Abstract
Objective
Depression symptom severity, the most commonly studied outcome in antidepressant treatment trials, accounts for only a small portion of burden related to major depression. While lost work productivity is the biggest contributor to depression’s economic burden, few studies have systematically evaluated the independent effect of treatment on work productivity and the relationship between changes in work productivity and longer-term clinical course.
Method
Work productivity was measured repeatedly by the Work Productivity and Activity Impairment (WPAI) self-report in 331 employed participants with major depression enrolled in the Combining Medications to Enhance Depression Outcomes (CO-MED) trial. Trajectories of change in work productivity during the first 6 weeks of treatment were identified and used to predict remission at 3 and 7 months.
Results
Participants reported reduced absence from work and increased work productivity with antidepressant treatment even after controlling for changes in depression severity. Three distinct trajectories of changes in work productivity were identified: 1) robust early improvement (24%), 2) minimal change (49%), and 3) high-impairment slight reduction (27%). As compared to other participants, those with robust improvement had 3–5 times higher remission rates at 3 months and 2–5 times higher remission rates at 7 months, even after controlling for select baseline variables and remission status at week 6.
Conclusions
In this secondary analysis, self-reported work productivity improved in depressed patients with antidepressant treatment even after accounting for depressive symptom reduction. Early improvement in work productivity is associated with much higher remission rates after 3 and 7 months of treatment.
Introduction
Major depressive disorder, the second leading cause of disability globally (1, 2), accounts for one-tenth of all years-lived-with-disability (3). Reduced work productivity accounts for over 80% of the financial costs attributed to depression (4) as depressed patients have substantial work productivity impairments (5, 6). Presenteeism (reduced productivity while at work) and absenteeism (absence from work) have been associated with the loss of 18.2 to 46.8 and 7.8 to 8.7 workdays per worker per year, respectively (7, 8). Amongst baseline clinical and sociodeomographic variables, depressive symptom severity was the strongest predictor of work productivity impairment after a year (9) in a community based cohort (10). However, several other factors have been shown to be associated with poor work productivity (e.g. part-time employment status (6), fair or poor general health (6), history of suicide attempt (5), anxious features, and earlier age of onset of depression (5). Impaired cognitive function has also been linked to disrupted work productivity in depressed patients (11, 12).
With effective antidepressant treatments, work productivity improves (5, 13–15) and this improvement is predicted by baseline levels of depression symptoms and work productivity (13) along with other clinical (e.g., recurrent episodes, melancholic or atypical features) and demographic factors (e.g., education level) (5). Outside of reduction in depression symptom severity, changes in cognitive function may be an important factor in improvement of work productivity. Recent data from the International Mood Disorders Collaborative Project suggest that cognitive function explains variability in workplace function to a greater extent than depression symptom severity (16). Importantly, both cognitive and workplace impairments are often still noted even with achievement of remission of depression symptoms (17).
The long-term deleterious effects of greater depression severity on work (9, 18–20) are well known. As a baseline factor, being employed (5, 6, 21, 22) has been shown to predict better clinical outcomes. However, whether changes in work productivity affect the typically chronic or recurrent course (23–25) of depression is unknown. Our knowledge of the relationship between depressive symptoms and work productivity is also limited by the fact that most studies of work productivity improvement during antidepressant treatment have measured changes only between baseline and post-treatment (5, 13–15). Multiple assessments over the course of treatment as used in this report would clarify better how depressive symptoms relate to work productivity improvement.
The present report, a secondary analysis, tested the hypothesis that early improvement in work productivity would be associated with better long term outcomes. Using data from the Combining Medications to Enhance Depression Outcomes (CO-MED) trial (26), a multi-site, single-blind, randomized controlled trial of outpatients with chronic and/or recurrent, nonpsychotic depression, we repeatedly measured and evaluated changes in work productivity associated with antidepressant treatment. The CO-MED trial included both an acute-phase of 12 weeks (3 months) and a continuation-phase of additional 16 weeks (total 7 months) for patients with a clinical response, thereby allowing for evaluation of the relationship between work productivity and antidepressant response over the course of 7 months of treatment (26). We addressed this issue by identifying subgroups of trial participants based on work productivity changes during the first six weeks of acute-phase treatment and estimated the association of these groups with depressive symptom status/improvement during the remaining 6 weeks of acute-phase treatment and the ensuing 16 weeks of continuation-phase treatment.
Methods
Study Overview and Participants
The COMED trial was approved by the Institutional Review Boards at UT Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, each participating regional center and clinical site, and monitored by an independent data safety and monitoring board. All participants provided written informed consent prior to completing study procedures.
Details of study design, measurements, and primary outcomes are available (26). From six primary and nine psychiatric care sites, CO-MED enrolled 665 participants with nonpsychotic chronic (current episode exceeded 2 years) or recurrent depression with current episode ≥2 months and the 17-item Hamilton Rating Scale (HRSD17) ≥16.
Participants, stratified by clinical sites, were assigned in a 1:1:1 ratio to: 1) escitalopram plus placebo (selective serotonin reuptake inhibitor (SSRI) monotherapy), 2) sustained-release bupropion plus escitalopram (bupropion combination), and 3) extended-release venlafaxine plus mirtazapine (venlafaxine combination). Measurement based care (27) was implemented by study physicians at each visit to tailor antidepressant dosage adjustments for each individual participant based on the scores on the Quick Inventory of Depressive Symptomatology Clinician-rated version (QIDS-C) (28) scale and the Frequency, Intensity, and Burden of Side Effects Rating scale (29).
Assessments
The following measures were obtained at baseline and each subsequent study visit of acute-phase (weeks 1, 2, 4, 6, 8, 10, and 12) and continuation-phase (weeks 16, 20, 24, and 28).
Quick Inventory of Depressive Symptomatology, clinician-rated (QIDS-C) and self-report (QIDS-SR): The total score of QIDS-C and QIDS-SR (range of 0–27) is based on the nine criterion symptom domains out of the 16 items, each of which is scored from 0–3 (28). The Pearson moment correlations between QIDS-SR and HRSD17 was 0.86 and between QIDS-C and HRSD17 was 0.93 in a previous report (30). The Cronbach’s α of QIDS-SR and QIDS-C have ranged from 0.86 to 0.87 in previous reports (28, 30, 31). The QIDS-SR served as the primary measure of depressive symptoms. The QIDS-C was completed by the clinician to monitor symptom changes and guide treatment decisions.
Work Productivity and Impairment (WPAI): This six-item self-report scale has good construct validity and test-retest reliability (32). Items include employment status (item 1), number of hours missed from work in the last week due to health reasons (range 0–80; item 2), number of hours missed from work due to other reasons such as vacation (item 3), number of hours worked in the last week (range 0–80; item 4), impairment resulting from health conditions while working using a scale of 0 to 10 where 0 indicates no impairment (item 5), and impairment in regular daily activities other than work or job (item 6).
Using the scoring guide by Reilly et al., absenteeism (percent of time missed from work), presenteeism (percent impairment while working), and work productivity loss (percent overall work impairment) were calculated using formulas based on items 2, 4, and 5 (33). These scores are expressed as percentages with higher scores reflecting greater impairment, and they comprised the primary work outcome measures for the current report.
In addition to these scores, the number of hours lost to impaired productivity while at work was calculated using the following formula: (item 4)*(item 5/ 10) and reported along with item 2 in the following categories: none (0 hours), greater than 0 to 4 hours (half a day of work), greater than 4 to 8 hours (whole day of work), greater than 8 to 20 hours (half a week of work) and greater than 20 hours (greater than half a week of work).
The Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ): The total score of this seven-item self-report questionnaire is calculated by adding the following items: motivation/interest, wakefulness/alertness, energy, ability to focus, ability to remember, ability to find words, and sharpness/mental acuity. The Pearson moment correlation between CPFQ and HRSD17 ranged from 0.24 to 0.29 in previous report (34). The Cronbach’s α of CPFQ has ranged from 0.89 to 0.91 in previous reports (34, 35).
Clinical Improvement: Symptomatic remission at 12 weeks, the primary outcome of CO-MED, was ascribed if out of the last two consecutive QIDS-SR score at least one was less than 6 while the other was less than 8. Per Rush et al. this definition of remission in CO-MED trial was chosen a priori “to ensure that a single ‘good week’ was not falsely signaling remission” (26) in lieu of the conventional criteria of remission (QIDS-SR score ≤5) for one week (36). Remission was measured at week 28 in a similar fashion.
Participation beyond 12 weeks (continuation phase) was contingent on whether participants received an acceptable benefit (defined as QIDS-C score of 9 or less by week 12) or had reached a score of 10–13 on QIDS-C and both the study physician and the participant decided to continue treatment because of substantial benefit.
Statistical analyses
The analytic sample for this report included all CO-MED participants who were employed when they enrolled in the study (331 out of 665 [49.8%]). Based on literature, we included the following baseline clinical and sociodemographic features to evaluate their role as predictors of change in work productivity outcomes: age, education (less than 12 years, 12 to 15 years, and 16+ years of schooling), monthly income (< $2000, $2000 to $4000, and greater than $4000), gender, race (white, black, other), Hispanic ethnicity, presence of anxious features at baseline, onset of depressive symptoms before age of 18, presence of suicidal ideations at baseline, and baseline levels of depressive symptom severity, cognitive functioning, and each work productivity outcome (5, 13–15).
As the participation in continuation-phase of CO-MED was based on clinical improvement (26), statistical analyses were conducted separately for acute- and continuation-phases. Note that because the treatment arms were not significantly different with respect to depression outcomes in the primary report (26), we did not hypothesize a differential effect of treatment arm on work productivity outcomes; however, we did include treatment arm in the analysis to evaluate whether such an effect was present.
We estimated the change over time in each work productivity outcome (absenteeism, presenteeism and work productivity loss) using repeated measures analysis of variance with visit as the within subject factor and all other variables as between subject factors. We used linear mixed model analyses with levels of QIDS-SR and CPFQ at each visit along with baseline covariates and levels of each work productivity outcome, CPFQ, and QIDS-SR. For the continuation-phase, we included scores of QIDS-SR, CPFQ, and each work productivity outcome at week 12 as additional covariates in the analyses. We estimated the correlation coefficients between work productivity outcome measures and depression severity as well as the nine symptom domains included in QIDS-SR. As the variability of observations between participants is usually higher as compared to the repeated observations in a single participant (37), we calculated the correlation coefficients based on repeated observations over time using PROC MIXED as implemented in SAS (38).
To develop groupings based on changes in work productivity during the first 6 weeks of acute-phase treatment, we used PROC TRAJ as implemented in SAS to estimate discrete mixture models with censored normal distribution (39). For both linear and non-linear (two-, three-, and four-degree polynomials) models, we increased the number of groupings in a stepwise fashion while evaluating changes in model fit using Bayesian information criteria and Akaike information criteria with the lowest value indicating best model fit (40). We selected final model based on fit and parsimony, group size, and interpretability. The groups were then compared with chi-square or general linear model for baseline clinical and sociodemographic features. Association of these groups with changes in depression severity after week 6 of CO-MED and remission at 3 months and 7 months were estimated using mixed models and logistic regression (first univariate and then multivariate analyses with backwards elimination), respectively, after controlling for the above-mentioned baseline clinical and sociodemographic variables.
We used Bonferroni correction for multiple comparisons when indicated, set the level of significance at 0.05 and have reported all p values after adjustment for multiple comparisons. All analyses were done using SAS 9.3 (SAS Inc., Cary, NC).
Results
Of the 665 CO-MED participants, 331 (49.8 %) were employed at baseline and did not differ on baseline sociodemographic or clinical variables among the three treatment arms (Table 1). During both acute- and continuation-phases, work productivity loss and presenteeism were very highly correlated (0.94 to 0.95), while the correlation coefficients of absenteeism and presenteeism ranged from 0.37 to 0.47. Using conventional criteria proposed by Cohen et al. (41), there was a medium (>0.3) correlation between presenteeism and work productivity loss with the following depressive symptom domains: sad mood, concentration, general interest, energy level, and psychomotor agitation/retardation; also see table 2.
Table 1.
Baseline sociodemographic and clinical characteristics of employed participants in CO-MED trial
| Total | SSRI monotherapy |
Bupropion combination |
Venlafaxine combination |
Test statistic | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Categorical variables | n | % | n | % | n | % | n | % | χ2 | df | ||
| Number | 331 | 99 | 119 | 113 | ||||||||
| Sex | 0.95 | 2 | 0.62 | |||||||||
| Male | 96 | 29 | 30 | 30.3 | 37 | 31.1 | 29 | 25.7 | ||||
| Female | 235 | 71 | 69 | 69.7 | 82 | 68.9 | 84 | 74.3 | ||||
| Race | 2.59 | 4 | 0.63 | |||||||||
| White | 228 | 68.9 | 70 | 70.7 | 86 | 72.3 | 72 | 63.7 | ||||
| Black | 75 | 22.7 | 20 | 20.2 | 24 | 20.2 | 31 | 27.4 | ||||
| Other | 28 | 8.4 | 9 | 9.1 | 9 | 7.6 | 10 | 8.9 | ||||
| Monthly income | 7.79 | 4 | 0.10 | |||||||||
| <$2000 | 150 | 49 | 45 | 48.4 | 51 | 48.6 | 54 | 55.1 | ||||
| $2000 – $4000 | 90 | 29.4 | 26 | 28 | 25 | 23.8 | 29 | 30.8 | ||||
| >$4000 | 66 | 21.6 | 22 | 23.6 | 29 | 27.6 | 15 | 14.1 | ||||
| Education | 1.28 | 4 | 0.87 | |||||||||
| <12 years | 33 | 10.3 | 9 | 9.6 | 12 | 10.4 | 12 | 10.9 | ||||
| 12–15 years | 170 | 53.3 | 52 | 55.3 | 64 | 55.6 | 54 | 49.1 | ||||
| >15 years | 116 | 36.4 | 33 | 35.1 | 39 | 24 | 44 | 40 | ||||
| Hispanic | 65 | 19.6 | 22 | 22.2 | 26 | 21.9 | 17 | 15.0 | 2.30 | 2 | 0.32 | |
| Anxious features | 238 | 72.1 | 65 | 66.3 | 93 | 78.2 | 80 | 70.8 | 2.89 | 2 | 0.14 | |
| Suicidal ideation | 196 | 59.2 | 55 | 55.6 | 71 | 59.7 | 70 | 61.9 | 0.91 | 2 | 0.64 | |
| Onset of depression before age 18 | 153 | 46.4 | 45 | 45.9 | 53 | 44.5 | 55 | 48.7 | 0.41 | 2 | 0.82 | |
| Continuous variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F value | df | ||
| Mean age in yrs. | 41.0 | 12.6 | 41.3 | 12.7 | 41.0 | 13.2 | 40.6 | 11.9 | 0.08 | 2 | 0.92 | |
| Mean QIDS-SR | 15.0 | 4.1 | 15.0 | 3.8 | 14.9 | 4.1 | 15.1 | 4.4 | 0.08 | 2 | 0.92 | |
CO-MED is Combining Medications to Enhance Depression Outcomes, SSRI is selective serotonin reuptake inhibitor, SSRI monotherapy refers to combination of escitalopram and placebo, Bupropion refers to combination of bupropion and escitalopram, Venlafaxine refers to venlafaxine and mirtazapine, SD is standard deviation, df is degrees of freedom, χ2 is chi-square and QIDS-SR is Quick Inventory of Depressive Symptomatology Self-Report.
Table 2.
Correlation coefficients over repeated observations of work productivity outcomes and self-reported depression symptoms
| Absenteeism | Presenteeism | Work Productivity Loss | |
|---|---|---|---|
| Coefficient | Coefficient | Coefficient | |
| Acute-phase of CO-MED | |||
| Total Score of QIDS-SR | 0.21 | 0.48 | 0.48 |
| Sad mood | 0.16 | 0.40 | 0.39 |
| Sleep | 0.13 | 0.25 | 0.26 |
| Appetite | 0.14 | 0.15 | 0.17 |
| Concentration | 0.14 | 0.43 | 0.42 |
| View of Self/Guilt | 0.11 | 0.27 | 0.27 |
| Thoughts of Death or Suicide | 0.12 | 0.24 | 0.22 |
| General Interest | 0.12 | 0.34 | 0.34 |
| Energy Level | 0.19 | 0.43 | 0.42 |
| Psychomotor agitation/retardation | 0.15 | 0.35 | 0.35 |
| Continuation-phase of CO-MED | |||
| Total Score of QIDS-SR | 0.23 | 0.52 | 0.50 |
| Sad mood | 0.19 | 0.34 | 0.34 |
| Sleep | 0.13 | 0.26 | 0.25 |
| Appetite | 0.03 | 0.09 | 0.08 |
| Concentration | 0.20 | 0.51 | 0.48 |
| View of Self/Guilt | 0.18 | 0.36 | 0.35 |
| Thoughts of Death or Suicide | 0.14 | 0.20 | 0.20 |
| General Interest | 0.21 | 0.38 | 0.39 |
| Energy Level | 0.27 | 0.51 | 0.50 |
| Psychomotor agitation/retardation | 0.15 | 0.32 | 0.31 |
CO-MED is Combining Medications to Enhance Depression Outcomes, QIDS-SR is Quick Inventory of Depressive Symptomatology Self-Report, the nine symptom domains of QIDS-SR are consistent with the diagnostic criteria of Major Depressive Disorder.
Work productivity improvement with antidepressant medications
Acute-Phase: The percentage of participants who missed at least one hour of work in last 7 days dropped from 40% at baseline to 14.1% at week 12. The percentage of participants who reported more than 20 hours of impairment while at work fell from 23.1% at baseline to 6.8% at week 12 (Figure 1). All work productivity outcomes significantly improved after 12 weeks of treatment; the mean rate of absenteeism (percent of time missed from work) reduced significantly (F=6.34, degrees of freedom (df)=7, p <0.0001) from 12.34 (standard deviation [SD]=22.8) hours at baseline to 4.17 (SD=13.9) hours at 12 weeks; mean rate of presenteeism (percent impairment while at work) decreased significantly (F=22.82, df=7, p <0.0001) from 40.4 (SD=31.5) hours at baseline to 19.8 (SD=22.5) hours at 12 weeks; and mean rate of work productivity loss (overall work impairment that includes absenteeism and/or presenteeism) lowered significantly (F=22.85, df=7, p <0.0001) from 44.9 (SD=32.4) hours at baseline to 21.0 (SD=27.4) hours at week 12. These reductions with time in absenteeism (F=4.34, df=6, p=0.0002), presenteeism (F=4.49, df=6, p=0.0002), and work productivity loss (F=5.79, df=6, p <0.0001) continued to be significant even after controlling for QIDS-SR and CPFQ at each visit, baseline clinical and sociodemographic variables along with baseline QIDS-SR, CPFQ and baseline absenteeism, presenteeism, and work productivity loss respectively.
Figure 1. Work Impairment in Depressed Outpatients After 12-Weeks of Antidepressant Treatment.
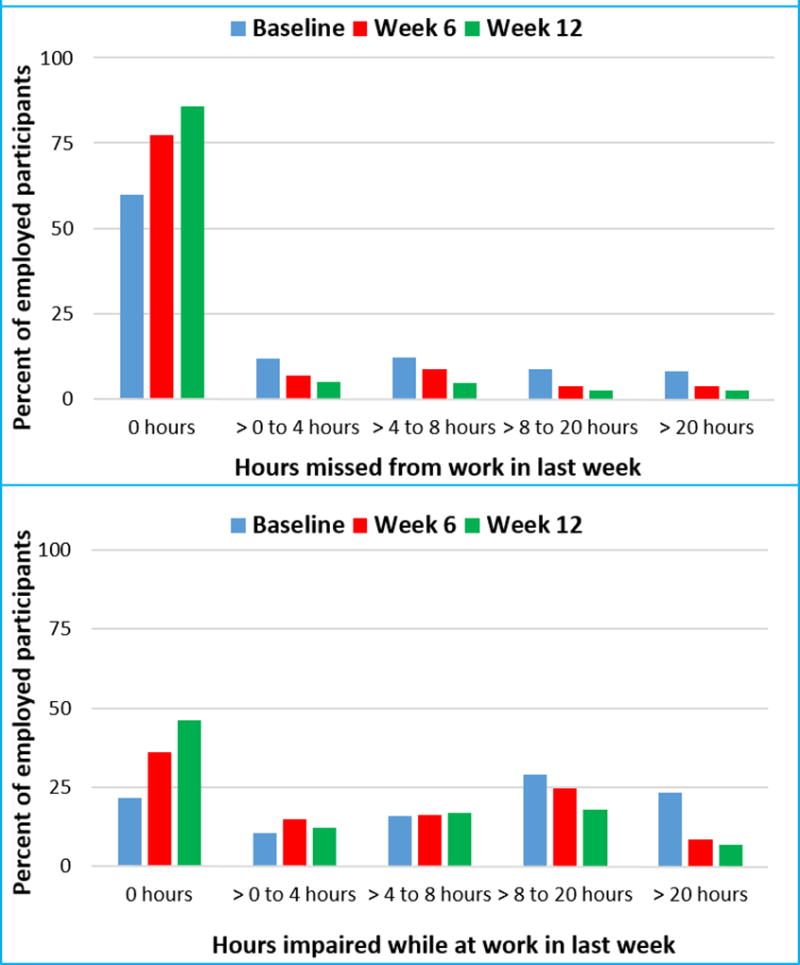
Continuation-Phase: Changes during the continuation-phase were not statistically significant after controlling for change in QIDS-SR, CPFQ, baseline clinical and sociodemographic variables along with baseline and 12 week levels of QIDS-SR, CPFQ and each work productivity outcome [absenteeism (F=2.13, df=3, p=0.10), presenteeism (F=1.31, df=3, p=0.27) or work productivity loss (F=0.93, df=3, p=0.43)].
During acute-phase, educational level of college or higher was associated with lower absenteeism, presenteeism and work productivity loss level. Other baseline variables, excluding baseline levels of each work productivity outcome, did not significantly predict change in work productivity outcomes during either acute- or continuation-phase, detailed results are presented in supplementary table 1. Changes in CPFQ were significantly associated with work productivity changes during both acute- and continuation-phases, also see supplementary table 1. Additionally, during both acute- and continuation-phases, the bupropion and venlafaxine combinations did not differ from SSRI monotherapy for any of the work productivity outcomes.
Groups based on change in work productivity within the first 6 weeks of treatment
The final model (Figure 2) identified three trajectories of change in work productivity during the first 6 weeks of acute-phase: 1) robust early improvement, 2) minimal change, and 3) high-impairment slight reduction with 23.8%, 49.2%, and 27.0% participants in each group respectively. The model fit estimates for linear and non-linear models with step-wise increase in number of groupings, and maximum likelihood estimates of final model parameters are presented in supplementary tables 2 and 3 respectively. At baseline, the three groups did not differ in clinical and sociodemographic variables, except depression severity; the high-impairment slight reduction group had higher baseline QIDS-SR scores than participants in robust early impairment and minimal change groups (supplementary table 4).
Figure 2. Data driven trajectories of changes in work productivity during the first 6 weeks of CO-MED trial.
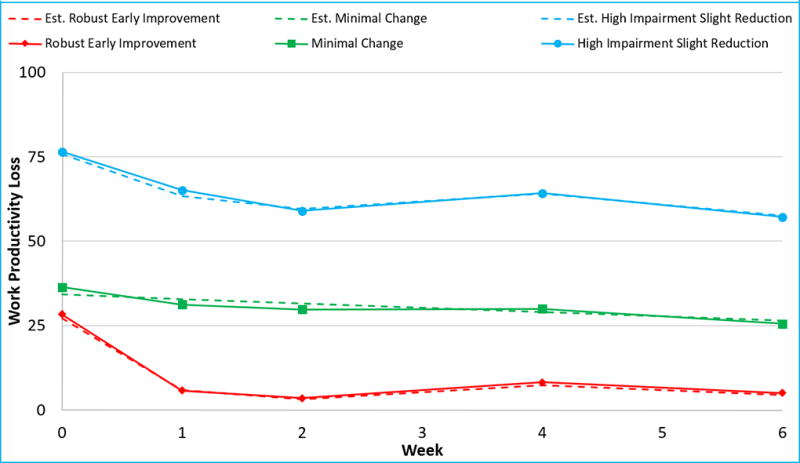
CO-MED is Combining Medications to Enhance Depression Outcomes, est. is estimate. Solid lines represent observed values and broken lines (dash) represent estimates obtained from the final selected model of data driven trajectory analyses, per SAS program PROC TRAJ. The three groups of changes identified using trajectory analyses are as follows: robust early improvement, minimal change group, and high impairment slight reduction.
Association of groups defined by early and robust change in work productivity with subsequent clinical improvement
Participants with robust early improvement in work productivity had significantly higher unadjusted remission rates at 3 and 7 months as compared to those in minimal change (3 months OR=3.1, 95% CI=1.5,6.6; 7 months OR=2.9, 95% CI=1.2,6.8) and high-impairment slight reduction (3 months OR=7.7, 95% CI=3.2,18.5; 7 months OR=7.1, 95% CI=2.7,19.2) groups. After controlling for remission status at week 6 and baseline clinical and sociodemographic variables, participants in robust early improvement group continued to have significantly higher remission rates at both 3 months (OR=5.4, 95% CI=1.8,15.9) and 7 months (OR=5.0, 95% CI=1.5,16.4) when compared to those in high-impairment slight reduction group. However, as compared to those in minimal change group, robust early improvement group had higher remission rates only at 3 months (OR=2.7, 95% CI=1.0,6.9) but not at 7 months (OR=2.2, 95% CI=0.8,6.1), also see supplementary table 5.
After controlling for baseline clinical and sociodemographic variables, participants in the three trajectory groups differed significantly (F=23.31, df=2, p <0.0001) in depression severity from week 6 to week 12 of acute-phase, with a significant effect of time (F=9.34, df=3, p <0.0001) and time-by- group interaction (F=3.20, df=6, p=0.004) (Figure 3). During the continuation-phase and after controlling for above-mentioned baseline variables, there was a significant difference in the levels of depression severity between the three groups (F=16.14, df=2, p <0.0001), but depression severity did not change with time (F=1.31, df=4, p=0.27) during this phase, nor was there a time-by-group interaction (F=1.19, df=8, p=0.30), also see figure 3.
Figure 3. Depression severity levels of employed depressed outpatients (n=331) in the CO-MED trial.
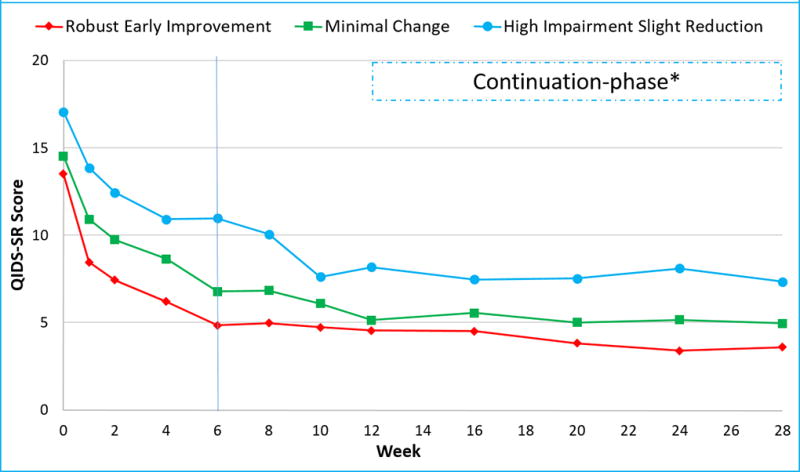
QIDS-SR is Quick Inventory of Depressive Symptoms Self-Report. * Participation in continuation-phase was restricted to participants with clinical response at week 12. Employed participants were divided in three groups (Robust early improvement, minimal change, and high impairment slight reduction) based on the trajectory of change in their work productivity during the first six weeks (as marked by the vertical line) of CO-MED trial.
Discussion
We found that work productivity improved with acute-phase antidepressant treatment even after accounting for change in depression severity and self-reported cognitive functioning. Secondly, the trajectories of this improvement in work productivity predicted long-term changes in depression severity and remission status. During the first 6 weeks of the trial, two groups (robust early improvement and minimal change) of depressed participants started at similar levels of work productivity and depression severity. One attained an early and sustained reduction in work productivity loss, while the other experienced minimal change. The group with early improvement in work productivity had markedly lower levels of depression severity throughout the duration of trial (28 weeks) and continued to be in remission at much higher rates at week 12, even after controlling for baseline clinical and sociodemographic variables and remission status at week 6. Early improvement in work productivity appears to be an important mediator of sustained remission.
These findings are consistent with prior reports that work productivity improves with antidepressant treatment (5, 13–15). In addition, the role of baseline levels of work productivity in predicting subsequent change with time (see supplementary table 5) is consistent with the findings of Beck et al. (13). As in Trivedi et al. (5), we also found that baseline educational level moderated work productivity improvement with antidepressant treatment.
The high positive correlation between work productivity loss and presenteeism implies that a large portion of variance in overall work productivity loss is accounted for by presenteeism – that is, while employees are at their job, their productivity is substantially impaired. This result is consistent with epidemiological findings that presenteeism, as compared to absenteeism, accounts for majority of the work productivity impairment (7, 8). Furthermore, our results that work productivity outcomes were similar for SSRI monotherapy and the bupropion and venlafaxine combinations are consistent with what was found with respect to depression symptom severity in the CO-MED trial.
Our findings that significant improvement in work productivity occurred even after accounting for baseline demographic and clinical factors, as well as changes in depression severity and self-reported cognitive functioning support the importance of measuring work productivity during antidepressant treatment. Measures of depression severity fail to adequately capture the burden of depression (42). Additionally, we found that changes in cognitive functioning were significantly associated with changes in work productivity. This report adds to the growing evidence regarding the importance of improving cognitive functioning in depressed patients. Our findings further support attainment of functional recovery as the ultimate outcome of antidepressant treatment (43, 44).
The results of this study may have practical implication if our findings are replicated. Work productivity may be an easy-to-administer patient-centered outcome that can identify patients early in course of treatment who may benefit with treatment change or augmentation. Amongst depressive symptom domains, energy and concentration are most strongly associated with work productivity outcomes. Hence, targeting these symptom domains with adjunct treatments may boost work productivity and improve long term outcomes. Adjunctive occupational therapy has been shown to improve depression severity in the long term (45). Similarly, telephone-based coaching interventions focused on improving work productivity have been reported to improve depressive symptom severity in patients with dysthymia (46). Perhaps such interventions could play a pivotal role in increasing the likelihood of sustained remission in patients with residual work impairment.
There are several limitations to our report. It is a secondary analysis. This study did not include any intervention that was meant to independently improve work productivity. Additionally, the work productivity measure in our study was subjective and based on self-report. Hence, it likely differs from objective measures of work productivity, such as those collected by employers. Further, the generalizability of our findings to routine clinical care may be restricted by the high quality of treatment received by participants in the CO-MED trial. While adopted by the major treatment guidelines for decades, measurement based care has not become routine practice.
In conclusion, we have demonstrated that work productivity outcomes improve significantly with antidepressant treatment and reflect burden of disease that is not captured adequately by depression severity alone. We have also demonstrated that early changes in work productivity are significant predictors of long-term clinical course. These findings highlight the multidimensional improvement with antidepressant treatment and argue for inclusion of work productivity assessments in routine clinical practice.
Supplementary Material
Acknowledgments
Funded by NIMH under contract N01 MH-90003 to the University of Texas Southwestern Medical Center at Dallas (principal investigators, A.J. Rush and M.H. Trivedi).
Forest Pharmaceuticals, GlaxoSmithKline, Organon, and Wyeth Pharmaceuticals provided medications for this trial at no cost. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. NIMH had no role in the drafting or review of the manuscript or in the collection or analysis of the data.
The authors thank the clinical staff at each clinical site for their assistance with this project; all of the study participants; Eric Nestler, M.D., Ph.D., Carol A. Tamminga, M.D., and Savitha Kalidas, Ph.D. for administrative support. The authors also thank Robert Freedman, M.D. and the reviewers for their feedback and suggestions for improvement of an earlier version of this manuscript. This work was also supported in part through the Center for Depression Research and Clinical Care (Principal Investigator: Madhukar H. Trivedi, MD) and Hersh Foundation.
Dr. Greer has received research funding from NARSAD and honoraria and/or consultant fees from H. Lundbeck A/S and Takeda Pharmaceuticals International, Inc. Dr. Rush has received consulting fees from the American Psychiatric Association, Brain Resource Ltd, H. Eli Lilly, Emmes Corp., Liva-Nova, Lundbeck A/S, Medavante, Inc, Montana State University, National Institute of Drug Abuse, Santium Inc.,Takeda USA,; speaking fees from the University of California at San Diego, Hershey Penn State Medical Center, the American Society for Clinical Psychopharmacology, the New York State Psychiatric Inst, Stanford Medical School; royalties from Guilford Publications and the University of Texas Southwestern Medical Center; and research support from Duke-National University of Singapore. Madhukar H. Trivedi, is or has been an advisor/consultant and received fee from: Alkermes, AstraZeneca, Cerecor, Eli Lilly & Company, Lundbeck, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., SHIRE Development and Takeda. In addition, he has received grants/research support from: National Institute of Mental Health and National Institute on Drug Abuse.
Footnotes
Disclosures: Drs. Jha, Minhajuddin, and Carmody have no potential conflicts of interest.
Previous presentation: The findings of this report have been accepted for presentation at the 2016 Annual Meeting of American Psychiatric Association and the 2016 Annual Meeting of the American Society of Clinical Psychopharmacology.
Clinicaltrials.gov identifier: NCT00590863
References
- 1.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekman M, Granström O, Omérov S, Jacob J, Landén M. The societal cost of depression: Evidence from 10,000 Swedish patients in psychiatric care. J Affect Disord. 2013;150(3):790–7. doi: 10.1016/j.jad.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi MH, Morris DW, Wisniewski SR, Lesser I, Nierenberg AA, Daly E, et al. Increase in work productivity of depressed individuals with improvement in depressive symptom severity. Am J Psychiatry. 2013;170(6):633–41. doi: 10.1176/appi.ajp.2012.12020250. [DOI] [PubMed] [Google Scholar]
- 6.Beck A, Crain AL, Solberg LI, Unutzer J, Glasgow RE, Maciosek MV, et al. Severity of depression and magnitude of productivity loss. Ann Fam Med. 2011;9(4):305–11. doi: 10.1370/afm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289(23):3135–44. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Heeringa S, Lakoma MD, Petukhova M, Rupp AE, Schoenbaum M, et al. Individual and societal effects of mental disorders on earnings in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2008;165(6):703–11. doi: 10.1176/appi.ajp.2008.08010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geraedts AS, Fokkema M, Kleiboer AM, Smit F, Wiezer NW, Majo MC, et al. The longitudinal prediction of costs due to health care uptake and productivity losses in a cohort of employees with and without depression or anxiety. J Occup Environ Med. 2014;56(8):794–801. doi: 10.1097/JOM.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 10.Penninx BW, Beekman AT, Smit JH, Zitman FG, Nolen WA, Spinhoven P, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17(3):121–40. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam RW, Michalak EE, Bond DJ, Tam EM, Axler A, Yatham LN. Which depressive symptoms and medication side effects are perceived by patients as interfering most with occupational functioning? Depress Res Treat. 2012;2012:630206. doi: 10.1155/2012/630206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godard J, Grondin S, Baruch P, Lafleur MF. Psychosocial and neurocognitive profiles in depressed patients with major depressive disorder and bipolar disorder. Psychiatry Res. 2011;190(2–3):244–52. doi: 10.1016/j.psychres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Beck A, Crain LA, Solberg LI, Unutzer J, Maciosek MV, Whitebird RR, et al. The effect of depression treatment on work productivity. Am J Manag Care. 2014;20(8):e294–301. [PMC free article] [PubMed] [Google Scholar]
- 14.Lam RW, Endicott J, Hsu MA, Fayyad R, Guico-Pabia C, Boucher M. Predictors of functional improvement in employed adults with major depressive disorder treated with desvenlafaxine. Int Clin Psychopharmacol. 2014;29(5):239–51. doi: 10.1097/YIC.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 15.Lam RW, Parikh SV, Ramasubbu R, Michalak EE, Tam EM, Axler A, et al. Effects of combined pharmacotherapy and psychotherapy for improving work functioning in major depressive disorder. Br J Psych. 2013;203(5):358–65. doi: 10.1192/bjp.bp.112.125237. [DOI] [PubMed] [Google Scholar]
- 16.McIntyre RS, Soczynska JZ, Woldeyohannes HO, Alsuwaidan MT, Cha DS, Carvalho AF, et al. The impact of cognitive impairment on perceived workforce performance: results from the International Mood Disorders Collaborative Project. Compr Psychiatry. 2015;56:279–82. doi: 10.1016/j.comppsych.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 17.Evans VC, Chan SSL, Iverson GL, Bond DJ, Yatham LN, Lam RW. Systematic review of neurocognition and occupational functioning in major depressive disorder. Neuropsychiatry. 2013;3(1):97–105. [Google Scholar]
- 18.Mojtabai R, Stuart EA, Hwang I, Susukida R, Eaton WW, Sampson N, et al. Long-term effects of mental disorders on employment in the National Comorbidity Survey ten-year follow-up. Soc Psychiatry Psychiatr Epidemiol. 2015 doi: 10.1007/s00127-015-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Z, Cowell AJ, Musuda YJ, Novak SP, Johnson EO. Course of major depressive disorder and labor market outcome disruption. J Ment Health Policy Econ. 2010;13(3):135–49. [PMC free article] [PubMed] [Google Scholar]
- 20.Zivin K, Campbell DG, Lanto AB, Chaney EF, Bolkan C, Bonner LM, et al. Relationships between mood and employment over time among depressed VA primary care patients. Gen Hosp Psychiatry. 2012;34(5):468–77. doi: 10.1016/j.genhosppsych.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Jakubovski E, Bloch MH. Prognostic subgroups for citalopram response in the STAR*D trial. J Clin Psych. 2014;75(7):738–47. doi: 10.4088/JCP.13m08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedhammer I, Malard L, Chastang JF. Occupational factors and subsequent major depressive and generalized anxiety disorders in the prospective French national SIP study. BMC Public Health. 2015;15:200. doi: 10.1186/s12889-015-1559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, et al. Psychosocial Disability During the Long-term Course of Unipolar Major Depressive Disorder. Arch Gen Psychiatry. 2000;57(4):375–80. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- 24.Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108(1):49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RMA, et al. Time to Recovery, Chronicity, and Levels of Psychopathology in Major Depression: A 5-Year Prospective Follow-up of 431 Subjects. Arch Gen Psychiatry. 1992;49(10):809–16. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- 26.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61–71. doi: 10.1016/j.drugalcdep.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 29.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12(2):71–9. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, et al. An Evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: A Sequenced Treatment Alternatives to Relieve Depression Trial Report. Biol Psychiatry. 2006;59(6):493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 32.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 33.Reilly MC. [accessed on May 17, 2015]; http://www.reillyassociates.net/WPAI_Scoring.html.
- 34.Baer L, Ball S, Sparks J, Raskin J, Dube S, Ferguson M, et al. Further evidence for the reliability and validity of the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ) Ann Clin Psych. 2014;26(4):270–80. [PubMed] [Google Scholar]
- 35.Fava M, Iosifescu DV, Pedrelli P, Baer L. Reliability and validity of the Massachusetts general hospital cognitive and physical functioning questionnaire. Psychother Psychosom. 2009;78(2):91–7. doi: 10.1159/000201934. [DOI] [PubMed] [Google Scholar]
- 36.Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology. 2006;31(9):1841–53. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 37.Bland JM, Altman DG. Statistics Notes: Correlation, regression, and repeated data. BMJ. 1994;308(6933):896. doi: 10.1136/bmj.308.6933.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamlett A, Ryan L, Wolfinger R. On the use of PROC MIXED to estimate correlation in the presence of repeated measures. [Accessed Apr, 2004];Proc Statistics and Data Analysis SAS Users Group International. 19:2006. http://www2.sascom/proceedings/sugi29/198-29.pdf. [Google Scholar]
- 39.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociological Methods & Research. 2007;35(4):542–71. [Google Scholar]
- 40.Ram N, Grimm KJ. Growth Mixture Modeling: A Method for Identifying Differences in Longitudinal Change Among Unobserved Groups. Int J Behav Dev. 2009;33(6):565–76. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 42.Cohen RM, Greenberg JM, IsHak WW. Incorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression to measure treatment impact and recovery in MDD. JAMA Psychiatry. 2013;70(3):343–50. doi: 10.1001/jamapsychiatry.2013.286. [DOI] [PubMed] [Google Scholar]
- 43.Greer TL, Kurian BT, Trivedi MH. Defining and Measuring Functional Recovery from Depression. CNS Drugs. 2010;24(4):267–84. doi: 10.2165/11530230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Lam RW, Parikh SV, Michalak EE, Dewa CS, Kennedy SH. Canadian Network for Mood and Anxiety Treatments (CANMAT) consensus recommendations for functional outcomes in major depressive disorder. Ann Clin Psychiatry. 2015;27(2):142–9. [PubMed] [Google Scholar]
- 45.Hees HL, de Vries G, Koeter MW, Schene AH. Adjuvant occupational therapy improves long-term depression recovery and return-to-work in good health in sick-listed employees with major depression: results of a randomised controlled trial. Occup Environ Med. 2013;70(4):252–60. doi: 10.1136/oemed-2012-100789. [DOI] [PubMed] [Google Scholar]
- 46.Adler DA, Lerner D, Visco ZL, Greenhill A, Chang H, Cymerman E, et al. Improving work outcomes of dysthymia (persistent depressive disorder) in an employed population. Gen Hosp Psychiatry. 2015;37(4):352–9. doi: 10.1016/j.genhosppsych.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


