Brink D. J., Smit J. E., Lee M. E., Möller A., Optical diffraction by the microstructure of the wing of a moth. Appl. Optics 34, 6049–6057 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Schachat S. R., Brown R. L., Color pattern on the forewing of Micropterix (Lepidoptera: Micropterigidae): Insights into the evolution of wing pattern and wing venation in moths. PLOS ONE 10, e0139972 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schachat S. R., Brown R. L., Forewing color pattern in Micropterigidae (Insecta: Lepidoptera): Homologies between contrast boundaries, and a revised hypothesis for the origin of symmetry systems. BMC Evol. Biol. 16, 116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D. A. Grimaldi, M. S. Engel, Evolution of the Insects (Cambridge Univ. Press, 2005). [Google Scholar]
- 55.Misof B., Liu S., Meusemann K., Peters R. S., Donath A., Mayer C., Frandsen P. B., Ware J., Flouri T., Beutel R. G., Niehuis O., Petersen M., Izquierdo-Carrasco F., Wappler T., Rust J., Aberer A. J., Aspöck U., Aspöck H., Bartel D., Blanke A., Berger S., Böhm A., Buckley T. R., Calcott B., Chen J., Friedrich F., Fukui M., Fujita M., Greve C., Grobe P., Gu S., Huang Y., Jermiin L. S., Kawahara A. Y., Krogmann L., Kubiak M., Lanfear R., Letsch H., Li Y., Li Z., Li J., Lu H., Machida R., Mashimo Y., Kapli P., McKenna D. D., Meng G., Nakagaki Y., Navarrete-Heredia J. L., Ott M., Ou Y., Pass G., Podsiadlowski L., Pohl H., von Reumont B. M., Schütte K., Sekiya K., Shimizu S., Slipinski A., Stamatakis A., Song W., Su X., Szucsich N. U., Tan M., Tan X., Tang M., Tang J., Timelthaler G., Tomizuka S., Trautwein M., Tong X., Uchifune T., Walzl M. G., Wiegmann B. M., Wilbrandt J., Wipfler B., Wong T. K. F., Wu Q., Wu G., Xie Y., Yang S., Yang Q., Yeates D. K., Yoshizawa K., Zhang Q., Zhang R., Zhang W., Zhang Y., Zhao J., Zhou C., Zhou L., Ziesmann T., Zou S., Li Y., Xu X., Zhang Y., Yang H., Wang J., Wang J., Kjer K. M., Zhou X., Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 736–767 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Bazinet A. L., Mitter K. T., Davis D. R., van Nieukerken E. J., Cummings M. P., Mitter C., Phylotranscriptomics resolves ancient divergences in the Lepidoptera. Syst. Entomol. 42, 305–316 (2017). [Google Scholar]
- 57.Galant R., Skeath J. B., Paddock S., Lewis D. L., Carroll S. B., Expression pattern of a butterfly achaete-scute homolog reveals the homology of butterfly wing scales and insect sensory bristles. Curr. Biol. 8, 807–813 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Yoshioka S., Kinoshita S., Polarization-sensitive color mixing in the wing of the Madagascan sunset moth. Opt. Exp. 15, 2691–2701 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Leertouwer H. L., Wilts B. D., Stavenga D. G., Refractive index and dispersion of butterfly chitin and bird keratin measured by polarizing interference microscopy. Opt. Exp. 19, 24061–24066 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Blakey R. C., Gondwana paleogeography from assembly to breakup: A 500 m.y. odyssey. Geol. Soc. Am. Spec. Pap. 441, 1–28 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/e1700988/DC1
fig. S1. Jurassic Lepidoptera from United Kingdom and China.
fig. S2. SEM image of forewing scales on Kazakhstan specimen PIN2239/607.
fig. S3. Jurassic Lepidoptera from Germany.
fig. S4. SEM image of forewing scales on Grimmen specimen LGA1500.
fig. S5. Reflectance color maps.
fig. S6. Structural color of extant Micropterigidae.


