Abstract
Methamphetamine (MA) addiction is a serious public health problem, and current methods to abate addiction and relapse are currently ineffective for mitigating this growing global epidemic. Development of a vaccine targeting MA would provide a complimentary strategy to existing behavioral therapies, but this has proven challenging. Herein, we describe optimization of both hapten design and formulation, identifying a vaccine that elicited a robust anti-MA immune response in mice, decreasing methamphetamine-induced locomotor activity.
Graphical abstract
Introduction
Methamphetamine (MA) is a highly addictive stimulant; administration of the drug results in an intense, euphoric high created by the release of multiple neurotransmitters in the brain. Frequent MA use leads to dependence, and attempted abstinence in dependent users precipitates severe withdrawal and a correspondingly high relapse rate.1-3 There are no approved medications to treat MA addiction,4, 5 with behavioral therapies providing only limited improvement to long-term abstinence rates.6, 7 With MA use (and the corresponding social and economic burden) on the rise globally,8, 9 it is vital that new treatments are discovered that can increase the chances of long-term abstinence. A vaccine against MA would decrease the likelihood of relapse through the sequestration of the stimulant from the bloodstream, limiting its psychoactive effects. However, MA, as other small molecules, cannot be recognized by the immune system alone. Conjugation to an immunogenic carrier is required to enable effective processing and recognition by leukocytes and ultimately the production of a long-lasting antibody response.10 This strategy has shown promise for both nicotine and cocaine, with vaccines reaching clinical trials,11-17 but application to treat MA abuse is still in its infancy.18
In order to successfully sequester MA, a vaccine must elicit a high concentration of high affinity MA-specific antibodies. The affinity and specificity of the antibodies generated is strongly dependent on identifying an effective hapten, which is a significant challenge given the small size of the drug and its limited chemical epitope. A range of MA haptens have been reported by various groups,19-26 but the drugs of abuse vaccine field suffers from variability in a number of important methodological factors that makes intra-study hapten design comparison innately flawed. These factors include carrier protein, adjuvant, formulation, vaccination schedule, and analytical methods, amongst others. We therefore sought to further our optimization of MA haptens, but with the added rigor of direct comparison with successful haptens from both our laboratory and others.
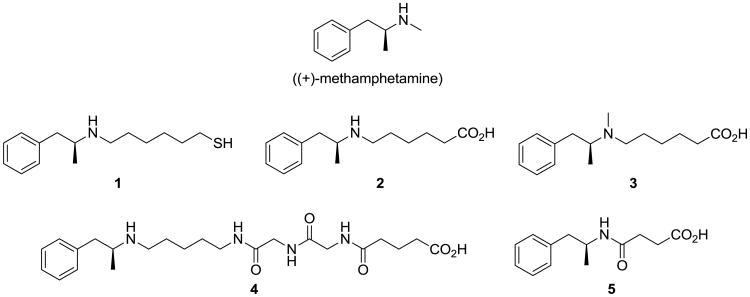
We have previously designed hapten 1, linked to the carrier protein via the N-methyl group, and shown that this can stimulate moderate affinities and concentrations of anti-MA antibodies, with an observed decrease in MA-induced locomotor activity (Figure 1).23 In this case we had selected a thiol-maleimide linkage due to the presence of a secondary amine in the structure, in order to ensure selectivity in the hapten-protein conjugation. However, we have observed that thiol addition to maleimide-activated proteins typically results in lower hapten densities than amide bond formation, with haptens that are less stable than their acid counterparts. Previous data had also demonstrated that the tertiary amine hapten 3 was moderately effective at eliciting anti-MA antibodies,26 and we wanted to clarify whether modification of the amine had an impact on the resulting immune response through direct comparison with its secondary amine hapten counterpart, 2. Additionally, we have recently found that the introduction of a diglycine linker into a nicotine hapten enhances both concentration and affinity of the resulting antibodies,27 with a postulated enhancement of antibody titers against oxycodone and hydrocodone upon the inclusion of diglycine and tetraglycine linkers.28, 29 Thus, we targeted hapten 4 as our first glycine-containing hapten. We also endeavored to directly compare our haptens with 5, a hapten that has been reported by Kosten and Orson to reduce MA-induced hyper- and hypo-locomotion in mice, and is currently progressing to clinical trials.25, 30
Figure 1.
Structures of haptens 1-5
Results and Discussion
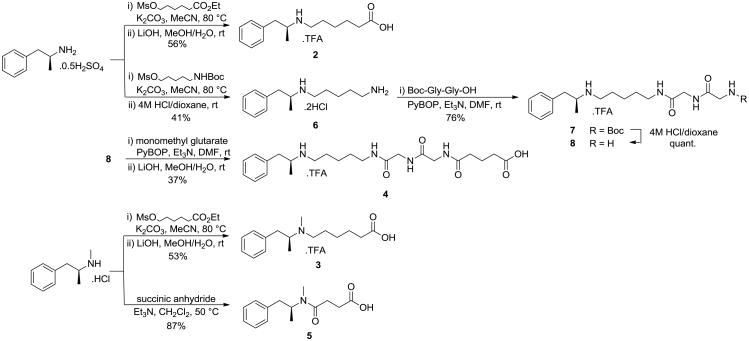
We began by synthesizing MA haptens 2 and 326 by alkylation of amphetamine and MA, respectively, followed by ester hydrolysis (Scheme 1). The diglycine variant, 4, could be synthesized using an analogous method to the strategy for nicotine hapten AM1(Gly)2.27 Alkylation of amphetamine with 5-((tert-butoxycarbonyl)amino)pentyl methanesulfonate31 and Boc-deprotection, followed by coupling with Boc-Gly-Gly-OH gave carbamate 7, which was subsequently deprotected and coupled with monomethyl glutarate, before ester hydrolysis. Hapten 5 was prepared as previously reported, by reaction of MA with succinic anhydride.25
Scheme 1.
Synthesis of haptens 2-5.
The four haptens were activated and conjugated to diphtheria toxoid (DT), an immunogenic protein commonly used as a carrier for conjugate vaccine haptens, with comparable conjugation efficiencies as determined by MALDI-TOF (Table S1, Supplementary Information). LCMS analysis confirmed that neither 2 nor 4 polymerized under extended activation times. Furthermore, the comparable hapten densities resulting from conjugation of 2 and 3 suggests that this was not a significant side-reaction.
These four conjugates were formulated with Sigma Adjuvant System (SAS), an oil-in-water emulsion adjuvant containing a Toll-like receptor 4 agonist, monophosphoryl lipid A (MPLA). SAS has been effectively used in previous campaigns to evaluate drugs of abuse vaccines.27, 32-34 Once formulated, the efficacies of the four vaccines were determined by vaccination of Swiss Webster mice (n=6). Antibody titers of mouse serum samples were determined by ELISA using 1-BSA22 as the coating antigen (Figure 2a). Titers induced by 2-DT, 3-DT and 4-DT were similar over the course of the vaccination schedule. The titers for 5-DT-vaccinated mice were significantly lower, although this could be partly attributed to the difference between the structures of the coating antigen and the hapten.
Figure 2.
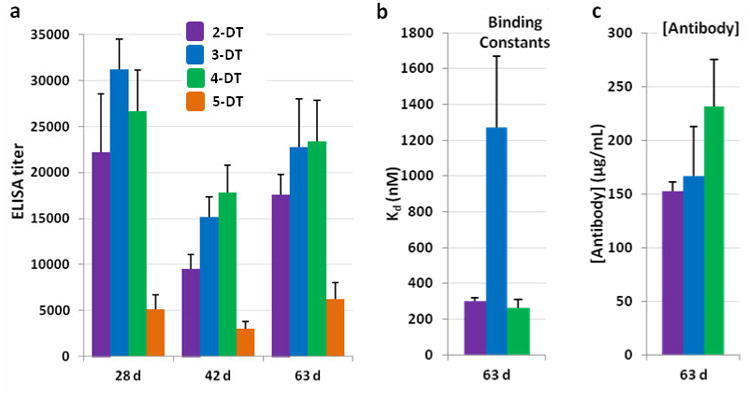
Anti-MA antibody titers, affinities and concentrations from 2-DT, 3-DT, 4-DT and 5-DT vaccinated mice (n = 6) with SAS as adjuvant. (a) Midpoint titers as determined by ELISA using 1-BSA as the coating antigen; (b) dissociation constants (Kds) as determined by competitive RIA using pooled sera; (c) antibody concentrations as determined by competitive RIA using pooled sera (63 d). RIA data were obtained in duplicate. Errors represent SEM.
Radioimmunoassay (RIA) analysis was performed to determine the antibody concentrations and their affinity for MA itself, enabling unbiased comparison of the four haptens (Figure 2b-c). 2-DT and 4-DT elicited antibodies with comparable affinity for MA, but the presence of the peptidic linker gave a moderate boost to the antibody concentration, paralleling results previously observed for the aforementioned peptidic nicotine hapten.27 Antibodies raised against 3-DT had much poorer MA affinity, demonstrating the detrimental effect of modification of the secondary amine to a tertiary amine. This hypothesis is further supported by the dramatic reduction in MA-binding capabilities observed for antibodies raised against 5-DT, the design of which converted the crucial secondary amine, protonated under physiological pH, to an uncharged tertiary amide. Full analysis for this hapten was not possible due to the low MA binding obtained; the highest serum concentration tested bound only 7.4% 3H-MA tracer, compared to 48.5% for 2-DT-elicited antibodies (Graph S1).
The ultimate goal of a MA vaccine is the ability to protect against MA administration in vivo. However, no modulation of MA-induced hyper-locomotion in mice was observed for any of these four vaccines. The presence of a secondary amine in the hapten appeared crucial for obtaining an effective immune response, and we thus focused in the next round of optimization on modulating the linker.
The introduction of a peptidic linker resulted in a moderate increase in antibody concentration, yet our first design concept required a lengthy synthesis, with an additional glutarate moiety necessary for conjugation. We therefore investigated simpler glycine and diglycine variants, with amides in the reverse direction to allow for direct conjugation to the carrier protein. Additionally, given that natural, peptidic linkers appear to generate a greater immune response, we targeted a hapten with a shorter alkyl spacer between the MA core and the glycine unit.
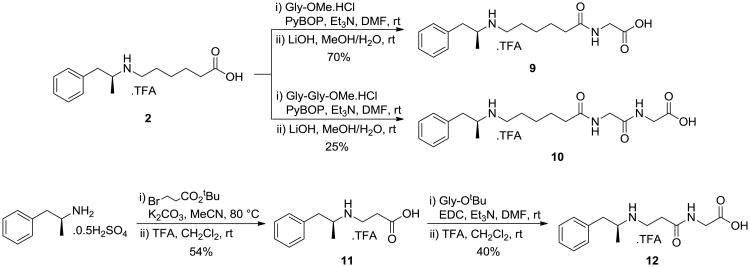
Haptens 9 and 10 were synthesized from 2 by coupling with the protected esters of the glycine and diglycine, respectively, followed by saponification to release the acids. Hapten 11 was synthesized directly from amphetamine through alkylation with tert-butyl 3-bromopropanoate and acid-mediated ester cleavage. Coupling with H-Gly-OtBu and subsequent deprotection then generated hapten 12.
The four haptens, 9-12, were activated, along with hapten 2 as a control, and conjugated to diphtheria toxoid (DT). Hapten 11 failed to conjugate due to immediate cyclization to the corresponding azetidinone. The three reverse-glycine-containing haptens conjugated to the carrier protein with comparable efficiency to hapten 2, suggesting that lactamization was not occurring.
The four successful conjugates were formulated with SAS, and 2-DT was also separately formulated with alum for comparison of adjuvants. Alum is a clinically approved adjuvant found in most human vaccines and is effective for boosting humoral responses to target antigens. The efficacies of the vaccines were again determined by vaccination of Swiss Webster mice (n=6). All four SAS formulations resulted in similar titers, with a slight increase using alum (Figure 3a). RIA analysis again allowed for unbiased hapten comparison, with similar antibody affinities and concentrations for the SAS formulations of 2-DT, 9-DT and 10-DT (Figure 3b-c). Excitingly, 12-DT elicited significantly higher affinity antibodies, suggesting we had identified a superior hapten design. Unfortunately, a significantly lower antibody concentration was obtained, but this variable could be more readily improved through adjuvant formulation optimization. In this study, we found that formulating 2-DT with alum resulted in higher antibody concentrations compared to SAS with a slight reduction in antibody affinity, and therefore a combination of 12-DT with alum was our starting point for further optimization.
Figure 3.
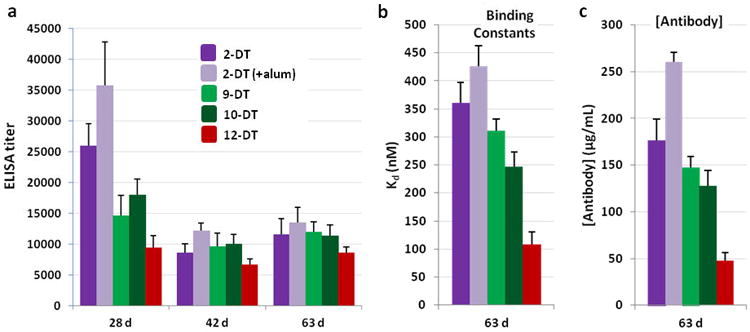
Anti-MA antibody titers, affinities and concentrations from 2-DT (with SAS or alum), 9-DT, 10-DT and 12-DT vaccinated mice (n = 6) with SAS (or alum) as adjuvant. (a) Midpoint titers as determined by ELISA using 1-BSA as the coating antigen; (b) dissociation constants (Kds) as determined by competitive RIA using pooled sera; (c) antibody concentrations as determined by competitive RIA using pooled sera (63 d). RIA data were obtained in triplicate. Errors represent SEM.
A decrease in ELISA titer was observed between days 28 and 42 for both studies, suggesting that improvements could be made to the vaccine formulation and schedule. In previous work, the use of CpG ODN 1826 has proved highly effective at increasing the immune response obtained for our heroin vaccine over using alum alone,35 and the use of tetanus toxoid (TT) rather than DT was preferred due to the potential for higher hapten densities. All of these components were therefore brought together for our final vaccination study. Hapten 12 was activated and conjugated to TT, resulting in a high hapten density, far greater than that attainable for DT due to the increased number of lysine residues present on the surface of the larger protein. The vaccine was formulated with alum and CpG ODN 1826 and again used to vaccinate Swiss Webster mice (n=6). On this occasion, the corresponding vaccine with unconjugated TT was used as a control for behavioral analysis.
The resulting ELISA analysis showed exceptionally high titers, suggesting that this new vaccine formulation was extremely effective at eliciting an immune response (Figure 4a). The new formulation succeeded in stimulating higher antibody concentrations, as determined by RIA, albeit with an unexpected reduction in antibody affinity (Figure 4b-c). However, the true test of the vaccine was the ability of the vaccine to reduce an in vivo response to MA administration.
Figure 4.
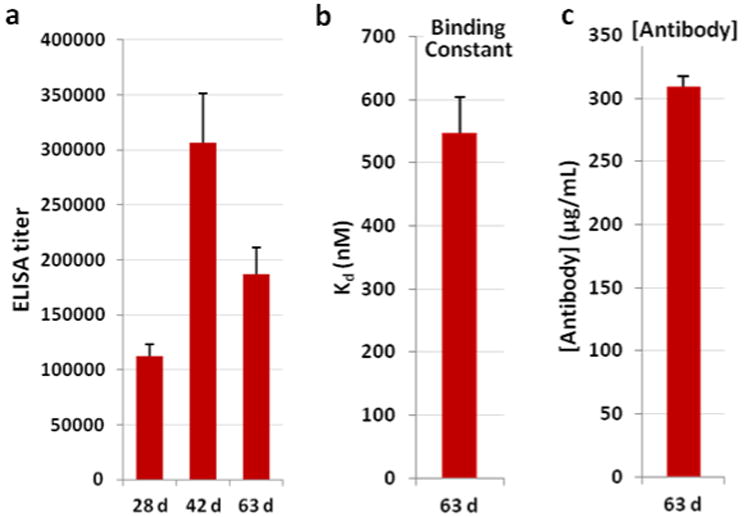
Anti-MA antibody titers, affinities and concentrations from 12-TT vaccinated mice (n = 6) with alum/CpG ODN 1826 as adjuvant. (a) Midpoint titers as determined by ELISA using 1-BSA as the coating antigen; (b) dissociation constants (Kds) as determined by competitive RIA using pooled sera; (c) antibody concentrations as determined by competitive RIA using pooled sera (63 d). RIA data were obtained in triplicate. Errors represent SEM.
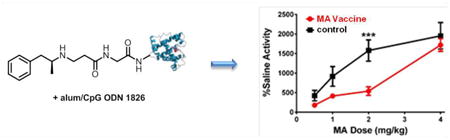
The effects of our lead MA vaccine were examined in a standard behavioral model for MA psychoactivity. Since MA is a stimulant, it produces a quantifiable increase in rodent locomotor activity. By using a video tracking system, we measured MA-induced locomotor activity in both vaccinated and control mice (n = 6). In the control group, a clear dose-response curve was observed at a wide range of MA doses (0.5-4.0 mg/kg); as expected, MA dosing varied directly with locomotor activity and inversely with time immobile (Figure 5a-b). Gratifyingly, 12-TT showed a significant shift in the dose-response curve for both locomotor activity [Dose × Vaccine Interaction: F3,30 = 4.88, p < 0.01] and immobility time [Dose × Vaccine Interaction: F4,40 = 2.85, p < 0.05], especially at 2 mg/kg, thus demonstrating a marked vaccine-mediated attenuation of the effects of MA. We attribute this successful result to a combination of successful MA hapten design and adjuvant formulation.
Figure 5.
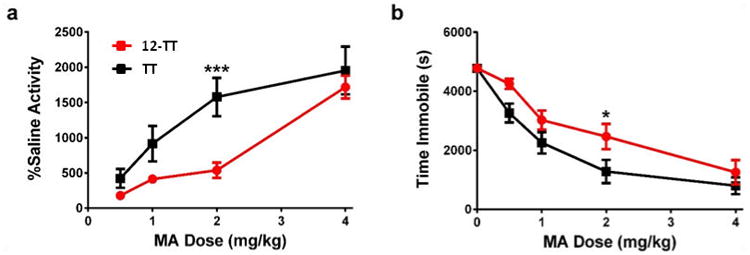
MA-induced locomotor activity in 12-TT vaccinated and TT only control mice (n = 6) using alum/CpG ODN 1826 as adjuvant. (a) Change in overall locomotor activity due to various doses of MA. Activity was measured as raw distances traveled using video tracking software for 90 min following injection, and represented as percent change from distance traveled during saline control session. (b) Time spent immobile during locomotor sessions. *p < 0.05, ***p < 0.001 versus TT control group. Errors represent SEM.
Conclusion
A series of comparative studies have demonstrated the importance of hapten design for drugs of abuse vaccines; an immune response will only be directed towards the drug of interest should the hapten be a true representation of the drug. In the case of MA, preservation of the secondary amine was essential for eliciting high affinity anti-MA antibodies; haptens containing a tertiary amine (hapten 3) or an amide (hapten 5)25, 30 failed to produce effective drug-binding antibodies. Additionally, the preference of peptidic rather than alkyl linkers for conjugation to carrier proteins was further supported. The choice of carrier protein and vaccine formulation were key in obtaining an efficacious vaccine in vivo, with 12-TT/alum+CpG attenuating MA-induced locomotion in mice. This vaccine is therefore a highly promising candidate for further development, with the ultimate aim of human clinical trials.
Experimental
Reactions were carried out under an argon atmosphere at rt using flame-dried glassware with dry solvents. Reagents were used as commercially supplied, unless otherwise specified. RP-HPLC was performed on an Agilent Technologies 1260 Infinity system using a Grace Vydac C18 column, 10-15 μm, 250 × 22 mm using the following method: [A = 0.1% TFA/H2O, B = 0.1% TFA/MeCN; λ = 254 and 210 nm; gradient 1% B (5 min), 1-15% B (15 min), 15-75% B (35 min), 75-95% B (10 min), 95% B (5 min)]. Flash chromatography was performed on silica gel (SiliaFlash® P60 40-63 μm, 230-400 mesh) according to the method of W. C. Still36. TLC was performed on glass-backed plates pre-coated with silica (EMD 60 F254, 0.25-1 mm) and developed using standard visualizing agents: UV fluorescence (254 nm) and KMnO4, cerium ammonium nitrate or ninhydrin with appropriate heating. 1H and 13C-NMR were performed on Bruker and Varian spectrometers, with the reference from the residual solvent peak for 1H-NMR (7.26 ppm for CDCl3, 3.31 ppm for CD3OD), and the solvent peak for 13C-NMR (77.1 ppm for CDCl3, 49.0 ppm for CD3OD). HRMS (ESI, positive ion mode) were performed on an Agilent 1100 Deries LC/MSD-TOF, protein concentration was determined by BCA assay (Pierce® BCA Protein Assay Kit) with analysis on a plate reader (Molecular Devices SpectraMax 250) at 562 nm,37 and determination of copy number of protein conjugates was carried out using comparative MALDI-MS, using sinapinic acid as the matrix on an Applied Biosystems Voyager-DE STR MS.38 All compounds had purity >95%.
(S)-6-((1-Phenylpropan-2-yl)amino)hexanoic acid, 2
Ethyl 6-((methylsulfonyl)oxy)hexanoate39 (323 mg, 1.36 mmol) was added to a suspension of D-amphetamine.0.5H2SO4 (200 mg, 1.09 mmol) and K2CO3 (450 mg, 3.26 mmol) in MeCN (7 mL) and the resulting mixture was heated to 80 °C (15 h). Upon cooling, the solvent was removed in vacuo, MeOH (3 mL) and 4M aq. LiOH (1 mL, 4.00 mmol) were added and the solution stirred at rt (5 h). Purification by preparative RP-HPLC (Rt = 34.4 min) followed by lyophilization gave the title compound as a colorless oil (352 mg, 89%), [α]D25 +5.8 (c 1.0, MeOH); δH (CD3OD, 600 MHz) 7.35 (t, J = 7.5 Hz, 2H), 7.30-7.25 (m, 3H), 4.89 (s, 2H), 3.52-3.45 (m, 1H), 3.21 (dd, J = 13.3, 4.5 Hz, 1H), 3.12-3.02 (m, 2H), 2.70 (dd, J = 13.3, 10.1 Hz, 1H), 2.34 (t, J = 7.3 Hz, 2H), 1.76-1.69 (m, 1H), 1.69-1.63 (m, 2H), 1.48-1.42 (m, 2H), 1.21 (d, J = 6.5 Hz, 3H); δC (CD3OD, 151 MHz) 177.2, 162.9 (q, J = 35.3 Hz), 137.3, 130.4, 129.9, 128.3, 118.16 (q, J = 292.5 Hz), 56.9, 46.1, 40.3, 34.4, 27.2, 27.0, 25.4, 16.0. Found: 250.1803. C15H23NO2 requires 250.1801.
(S)-N1-(1-Phenylpropan-2-yl)pentane-1,5-diamine, 6
A solution of D-amphetamine.0.5H2SO4 (200 mg, 1.09 mmol), 5-((tert-butoxycarbonyl)amino)pentyl methanesulfonate31 (458 mg, 1.63 mmol) and K2CO3 (450 mg, 3.26 mmol) in MeCN (6 mL) was heated to 80 °C (24 h), whereupon the solution was cooled to rt and diluted with CH2Cl2 (20 mL). The solution was washed with brine (20 mL) and the aqueous layer extracted with CH2Cl2 (2 × 20 mL), the combined organic layers dried (MgSO4), filtered and the solvent removed in vacuo. Purification by preparative RP-HPLC (C18 column, Rt = 39.9 min) followed by lyophilization gave intermediate carbamate as a colorless oil (192 mg, 41%), [α]D25 +9.2 (c 2.0, CHCl3); δH (CDCl3, 600 MHz) 9.22 (s, 2H), 7.30 (t, J = 7.4 Hz, 2H), 7.24 (t, J = 7.3 Hz, 1H), 7.15 (d, J = 7.2 Hz, 2H), 3.39-3.32 (m, 1H), 3.29 (dd, J = 13.1, 3.6 Hz, 1H), 3.06-2.97 (m, 3H), 2.97-2.90 (m, 1H), 2.68 (dd, J = 12.8, 10.8 Hz, 1H), 1.78-1.71 (m, 2H), 1.46-1.39 (m, 11H), 1.39-1.33 (m, 2H), 1.23 (d, J = 6.5 Hz, 3H); δC (CDCl3, 151 MHz) 161.8 (q, J = 37.4, 36.7 Hz), 156.3, 136.1, 127.4, 116.4 (q, J = 292.1 Hz), 79.4, 56.1, 45.1, 40.1, 39.6, 29.6, 28.5, 25.9, 23.8, 15.5. Found: 321.2536. C19H33N2O2 requires 321.2536. A solution of intermediate carbamate (50 mg, 0.115 mmol) in 4M HCl in dioxane (1.5 mL) was stirred at rt (1.5 h) before the solvent was removed in vacuo to give the title compound as a pale yellow oil (34 mg, 100%), [α]D25 +5.8 (c 2.0, MeOH); δH (CD3OD, 600 MHz) 7.34 (t, J = 7.0 Hz, 2H), 7.29 (d, J = 7.3 Hz, 2H), 7.27 (t, J = 7.0 Hz, 1H), 3.74 (t, J = 5.5 Hz, 1H), 3.69-3.65 (m, 1H), 3.58 (t, J = 4.6 Hz, 1H), 3.52 (s, 1H), 3.30 (s, 1H), 3.11 (s, 2H), 2.98 (s, 1H), 2.75 (t, J = 10.6 Hz, 2H), 1.82 (s, 2H), 1.76 (s, 2H), 1.54 (s, 2H), 1.24 (s, 3H); δC (CD3OD, 151 MHz, mixture of rotamers) 137.4, 130.5, 129.9, 128.2, 73.5, 72.4, 62.1, 57.1, 46.1, 43.8, 40.5, 40.2, 27.9, 26.9, 24.5, 16.0. Found: 221.2013. C14H25N2 requires 221.2012.
tert-Butyl(S)-(2-oxo-2-((2-oxo-2-((5-((1-phenylpropan-2-yl)amino)pentyl)amino)ethyl)amino)ethyl)carbamate, 7
A solution of amine 6 (16 mg, 0.0546 mmol), Boc-Gly-Gly-OH (14 mg, 0.0600 mmol), PyBOP (34 mg, 0.0655 mmol) and Et3N (34 μL, 0.245 mmol) in DMF (500 μL) was stirred at 0 °C (1.5 h). The reaction was quenched with H2O (10 μL) and the reaction stirred at rt (5 min) before the solvent was removed in vacuo. Purification by preparative RP-HPLC (Rt = 39.0 min) followed by lyophilization gave the title compound as a colorless oil (22 mg, 74%), [α]D25 +3.3 (c 2.0, CHCl3); δH (CD3OD, 600 MHz, mixture of rotamers) 7.38-7.33 (m, 2H), 7.30-7.25 (m, 3H), 3.83 (s, 2H), 3.72 (s, 2H), 3.53-3.45 (m, 1H), 3.36-3.34 (m, 0.5H), 3.27-3.22 (m, 2H), 3.22-3.19 (m, 0.5H), 3.11-3.01 (m, 2H), 2.73-2.67 (m, 1H), 1.75-1.67 (m, 2H), 1.62-1.55 (m, 2H), 1.48-1.39 (m, 11H), 1.24-1.20 (m, 3H); δC (CD3OD, 151 MHz, mixture of rotamers) 173.4, 171.7, 162.3 (q, J = 35.3 Hz), 158.9, 137.3, 130.4, 130.0, 128.4, 117.9 (q, J = 292.3 Hz), 81.0, 56.9, 46.2, 45.1, 43.6, 40.3, 39.8, 29.6, 28.7, 27.0, 24.6, 16.0. Found: 435.2966. C23H39N4O4 requires 435.2966.
(S)-2-Amino-N-(2-oxo-2-((5-((1-phenylpropan-2-yl)amino)pentyl)amino)ethyl)acetamide, 8
A solution of carbamate 7 (22 mg, 0.0413 mmol) in 4M HCl in 4M HCl in dioxane/MeOH (5:3, 800 μL) was stirred at rt (1 h) before the solvent was removed in vacuo to give the title compound as a colorless oil (16.8 mg, 100%), [α]D25 +3.7 (c 1.5, CHCl3); δH (CDCl3, 600 MHz) 7.37 (t, J = 7.3 Hz, 2H), 7.33-7.28 (m, 2H), 3.95 (s, 2H), 3.81 (s, 2H), 3.53 (s, 1H), 3.32-3.25 (m, 3H), 3.10 (s, 2H), 2.76 (t, J = 11.0, 1H), 1.78 (s, 2H), 1.62 (s, 2H), 1.47 (s, 2H), 1.25 (d, J = 5.1 Hz, 2H); δC (CDCl3, 151 MHz, mixture of rotamers) 171.2, 167.9, 137.4, 130.4, 129.9, 128.3, 73.5, 72.4, 62.1, 57.0, 46.3, 43.4, 41.7, 40.2, 40.0, 29.7, 27.1, 24.8, 16.0. Found: 335.2442. C18H31N4O2 requires 335.2441.
(S)-2-Methyl-10,13,16-trioxo-1-phenyl-3,9,12,15-tetraazaicosan-20-oic acid, 4
PyBOP (29 mg, 0.0552 mmol) and Et3N (23 μL, 0.166 mmol) were added to a solution of mono-methylglutarate (7 μL, 0.0552 mmol) in DMF (200 μL) and stirred at rt (5 min) before the addition of a solution of amine 8 (15 mg, 0.0368 mmol) in DMF (100 μL) and the solution stirred at rt (45 min). The reaction was quenched with H2O (50 μL) and the reaction stirred at rt (10 min) before the solvent was removed in vacuo. The residue was dissolved in MeOH (500 μL), 4M aq. LiOH (100 μL) added and the reaction stirred at rt (1.5h). Purification by preparative RP-HPLC (Rt = 33.4 min) followed by lyophilization gave the title compound as a white solid (7.5 mg, 36%), [α]D25 +4.0 (c 0.75, CD3OD); δH (CD3OD, 600 MHz) 7.36 (t, J = 7.6 Hz, 2H), 7.30-7.25 (m, 3H), 3.84-3.82 (m, 4H), 3.53-3.47 (m, 1H), 3.25 (t, J = 6.8, 2H), 3.21 (dd, J = 13.4, 4.6 Hz, 1H), 3.11-3.02 (m, 2H), 2.70 (dd, J = 13.2, 10.0 Hz, 1H), 2.36 (q, J = 7.6 Hz, 4H), 1.94-1.89 (m, 2H), 1.74-1.67 (m, 2H), 1.59 (p, J = 6.9 Hz, 2H), 1.43 (p, J = 7.7 Hz, 2H), 1.22 (d, J = 6.5 Hz, 3H); δC (CD3OD, 151 MHz, mixture of rotamers) 176.6, 176.5, 172.6, 171.7, 161.9 (q, J = 38.6, 36.9 Hz), 137.3, 130.4, 130.0, 128.4, 128.4, 117.7 (d, J = 293.0 Hz), 56.9, 46.2, 44.2, 43.7, 40.3, 39.8, 35.7, 34.1, 29.6, 27.0, 24.6, 21.9, 16.0. Found: 449.2758. C23H37N4O5 requires 449.2758.
(S)-4-Oxo-4-((1-phenylpropan-2-yl)amino)butanoic acid, 5
According to the method of Kosten,25 a solution of (+)-methamphetamine.HCl (30 mg, 0.162 mmol), succinic anhydride (24 mg, 0.242 mmol) and Et3N (68 μL, 0.485 mmol) in CH2Cl2 (1 mL) was heated to 50 °C (1 h) before the solvent was removed in vacuo. Purification by preparative RP-HPLC (Rt = 40.0 min) followed by lyophilization gave the title compound as a colorless oil (35 mg, 87%), [α]D25 +34.9 (c 2.0, CDCl3); δH (CD3OD, 600 MHz, mixture of rotamers) 7.30-7.17 (m, 3H), 7.15 (d, J = 7.2 Hz, 1H), 7.09 (d, J = 7.2 Hz, 1H), 5.01-4.93 (m, 0.5H), 4.13-4.06 (m, 0.5H), 2.86 (s, 1.5H), 2.82-2.76 (m, 2H), 2.76-2.69 (m, 1.5H), 2.61-2.53 (m, 1.5H), 2.50-2.42 (m, 1.5H), 2.38-2.31 (m, 0.5H), 2.07-2.00 (m, 0.5H), 1.26 (d, J = 6.7 Hz, 1.5H), 1.11 (d, J = 6.8 Hz, 1.5H); δC (CD3OD, 151 MHz, mixture of rotamers) 177.1, 177.0, 172.3, 172.2, 138.2, 138.0, 128.9, 128.9, 128.8, 128.4, 127.0, 126.5, 54.8, 50.3, 40.6, 40.1, 29.7, 29.7, 29.4, 28.8, 27.8, 27.0, 18.8, 17.2. Found: 250.1437. C14H20NO3 requires 250.1438.
(S)-(6-((1-Phenylpropan-2-yl)amino)hexanoyl)glycine, 9
Et3N (41 μL, 0.295 mmol) was added to a solution of 2 (20 mg, 0.0491 mmol), and glycine methyl ester.HCl (18 mg, 0.147 mmol) in DMF (300 μL) and stirred at rt (5 min) before the addition of PyBOP (38 mg, 0.0736 mmol) and the solution stirred at rt (1 h). The reaction was quenched with H2O (20 μL) and the reaction stirred at rt (10 min) before the solvent was removed in vacuo. The residue was dissolved in MeOH (500 μL), 4M aq. LiOH (200 μL, 0.800 mmol) added and the reaction stirred at rt (2.5h). Purification by preparative RP-HPLC (Rt = 32.8 min) followed by lyophilization gave the title compound as a white solid (14.5 mg, 70%), [α]D25 +4.4 (c 0.5, MeOH); δH (CD3OD, 600 MHz) 7.38 (t, J = 7.4 Hz, 2H), 7.33-7.27 (m, 3H), 3.93 (d, J = 1.3 Hz, 2H), 3.54-5.48 (m, 1H), 3.23 (dd, J = 13.4, 4.5 Hz, 1H), 3.15-3.05 (m, 2H), 2.72 (m, 1H), 2.35-2.31 (m, 2H), 1.78-1.68 (m, 4H), 1.49 (p, J = 7.7 Hz, 2H), 1.24 (d, J = 6.4 Hz, 3H); δC (CD3OD, 151 MHz) 174.4, 171.2, 159.7 (q, J = 37.0 Hz), 135.4, 128.5, 128.1, 126.5, 115.6 (q, J = 289.2 Hz), 55.0, 44.2, 39.8, 38.4, 34.2, 25.2, 25.0, 24.0, 14.1. Found: 307.2017. C17H27N2O3 requires 307.2016.
(S)-(6-((1-Phenylpropan-2-yl)amino)hexanoyl)glycylglycine, 10
Et3N (41 μL, 0.295 mmol) was added to a solution of 2 (20 mg, 0.0491 mmol), and methyl((aminoacetyl)amino)acetate. HCl (27 mg, 0.147 mmol) in DMF (300 μL) and stirred at rt (5 min) before the addition of PyBOP (38 mg, 0.0736 mmol) and the solution stirred at rt (1 h). The reaction was quenched with H2O (20 μL) and the reaction stirred at rt (10 min) before the solvent was removed in vacuo. The residue was dissolved in MeOH (500 μL), 4M aq. LiOH (200 μL, 0.800 mmol) added and the reaction stirred at rt (2.5h). Purification by preparative RP-HPLC (Rt = 31.7 min) followed by lyophilization gave the title compound as a white solid (6 mg, 25%), [α]D25 +4.5 (c 0.6, MeOH); δH (CD3OD, 600 MHz) 7.35 (t, J = 7.5 Hz, 2H), 7.30-7.25 (m, 3H), 3.93 (s, 2H), 3.92 (s, 2H), 3.52-3.46 (m, 1H), 3.21 (dd, J = 13.3, 4.4 Hz, 1H), 3.13-3.04 (m, 2H), 2.69 (dd, J = 13.2, 10.1 Hz, 1H), 2.32 (t, J = 7.2 Hz, 2H), 1.76-1.67 (m, 4H), 1.47 (p, J = 7.7 Hz, 2H), 1.22 (d, J = 6.6 Hz, 3H); δC (CD3OD, 151 MHz) 176.3, 172.8, 172.1, 162.6 (q, J = 36.2 Hz), 137.3, 130.4, 130.0, 128.4, 118.1 (q, J = 290.6 Hz), 57.0, 46.1, 43.2, 41.8, 40.3, 36.2, 27.1, 26.9, 25.7, 16.0. Found: 364.2231. C19H30N3O4 requires 364.2231.
(S)-3-((1-Phenylpropan-2-yl)amino)propanoic acid, 11
tert-Butyl 3-bromo-propionate (25 mg, 0.119 mmol) was added to a suspension of D-amphetamine.0.5H2SO4 (20 mg, 0.109 mmol) and K2CO3 (45 mg, 0.329 mmol) in MeCN (700 μL) and the resulting mixture was heated to 80 °C (18 h). Upon cooling, the solvent was removed in vacuo and purification by preparative TLC using MeOH/CH2Cl2 (1:9) gave the intermediate ester (16 mg, 56%). A solution of intermediate ester (16 mg, 0.0607 mmol) in TFA/CH2Cl2 (500 μL) was stirred (2 h), whereupon the solvent was removed in vacuo to give the title compound as a colorless gum (19 mg, 97%), [α]D25 +13.6 (c 1.5, CHCl3); δH (CHCl3, 600 MHz) 9.92 (br s, 2H), 8.44 (br s, 2H), 7.30 (t, J = 7.3 Hz, 2H), 7.26-7.23 (m, 1H), 7.18 (d, J = 7.3 Hz, 2H), 3.52 (br s, 1H), 3.36 (br s, 1H), 3.30-3.22 (m, 2H), 2.92-2.87 (m, 2H), 2.83-2.77 (m, 1H), 1.32 (d, J = 6.4 Hz, 3H); δC (CHCl3, 151 MHz) 174.5, 161.7 (q, J = 38.2 Hz), 135.4, 129.3, 129.1, 127.6, 115.9 (q, J = 291.6, 290.8 Hz), 57.1, 41.2, 39.3, 30.3, 15.7. Found: 208.1332. C12H18NO2 requires 208.1332.
(S)-(3-((1-Phenylpropan-2-yl)amino)propanoyl)glycine, 12
EDC.HCl (4 mg, 0.0205 mmol) and Et3N (6 μL, 0.0411 mmol) were added to a solution of 11 (4.4 mg, 0.0137 mmol) in CH2Cl2 (400 μL) and stirred at rt (5 min) before the addition of a solution of tert-butyl glycinate (67 mg, 0.242 mmol) in CH2Cl2 (200 μL) and the solution stirred at rt (1 h). The reaction was quenched with H2O (10 μL) and the reaction stirred at rt (5 min) before the solvent was removed in vacuo. Purification by preparative TLC using MeOH/CH2Cl2 (1:9) gave the intermediate ester. A solution of intermediate ester in TFA/CH2Cl2 (500 μL) was stirred (2 h), whereupon the solvent was removed in vacuo to give the title compound as a colorless gum (2.1 mg, 90%), [α]D25 +5.5 (c 0.2, CH3OH); δH (CD3OD, 600 MHz) 7.39-7.36 (m, 2H), 7.32-7.28 (m, 3H), 3.97 (s, 2H), 3.60-3.54 (m, 1H), 3.45-3.34 (m, 2H), 3.21 (dd, J = 13.4, 5.0 Hz, 1H), 2.81-2.74 (m, 3H), 1.27 (d, J = 6.5 Hz, 3H); δC (CD3OD, 151 MHz) 173.2, 172.5, 137.1, 130.4, 130.0, 128.4, 57.1, 42.3, 41.7, 40.3, 31.7, 16.0. Found: 265.1547. C14H21N2O3 requires 265.1547.
Conjugation of haptens
Acids
A solution of hapten (150 mM), EDC.HCl (4.5 eq) and sulfo-NHS (1.5 eq) in 10% H2O/DMF was agitated at rt until complete activation was observed by LCMS analysis (adding further EDC.HCl if necessary). DT or BSA in PBS (pH 7.4) was added to the activated hapten 2, 3, 4, 5, 9, 10 or 12 (ca. 250 eq for DT, 1500 eq for TT) to give a final concentration of 3 mg/mL in PBS (pH 7.4) and the solutions mixed (rt, 6-8h then 4 °C, 16 h).
Thiol
A solution of sulfo-GMBS (0.5 mg per mg protein) and BSA (5 mg/mL in PBS (pH 7.4)) were mixed (rt, 1.5 h) before excess sulfo-GMBS was removed (Zeba desalting column). A solution of hapten 122 (ca. 250 eq) in 10% DMSO in maleimide conjugation buffer (Imject, Thermo Pierce) was added dropwise to the activated BSA solution to give a final concentration of 2 mg/mL protein, which was mixed (rt, 6 h then 4 °C, 16 h). The resulting protein conjugates were dialyzed into PBS (pH 7.4) at 4 °C. DT and TT conjugates were used for immunization; BSA conjugates were used for ELISA plate coating.
Active Immunization Protocol
All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. No adverse reactions to the vaccines were observed and all mice maintained a healthy weight throughout the vaccine trial. 2-DT, 3-DT, 4-DT, 5-DT, 9-DT, 10-DT, 12-DT and 12-TT in PBS (pH 7.4) (100 μL, 1 mg/mL for DT conjugates, 75 μL, 1 mg/mL for TT conjugates) were formulated with Sigma Adjuvant System® (SAS, 100 μL) or Alhydrogel® (Invivogen, 10 mg/mL) (100 μL for DT conjugates, 75 μL for TT conjugates) with or without phosphorothioated CpG ODN 1826 (Eurofins MWG Operon) (75 μL) were used to immunize groups of Swiss Webster mice (n = 6) via subcutaneous injection on days 0, 21 and 35 for DT conjugates or days 0, 14 and 28 for TT conjugates. Serum was collected via tail bleed on days 28 and 42 and via cardiac bleed on day 63.
ELISA
Production of anti-MA IgG was evaluated by ELISA. Microtiter plates (Costar 3690) were incubated with coating antigen 1-BSA in PBS (5 μg/mL, 25 μL) (18 h, 37 °C). 5% Non-fat milk in PBS (30 min, 37 °C) was added to block non-specific binding. Mouse sera in 1% BSA were serially diluted across the plate before incubation in a moist chamber (1.5 h, 37 °C). The plate was washed with dH2O or PBS before incubation with peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) in a moist chamber (30 min, 37 °C). The plates were further washed with dH2O or PBS before being developed with the TMB substrate kit (Thermo Pierce) and the absorbance at 450 nm measured on a microplate reader (SpectraMax M2e Molecular Devices). Titers were calculated as the dilution corresponding to 50% of the maximum absorbance from a plot of the absorbance versus log(dilution) using GraphPad Prism 6.
Radioimmunoassay (RIA)
Dissociation constants and antibody concentrations were determined using competitive RIA. Mouse sera were pooled and diluted into 2% BSA, giving a concentration that was determined to bind ca. 30-40% (+)-[2′,6′-3H(n)] methamphetamine tracer (20,000 dpm, 39 Ci/mmol (National Institute on Drug Abuse, Bethesda, MD)). Diluted serum (60 μL) and methamphetamine tracer (60 μL) were added to the sample chamber of a 5 kDa MWCO 6-well Equilibrium Dialyzer (Harvard Apparatus), and unlabelled (+)-methamphetamine (120 μL) at varying concentrations in 1% BSA was added to the buffer chamber. After equilibration on a plate rotator (Harvard Apparatus) at rt (22-26 h), a sample from each chamber (60 μL) was diluted into Ecolite(+)™ liquid scintillation cocktail (5 mL, MP Biomedicals) and the radioactivity measured using a Beckman LS 6500 Scintillation Counter. Kds and antibody concentrations were calculated according to Müller 40.
Locomotion
Mice were allowed to acclimate for one hour in a plastic cage (10.5 × 19 × 8 inch) with clear ventilated acrylic top. Mice were then quickly removed and injected with either saline, 0.5, 1, 2, or 4 mg/kg MA before being returned to their cages; cages were wiped down with dry paper towels to remove excess debris while mice were being injected. The mice were then returned to the cage to be recorded and tracked by overhead camera using ANY-Maze video tracking software (Stoelting Co., Wood Dale, IL). Sessions were run during the middle of the dark cycle in a 4.6 × 4.6 m room with a single 30 W upward-directed light source, and repeated on mice after a two-day washout period until all mice received all MA doses. Distance travelled (m) and time spent immobile (s; 80% pixel consistency for at least 5 s threshold) were measured and analyzed via Prism 6 software, using a two-way repeated-measures ANOVA with Bonferroni post-hoc corrected comparisons. Significance was set at α = 0.05 and data is represented as mean ± SEM.
Supplementary Material
Scheme 2.
Synthesis of haptens 9-12.
Acknowledgments
We acknowledge the support of The Scripps Research Institute, Skaggs Institute for Chemical Biology, and the National Institute on Drug Abuse under grants R01-DA024705, F31-DA037709. This is manuscript #29277 from The Scripps Research Institute.
Abbreviations
- BCA
bicinchoninic acid assay
- BSA
bovine serum albumin
- CpG ODN
cytosine-guanine oligodeoxynucleotide
- dH2O
deionized water
- DMF
dimethylformamide
- DT
diphtheria toxoid
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- ELISA
enzyme-linked immunosorbent assay
- Ig
immunoglobulin
- Kd
dissociation constant
- KLH
keyhole limpet hemocyanin LCMS, liquid chromatography mass spectrometry
- MA
methamphetamine
- MALDI-MS
matrix-assisted laser desorption/ionization mass spectrometry
- MWCO
molecular weight cut-off
- NMR
nuclear magnetic resonance
- PBS
phosphate-buffered saline
- PyBOP
benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate
- RIA
radioimmunoassay
- RP-HPLC
reversed-phase high-performance liquid chromatography
- SAS
Sigma Adjuvant System
- SEM
standard error of the mean
- sulfo-GMBS
N-(γ-maleimidobutyryloxy) sulfosuccinimide ester sulfo-NHS, Nhydroxysulfosuccinimide
- TFA
trifluoroacetic acid
- TLC
thin layer chromatography
- TMB
3,3′,5,5′-tetramethylbenzidine
- TT
tetanus toxoid
- UV
ultra-violet
Footnotes
Supporting Information: Additional figures showing hapten densities, antibody titers, binding constants and concentrations, NMR data for haptens 1 and 3, NMR spectra and MALDI traces. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of Neurotransmitter Release by Amphetamines: A Review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Rawson RA Center for Substance Abuse Treatment. Treatment for Stimulant Use Disorders. Substance Abuse and Mental Health Services Administration (US); Rockville (MD): 1999. [PubMed] [Google Scholar]
- 3.Rawson RA, Anglin MD, Ling W. Will the Methamphetamine Problem Go Away? J Addict Dis. 2002;21:5–19. doi: 10.1300/j069v21n01_02. [DOI] [PubMed] [Google Scholar]
- 4.Vocci FJ, Appel NM. Approaches to the Development of Medications for the Treatment of Methamphetamine Dependence. Addiction. 2007;102:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen YH, Chen CH. The Development of Antibody-based Immunotherapy for Methamphetamine Abuse: Immunization, and Virus-Mediated Gene Transfer Approaches. Curr Gene Ther. 2013;13:39–50. doi: 10.2174/156652313804806552. [DOI] [PubMed] [Google Scholar]
- 6.Carroll KM, Onken LS. Behavioral Therapies for Drug Abuse. Am J Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKetin R, Najman JM, Baker AL, Lubman DI, Dawe S, Ali R, Lee NK, Mattick RP, Mamun A. Evaluating the Impact of Community-based Treatment Options on Methamphetamine Use: Findings from the Methamphetamine Treatment Evaluation Study (MATES) Addiction. 2012;107:1998–2008. doi: 10.1111/j.1360-0443.2012.03933.x. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales R, Mooney L, Rawson RA. The Methamphetamine Problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States, 2005. RAND Corporation; Santa Monica, CA: 2009. [accessed September 27 2015]. http://www.rand.org/pubs/monographs/MG829. [Google Scholar]
- 10.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease, Chapter 9 The Humoral Immune Response; B-cell Activation by Armed Helper T Cells. Garland Science; New York: 2001. [Google Scholar]
- 11.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Müller P, Willers J, Maurer P, Bachmann MF, Cerny T. A Vaccine Against Nicotine for Smoking Cessation: A Randomized Controlled Trial. PLoS ONE. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim REF, Kessler PD, Niknian M, Kalnik MW, Rennard SI. Immunogenicity and Smoking-Cessation Outcomes for a Novel Nicotine Immunotherapeutic. Clin Pharmacol Ther. 2011;89:392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Tracie G, Kosten TR. Cocaine Vaccine for the Treatment of Cocaine Dependence in Methadone-maintained Patients: A Randomized, Double-blind, Placebo-controlled Efficacy Trial. Arch Gen Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosten TR, Biegel D. Therapeutic Vaccines for Substance Dependence. Expert Rev Vaccines. 2002;1:365–371. doi: 10.1586/14760584.1.3.365. [DOI] [PubMed] [Google Scholar]
- 15.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine Pharmacotherapy for the Treatment of Cocaine Dependence. Biol Psychiat. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-Specific Antibodies Blunt the Subjective Effects of Smoked Cocaine in Humans. Biol Psychiat. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, Horwith G, Pentel PR. Safety and Immunogenicity of a Nicotine Conjugate Vaccine in Current Smokers. Clin Pharmacol Ther. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kinsey BM, Jackson DC, Orson FM. Anti-drug Vaccines to Treat Substance Abuse. Immunol Cell Biol. 2009;87:309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- 19.Byrnes-Blake KA, Carroll FI, Abraham P, Owens SM. Generation of Anti-(+)methamphetamine Antibodies is Not Impeded by (+)Methamphetamine Administration During Active Immunization of Rats. Int Immunopharmacol. 2001;1:329–338. doi: 10.1016/s1567-5769(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 20.Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, Sanderson SD. Immune Responses to Methamphetamine by Active Immunization with Peptide-based, Molecular Adjuvant-containing Vaccines. Vaccine. 2009;27:2981–2988. doi: 10.1016/j.vaccine.2009.02.105. [DOI] [PubMed] [Google Scholar]
- 21.Carroll FI, Blough BE, Pidaparthi RR, Abraham P, Gong PK, Deng L, Huang X, Gunnell M, Lay JO, Peterson EC, Owens SM. Synthesis of Mercapto-(+)-methamphetamine Haptens and Their Use for Obtaining Improved Epitope Density on (+)-Methamphetamine Conjugate Vaccines. J Med Chem. 2011;54:5221–5228. doi: 10.1021/jm2004943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno AY, Mayorov AV, Janda KD. Impact of Distinct Chemical Structures for the Development of a Methamphetamine Vaccine. J Am Chem Soc. 2011;133:6587–6595. doi: 10.1021/ja108807j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Jr, Janda KD, Taffe MA. A Methamphetamine Vaccine Attenuates Methamphetamine-Induced Disruptions in Thermoregulation and Activity in Rats. Biol Psychiat. 2013;73:721–728. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rüedi-Bettschen D, Wood SL, Gunnell MG, West CM, Pidaparthi RR, Carroll FI, Blough BE, Owens SM. Vaccination Protects Rats from Methamphetamine-induced Impairment of Behavioral Responding for Food. Vaccine. 2013;31:4596–4602. doi: 10.1016/j.vaccine.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen XY, Kosten TA, Lopez AY, Kinsey BM, Kosten TR, Orson FM. A Vaccine against Methamphetamine Attenuates its Behavioral Effects in Mice. Drug Alcohol Depen. 2013;129:41–48. doi: 10.1016/j.drugalcdep.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins KC, Schlosburg JE, Lockner JW, Bremer PT, Ellis BA, Janda KD. Lipid Tucaresol as an Adjuvant for Methamphetamine Vaccine Development. Chem Commun. 2014;50:4079–4081. doi: 10.1039/c4cc00682h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins KC, Janda KD. Investigating Hapten Clustering as a Strategy to Enhance Vaccines against Drugs of Abuse. Bioconjugate Chem. 2014;25:593–600. doi: 10.1021/bc500016k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. An Oxycodone Conjugate Vaccine Elicits Drug-specific Antibodies that Reduce Oxycodone Distribution to Brain and Hot-plate Analgesia. J Pharmacol Exp Ther. 2012;341:225–232. doi: 10.1124/jpet.111.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, Pentel PR. Reduced Antinociception of Opioids in Rats and Mice by Vaccination with Immunogens Containing Oxycodone and Hydrocodone Haptens. J Med Chem. 2013;56:915–923. doi: 10.1021/jm3013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora R, Kosten TA, Bennett RS, Kinsey BM, Orson FM, Kosten TR. Preclinical Efficacy of an Anti-methamphetamine Vaccine with an E6020 Adjuvant. Drug Alcohol Depen. 2015;146:e211. [Google Scholar]
- 31.Leit De Moradei SM, Tessier P, Smil D, Wahhab A, Deziel R, Manku S, Mancuso J, Therrien E, Allan M, Chantihny YA, Ajamian A, Beaulieu P. Inhibitors of Histone Deacetylase. WO 102760 (A1) 2006 [Google Scholar]
- 32.Cai X, Tsuchikama K, Janda KD. Modulating Cocaine Vaccine Potency through Hapten Fluorination. J Am Chem Soc. 2013;135:2971–2974. doi: 10.1021/ja400356g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai X, Whitfield T, Hixon MS, Grant Y, Koob GF, Janda KD. Probing Active Cocaine Vaccination Performance through Catalytic and Noncatalytic Hapten Design. J Med Chem. 2013;56:3701–3709. doi: 10.1021/jm400228w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lockner JW, Ho SO, McCague KC, Chiang SM, Do TQ, Fujii G, Janda KD. Enhancing Nicotine Vaccine Immunogenicity with Liposomes. Bioorg Med Chem Lett. 2013;23:975–978. doi: 10.1016/j.bmcl.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bremer PT, Schlosburg JE, Lively JM, Janda KD. Injection Route and TLR9 Agonist Addition Significantly Impact Heroin Vaccine Efficacy. Mol Pharm. 2014;11:1075–1080. doi: 10.1021/mp400631w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Still WC, Kahn M, Mitra A. Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. J Org Chem. 1978;43:2923–2925. [Google Scholar]
- 37.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of Protein using Bicinchoninic Acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 38.Wengatz I, Schmid RD, Kreiβig S, Wittmann C, Hock B, Ingendoh A, Hillenkamp F. Determination of the Hapten Density of Immuno-conjugates by Matrix-assisted UV Laser Desorption/Ionization Mass Spectrometry. Anal Lett. 1992;25:1983–1997. [Google Scholar]
- 39.Ekwuribe NN, Price CH, Ansari AM, Radhakrishnan B, Odenbaugh AL. Mixtures of Insulin Drug-oligomer Conjugates Comprising Polyalkylene Glycol, Uses thereof, and Methods of Making Same. WO 0027748 (A1) 2003 [Google Scholar]
- 40.Müller R. Determination of Affinity and Specificity of Anti-hapten Antibodies by Competitive Radioimmunoassay. Methods Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.