Abstract
Background
Rates of pregnancy and HIV infection are high among adolescents. However, their engagement in prevention of mother-to-child HIV transmission (PMTCT) services is poorly characterized. We compared engagement in the PMTCT cascade between adult and adolescent mothers in Kenya.
Methods
We conducted a nationally representative cross-sectional survey of mother–infant pairs attending 120 maternal child health clinics selected by probability proportionate to size sampling, with a secondary survey oversampling HIV-positive mothers in 30 clinics. Antenatal care (ANC) attendance, HIV testing, and antiretroviral (ARV) use were compared between adolescent (age ≤19 years) and adult mothers using χ2 tests and logistic regression.
Results
Among 2521 mothers, 278 (12.8%) were adolescents. Adolescents were less likely than adults to be employed (16.5% vs. 37.9%), married (66.1% vs. 88.3%), have intended pregnancy (40.5% vs. 58.6%), or have disclosed their HIV status (77.5% vs. 90.7%) (P < 0.01 for all). Adolescents were less likely than adults to attend ≥4 ANC visits (35.2% vs. 45.6%, P = 0.002). This effect remained significant when adjusting for employment, household crowding, pregnancy intention, gravidity, and HIV status [adjusted odds ratio (95% confidence interval) = 0.54 (0.37 to 0.97), P = 0.001]. Among 2359 women without previous HIV testing, 96.1% received testing during pregnancy; testing levels did not differ between adolescents and adults. Among 288 HIV-positive women not on antiretroviral therapy before pregnancy, adolescents were less likely than adults to be on ARVs (65.0% vs. 85.8%, P = 0.01) or to have infants on ARVs (85.7% vs. 97.7%, P = 0.005).
Conclusions
Adolescent mothers had poorer ANC attendance and uptake of ARVs for PMTCT. Targeted interventions are needed to improve retention of this vulnerable population in the PMTCT cascade.
Keywords: PMTCT, cascade, adolescent, antiretroviral, ANC
INTRODUCTION
Adolescence, defined by the World Health Organization as the second decade of life (age 10–19 years), is a time of enormous developmental, social, and biological transition. This multifaceted transition creates a combination of risk factors for several health outcomes. Sexual debut during this period brings with it a risk of pregnancy, as well as sexually transmitted infections, and the psychological, socioeconomical, and legal circumstances of adolescents render this group especially vulnerable to the consequences of these events. Globally, 11% of all births are to adolescent mothers, with much of this burden in low-income countries. In Kenya, for example, the 2014 demographic health survey reported that 40% of 19-year olds had already begun childbearing.1 The risks associated with these pregnancies are particularly pronounced: adolescents bear 23% of the global burden of disease due to pregnancy and childbirth.2 Many of the countries with the highest rates of adolescent pregnancy also have high HIV prevalence, creating parallel risks of pregnancy and HIV for adolescent women, and of vertical HIV transmission to their infants. Indeed, approximately 2.1 million adolescents were HIV infected globally in 2013, and the majority of these (56%) were female.3 Adolescents and young adults show low rates of HIV testing,4 poor linkage to care,5 and high loss to follow-up from antiretroviral therapy (ART) programs.6,7 These disparities are thought to be responsible for the alarming observation that although overall AIDS-related deaths decreased by 24% between 2004 and 2011, they increased by 50% in adolescents during the same period.8 Adolescents therefore need improved delivery of HIV prevention and treatment services, including prevention of mother-to-child transmission (PMTCT) of HIV services.
The “PMTCT cascade” refers to the series of events that enable identification of an HIV-infected pregnant woman and prevention of HIV transmission to her infant.9 The cascade begins with antenatal care (ANC), followed by HIV testing, and use of antiretrovirals (ARVs) by the HIV-positive mother and her HIV-exposed infant. Optimization of the PMTCT cascade is not only critical to the Joint United Nations Programme on HIV/AIDS goal of eliminating mother-to-child transmission10 but also an opportunity to integrate pregnancy-related care and linkage of HIV-positive women to treatment and prevention services. Although the need for greater attention to pregnant adolescents in the HIV response is recognized,3 analysis of engagement of pregnant adolescents in the PMTCT cascade has been limited. A number of studies in sub-Saharan Africa have reported lower ARV uptake and higher loss to follow up from PMTCT programs in young women, aged 24 years or younger.11–14 Three studies have reported lower coverage of ARVs and higher rates of mother-to-child transmission of HIV in adolescent women, aged 19 years or younger.15–17 No studies have characterized the correlates of lower PMTCT uptake among adolescent mothers.
We compared uptake of ANC, HIV testing, and maternal and infant ARVs by adolescent women with that by adults and determined the correlates of engagement in the PMTCT cascade, overall and within adolescents in 2 surveys including a total of 141 clinics in Kenya.
METHODS
Study Design and Population
Data for this study were from a national evaluation of Kenya’s PMTCT program, the Collaborative HIV Impact on MCH Evaluation study. Mobile teams performed 2 facility-based cross-sectional surveys conducted from June to December 2013. The primary national survey sampled 120 maternal child health (MCH) clinics in 7 of 8 provinces in Kenya (see Supplemental Digital Content, Fig. S1, http://links.lww.com/QAI/A917). Facilities were selected by probability proportionate to size sampling. All women bringing their infants for 6-week or 9-month infant immunizations to the selected clinics within a fixed 5-day period were recruited to participate, regardless of HIV status. The secondary Nyanza oversample survey purposively sampled HIV-positive women attending 30 large (≥1000 annual antenatal care visits) facilities in Nyanza, the province with the highest HIV prevalence. All HIV-positive women bringing their infants for 6-week or 9-month infant immunizations to the selected clinics during a fixed 10-day period were recruited to participate. Across both surveys, 141 clinics were sampled (9 were included in both surveys).
Data Collection
Study staff administered a questionnaire using Open Data Kit (opendatakit.org) on tablet computers. The same questionnaire was used in both surveys. Data were based on self-report and verified by the mother’s Maternal Child Health Booklet, when available. The questionnaire included uptake of ANC, maternal HIV testing, and the use of ARVs, and maternal and paternal demographics, household characteristics, reproductive and family planning history, depression, intimate partner violence (IPV), and use of ARVs and HIV testing in HIV-exposed infants.
Ethics Approval
Written informed consent was obtained from all study participants. Ethical approval was obtained from the ethical review committees at the Kenya Medical Research Institute, the University of Washington, and the US Centers for Disease Control and Prevention.
Statistical Analyses
Analyses included subsets of women, based on the outcome of interest. For analysis of ANC attendance, all women in the primary survey were included irrespective of their HIV status. For analysis of HIV testing, all women in the primary survey who reported attending any ANC were included, irrespective of HIV status. For analysis of infant ARV use, all HIV-positive women in both the primary and secondary surveys were included to achieve maximal power. For analysis of maternal ARV use, all HIV-positive women in both the primary and secondary surveys who did not report initiating ART before pregnancy were included. Adolescent age was defined as ≤19 years old, as defined by the World Health Organization.18
Standardized tools were used to measure IPV (HITS) in the year before study participation19 and depression (PHQ9) in the 2 weeks before study participation.20 For IPV, responses to the HITS instrument were scored 1–5 and summed; a score of >10 was considered positive.19 For depression, responses to the PHQ9 instrument were scored 0–3 and summed; a score of ≥5 (mild depression or greater) was considered positive.20 Household crowding was defined as ≥3 people per room. Characteristics of adolescent and nonadolescent participants were compared by χ2 test or t test as appropriate. Associations of participant characteristics with ANC and PMTCT outcomes were determined by logistic regression. Variables showing an effect on ANC and PMTCT outcomes in univariable analysis at a significance of P < 0.10 were included in multivariable analysis. All proportions, means, and regression coefficients were adjusted for clinic-level clustering effects. Analyses of the primary survey also included inverse-probability adjustment for sampling weights. We determined a priori to adjust all analyses for clinic location in Nyanza province (the high HIV-prevalence region that was oversampled in the secondary survey). Analyses presented are based on self-reported data; analyses restricted to data confirmed by Maternal & Child Health Booklet (available for 78% of women’s reported ANC visit numbers, 80% of reported maternal ARV use, and 50% of reported infant ARV use) yielded no significant differences from the overall data. Data were analyzed using Stata/SE 13.1 (Stata Corporation, College Station, TX).
RESULTS
Characteristics of Adult and Adolescent Mothers
Between June and December 2013, 2521 women attending 6-week and 9-month infant immunization visits at 120 clinics were enrolled in the primary national survey. Among the women in the primary survey, 278 (12.8%) were adolescents, aged 19 years or younger. Table 1 presents the sociodemographic characteristics of women in the study. Compared with adults, adolescents had lower rates of employment (16.5% for adolescents vs. 37.9% for adults, P < 0.001); less partnership stability (66.1% of adolescents vs. 88.3% of adults were married, P < 0.001); and less experience of IPV (5.1% vs. 10.2%, P = 0.04). More adolescent mothers reported that their most recent pregnancy was their first (77.8% vs. 27.4%, P < 0.001) and fewer were intended (40.5% vs. 58.6%, P < 0.001). Fewer adolescents had disclosed their HIV status (either positive or negative) to their current intimate partner or to their most recent partner if they did not currently have one (77.5% vs. 90.7%, P < 0.001).
TABLE 1.
Characteristics of Adult and Adolescent Mothers
| n (Weighted %) or Weighted Mean (95% CI) | n (%) or Mean (95% CI) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| All Women, Primary Survey | HIV+ Women, Primary and Secondary Surveys | |||||
|
|
|
|||||
| Adolescent (n = 278) | Adult (n = 2243) | P | Adolescent (n = 21) | Adult (n = 477) | P | |
| Age, yrs | 17.9 (17.7 to 18.1) | 26.7 (26.4 to 27.1) | 18.2 (17.9 to 18.5) | 28.6 (28.2 to 29.1) | ||
| Employed | 43 (16.5) | 887 (37.9) | <0.001 | 3 (14.3) | 204 (42.8) | 0.02 |
| Crowding | 173 (58.6) | 1330 (56.6) | 0.66 | 13 (61.9) | 210 (44.0) | 0.13 |
| Depression | 22 (8.6) | 243 (11.3) | 0.28 | 0 (0.0) | 116 (24.3) | 0.02 |
| IPV | 9 (5.1) | 189 (10.2) | 0.04 | 3 (16.7) | 84 (18.5) | 0.84 |
| Partnership | ||||||
| No partner | 81 (26.9) | 206 (9.1) | <0.001 | 8 (38.1) | 70 (14.7) | 0.002 |
| Unmarried | 22 (7.0) | 47 (2.5) | 0.007 | 0 (0.0) | 11 (2.3) | 0.44 |
| Married/cohabiting | 175 (66.1) | 1990 (88.3) | <0.001 | 13 (61.9) | 396 (83.0) | 0.007 |
| One or both parents deceased | 84 (30.6) | 859 (37.8) | 0.06 | 12 (57.1) | 261 (54.8) | 0.84 |
| Primigravida | 220 (77.8) | 682 (27.4) | <0.001 | 11 (52.4) | 50 (10.5) | <0.001 |
| Pregnancy intended | 108 (40.5) | 1362 (58.6) | <0.001 | 12 (57.1) | 261 (54.7) | 0.90 |
| Facility delivery | 207 (73.6) | 1745 (72.9) | 0.86 | 16 (76.2) | 395 (82.8) | 0.41 |
| HIV status disclosed | 207 (77.5) | 2022 (90.7) | <0.001 | 15 (71.4) | 396 (83.0) | 0.15 |
| HIV status | ||||||
| Negative | 257 (97.2) | 2017 (92.2) | 0.003 | |||
| Positive pre-pregnancy | 1 (0.4) | 113 (4.8) | 0.002 | 6 (28.6) | 300 (62.9) | <0.001 |
| Positive in pregnancy | 9 (2.4) | 77 (3.0) | 0.55 | 15 (71.4) | 177 (37.1) | <0.001 |
Bold type highlights P values > 0.05.
A total of 498 HIV-positive women were sampled across the primary national and secondary Nyanza oversample surveys (200 women in the primary survey and 298 in the secondary). Of these, 21 (4.2%) were adolescents. Comparison of HIV-positive adolescents and adults showed similar trends to those in the primary survey, with the following exceptions. Fewer HIV-positive adolescents than adults reported symptoms of at least mild depression (0.0% vs. 24.3%, P = 0.02), the proportion of HIV-positive adolescents reporting IPV did not differ from their adult counterparts (16.7% vs. 18.5%) and the proportion of intended pregnancies did not differ between HIV-positive adolescents and adults (57.1% vs. 54.7%).
Lower Adolescent Engagement in the PMTCT Cascade
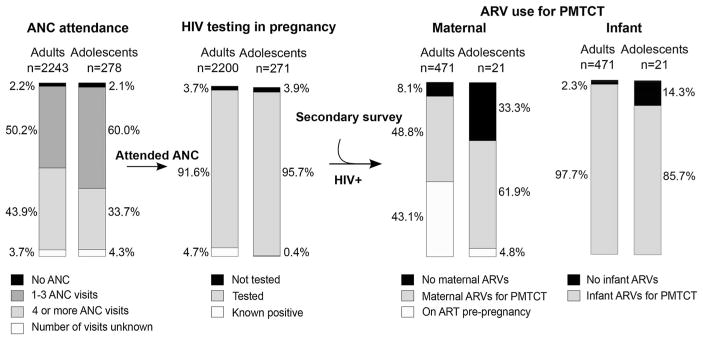
Figure 1 summarizes the proportion of adult and adolescent mothers engaged in each step of the PMTCT cascade. Among all participants in the primary survey (HIV-positive and negative), almost all adults and adolescents reported attending at least one ANC visit (97.8% and 97.9% respectively). The mean gestational age at first ANC was 23.4 week [95% confidence interval (CI) 22.9 to 23.8 weeks] and did not differ significantly between adolescents and adults (23.8 vs. 23.3 weeks, P = 0.48). However, the proportion of women attending at least 4 ANC visits, as recommended by national guidelines, was significantly lower in adolescents than adults (35.2% vs. 45.6%, P = 0.002). Logistic regression analysis evaluating the association between engagement in each step of the PMTCT cascade and adolescent (vs. adult) status is summarized in Table 2. Being an adolescent was associated with a significantly lower likelihood of attending 4 or more ANC visits [odds ratio (OR) 0.64 (95% CI: 0.49 to 0.85), P = 0.002]. In addition to being an adolescent, a number of other covariates showed significant associations with ANC attendance in univariable analysis (summarized in Supplemental Digital Content, Table 1, http://links.lww.com/QAI/A918). In a multivariable model adjusted for employment, household crowding, pregnancy intention, gravidity, and HIV status, being an adolescent remained a significant predictor of lower attendance of complete ANC [adjusted OR (aOR) 0.54 (95% CI: 0.37 to 0.79), P = 0.001].
FIGURE 1.
Comparison of adult and adolescent engagement in the PMTCT cascade. The proportion of adult and adolescent women engaged and not engaged in each step of the PMTCT cascade is displayed. All women in the primary national survey (HIV positive and HIV negative) are included in the ANC attendance step; all women in the primary national survey who attended any ANC are included in the HIV testing step; all HIV-positive women in the primary and Nyanza oversample surveys who attended any ANC are included in the ARV use steps. Proportions displayed for analyses of the primary survey are weighted for survey design.
TABLE 2.
Association Between Adolescent Age and Engagement in the PMTCT Cascade
| Attended ≥4 ANC* | Received HIV Testing† | Used Maternal ARVs for PMTCT‡ | Used Infant ARVs for PMTCT§ | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Univariable | 0.64 (0.49 to 0.85) | 0.002 | 0.95 (0.19 to 4.71) | 0.95 | 0.31 (0.12 to 0.80) | 0.02 | 0.15 (0.03 to 0.65) | 0.01 |
| Multivariable|| | 0.54 (0.37 to 0.79) | 0.001 | 1.38 (0.23 to 8.36) | 0.72 | 0.32 (0.11 to 0.92) | 0.03 | 0.21 (0.03 to 1.52) | 0.12 |
Among women in primary survey with known number of ANC visits. Multivariable adjusted for employment, crowding, pregnancy intention, gravidity, HIV status, and attendance ≥4 ANC visits.
Women in primary survey ≥1 ANC visit and not known HIV+ pre-pregnancy. Adjusted for IPV, depression, and pregnancy intention. Multivariable adjusted for IPV, depression, and pregnancy intention.
Among HIV+ women in primary and secondary surveys who attended ≥1 ANC visit and were not on ART before pregnancy. Multivariable adjusted for HIV status disclosure, marital status, and attendance ≥4 ANC visits.
Among HIV+ women in primary and secondary surveys who attended ≥1 ANC visit. Multivariable adjusted for timing of HIV diagnosis, HIV status disclosure, facility delivery, and attendance ≥4 ANC visits.
Multivariable analyses include all variables showing an effect in univariate analysis at a significance of P < 0.1.
Bold type highlights P values > 0.05.
Among women who attended at least one ANC visit, almost all were either tested for HIV in pregnancy if their status was negative or unknown (95.7% of adolescents vs. 91.6% of adults) or they already knew they were HIV positive before pregnancy (0.4% vs. 4.7%). There were no significant differences between adults and adolescents in coverage of HIV testing in pregnancy [OR 0.95 (95% CI: 0.19 to 4.71), P = 0.95].
ARV use for PMTCT was evaluated in all HIV-positive women attending at least one ANC visit, from the 2 surveys combined. A lower proportion of adolescent women were on ART for their own health before pregnancy than adult women (4.8% vs. 43.1%, respectively). Of the women who were not on ART pre-pregnancy (n = 288), 85.8% of adults but only 65.0% of adolescents took ARVs for PMTCT (P = 0.01 for the comparison). Adolescents had one-third odds of using maternal ARVs for PMTCT compared with adults [OR 0.31 (95% CI: 0.12 to 0.80), P = 0.02]. In a model adjusted for marital status, HIV status disclosure and attendance of at least 4 ANC visits, being an adolescent remained a significant predictor of lower maternal ARV use [aOR 0.32 (95% CI: 0.11 to 0.92)] (see Table 2 and Supplemental Digital Content, Table S1, http://links.lww.com/QAI/A918). The most common reasons women gave for not using ARVs for PMTCT were not receiving them from the health care provider (52.6% of adults who had not received ARVs and 57.1% of adolescents who had not received ARVs), and being diagnosed with HIV late in pregnancy or after delivery (18.4% of adults and 42.9% of adolescents).
Among HIV-positive women who attended at least one ANC visit (Fig. 1), 97.7% of infants born to adult women received ARVs for PMTCT compared with 85.7% of infants born to adolescent women (P = 0.005). Being an adolescent was negatively associated with use of infant ARVs [OR 0.15 (95% CI: 0.03 to 0.65), P = 0.01]. In a model adjusted for timing of HIV diagnosis, HIV status disclosure, and facility delivery, a trend persisted for the association between being an adolescent and infant ARV use [aOR 0.21 (95% CI: 0.03 to 1.52), P = 0.12] (see Table 2 and Supplemental Digital Content, Table S1, http://links.lww.com/QAI/A918). Notably, infant ARV uptake in both adults and adolescents was higher than maternal ARV uptake. The most common reasons that women gave for their infants not using ARVs were not receiving them from their health care provider (18.2% of adults, 66.6% of adolescents), being diagnosed with HIV after delivery (27.3% of adults, 33.3% of adolescents) and not disclosing their HIV status (27.3% of adults, not cited by adolescents).
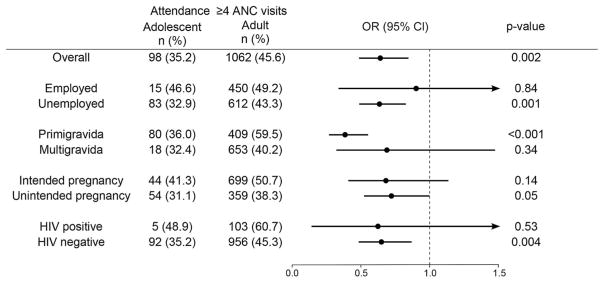
As an additional assessment of possible mediation or modification of the effect of being an adolescent on engagement in the PMTCT cascade by sociodemographic characteristics of adolescent women, analyses were stratified by characteristics that differed between adolescents and adults and showed a significant association with our outcome of interest. Figure 2 summarizes the association between being an adolescent and attendance of 4 or more ANC visits, stratified by employment, gravidity, pregnancy intention, and HIV status. In most strata, the association between being an adolescent and incomplete ANC attendance persisted, although CIs were large in analyses of employed women, women who were not in their first pregnancy, and HIV-positive women. These were the smallest strata, with fewer than 20 observations in the adolescent group. Given the small number of HIV-positive adolescents in our study (n = 21), we did not have sufficient power to conduct stratified analyses of maternal or infant ARV uptake.
FIGURE 2.
Stratified analysis of association between adolescent age and attendance of complete ANC. The proportion and odds ratio of adolescent attendance of ≥4 ANC visits are displayed overall and in the subgroups listed. Arrow heads signify that the confidence interval extends beyond 1.5.
Correlates of ANC Attendance Within Adolescents
To explore the adolescent-specific correlates of engagement in the PMTCT cascade, characteristics of adolescents who were engaged in the PMTCT cascade were compared with those who were not. Given the small sample size of HIV-positive adolescents, ANC attendance among adolescents of all HIV statuses was evaluated. Table 3 summarizes correlates of adolescent attendance of 4 or more ANC visits. Although 74.3% of adolescents who attended fewer than 4 ANC visits had disclosed their HIV status to their partner, 82.8% of adolescents who attended at least 4 ANC visits had disclosed. In logistic regression, disclosure had a borderline association with attendance of at least 4 ANC visits [OR 1.67 (95% CI: 0.98 to 2.84), P = 0.06]. Similarly, although 63.3% of adolescents who attended fewer than 4 ANC visits were married, 71.8% of adolescents who attended at least 4 ANC visits were married, and this also had a borderline association by logistic regression [OR 1.66 (95% CI: 0.91 to 3.04), P = 0.10].
TABLE 3.
Correlates of Complete ANC Attendance Among Adolescents
| ≥4 ANC visits (n = 98), n (%) | <4 ANC visits (n = 169), n (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Employed | 15 (21.9) | 25 (13.6) | 1.79 (0.64 to 5.04) | 0.27 |
| Crowding (≥3 people/room) | 40 (38.6) | 66 (35.11) | 1.16 (0.63 to 2.13) | 0.62 |
| Partnership | ||||
| No partner | 24 (21.5) | 55 (30.6) | Referent | |
| Unmarried | 7 (6.7) | 14 (6.8) | 1.39 (0.36 to 5.46) | 0.63 |
| Married/cohabiting | 67 (71.8) | 101 (63.3) | 1.66 (0.91 to 3.04) | 0.10 |
| Intimate partner violence | 5 (7.5) | 4 (4.1) | 1.98 (0.34 to 11.43) | 0.44 |
| Depression | 10 (10.1) | 10 (6.7) | 1.56 (0.46 to 5.30) | 0.47 |
| Primigravida | 80 (79.2) | 132 (76.4) | 1.18 (0.53 to 2.62) | 0.69 |
| Pregnancy intended | 44 (46.9) | 60 (36.2) | 1.56 (0.81 to 2.98) | 0.18 |
| HIV status | ||||
| Negative | 92 (96.0) | 155 (97.7) | Referent | |
| Positive pre-pregnancy | 1 (1.2) | 0 (0.0) | — | |
| Positive in pregnancy | 4 (2.8) | 5 (2.3) | 1.24 (0.27 to 5.73) | 0.79 |
| HIV status disclosed | 80 (82.8) | 118 (74.3) | 1.67 (0.98 to 2.85) | 0.06 |
DISCUSSION
Pregnant adolescent women represent a vulnerable group with respect to HIV incidence, HIV testing coverage, and HIV-related mortality.4,21 Integrated PMTCT services in routine antenatal care present a crucial opportunity to identify young HIV-infected pregnant women and link them to HIV care and PMTCT services.
In this study, we evaluated engagement of adolescent and adult women in the PMTCT cascade, using data from a national evaluation of the Kenyan PMTCT program. We found that 12.8% of women of any HIV status and 4.2% of HIV-positive women were 19 years old or younger, and these adolescent women showed lower engagement at 3 steps in the PMTCT cascade: attendance of 4 or more ANC visits by women of any HIV status, use of maternal ARVs by HIV-positive women, and use of infant ARVs by HIV-positive women. We found that attendance of at least 4 ANC visits was significantly associated with maternal and infant ARV uptake (Supplemental Digital Content, Table S1, http://links.lww.com/QAI/A918), highlighting the interrelatedness of the steps in the PMTCT cascade.
Our findings are consistent with previous studies reporting lower PMTCT coverage in adolescent mothers in other African contexts.15–17,22 Furthermore, our analysis of individual steps in the PMTCT cascade helps define the points at which adolescents are underserved. We found no significant difference between adults and adolescents in attendance of at least one ANC visit, gestational age at first ANC, or HIV testing in pregnancy. This suggests that initial presentation at ANC and HIV testing is similar across age groups, but adolescents show lower retention in ANC irrespective of HIV status, and HIV-positive adolescents show lower uptake of ARVs for PMTCT. Echoing the calls for improved HIV testing and treatment of adolescents,23 these findings argue that adolescents face unique challenges in uptake of PMTCT services, specifically in returning for the recommended number of ANC visits and using ARVs for PMTCT.
Our findings suggest that maternal age alone, a characteristic routinely ascertained in clinical care, can act as an indicator of risk and need for targeted support. Furthermore, we observed that >70% of HIV-positive adolescents were diagnosed during pregnancy, highlighting the importance of antenatal care as an opportunity to identify HIV-positive women in this group and link them to prevention and care.
Development of interventions that improve adolescent engagement in the PMTCT cascade requires an understanding of the barriers experienced by adolescent pregnant women. Our analysis offers some insight into factors associated with adolescent engagement. In multivariable and stratified analyses, we found that being an adolescent remained significantly associated with incomplete ANC attendance independent of employment, household crowding, pregnancy intention, gravidity and HIV status. This suggests that lower attendance by adolescents is not explained by differences in the other maternal predictors of attendance we identified. Similarly, maternal ARV use was independently associated with being an adolescent in multivariable analysis adjusted for marital status and HIV status disclosure, suggesting lower uptake of ARVs by adolescents was not explained by these characteristics. The association of being an adolescent with infant ARV showed borderline statistical significance in multivariable analysis, suggesting it may partly be explained by differences in HIV status disclosure or facility delivery between the groups (although this may also be due to limited statistical power).
A number of factors beyond the demographic and obstetric characteristics that we measured may be responsible for our observation of lower adolescent engagement in the PMTCT cascade. When asked why they had not received maternal or infant ARVs, both adult and adolescent women reported not being prescribed ARVs by their provider and being diagnosed with HIV late, suggesting health system factors may be at play. Our sample size was too small for a formal comparison of reasons between age groups. Previous studies examining PMTCT uptake in women of all ages have suggested both individual level and systemic facilitators and barriers (reviewed in Refs. 22,24,25). Facilitators include knowledge of HIV and PMTCT, skills to manage practical demands of taking medication and access to social support; barriers include transport to clinic, stigma, and negative interactions with health care workers. Few studies have investigated adolescent-specific barriers to ANC and PMTCT uptake; those that have report negative experiences with clinic staff26 and stigma related to both HIV status and adolescent pregnancy.27 Several of the barriers experienced by all women (transport, lack of HIV knowledge, lack of skills managing medication, and lack of social support) are likely heightened in younger women due to their psychological development, limited experience, and diminished power and agency. Interestingly, although we observed a disparity between adults and adolescents in uptake of both maternal and infant ARVs, levels of infant ARV use were overall higher than maternal ARV use, suggesting that barriers to maternal ARV use may be especially challenging.
Our analysis of the correlates of ANC attendance within adolescent women provides support for the role of social support in enabling PMTCT uptake. We observed a trend for adolescents who had disclosed their HIV status to their partner (whether positive or negative) and married adolescents being more likely to attend 4 or more ANC visits. Our statistical power was limited for this analysis, and our sample size too small to explore correlates of ARV use in HIV-positive adolescent women. However, these findings suggest that supportive partnerships in which women feel comfortable disclosing their HIV status act as an enabler to ANC attendance in adolescents. Of note, in analysis of the overall cohort, marital status was not significantly associated with ANC attendance, pointing to partner support as being especially important in adolescent women. Interventions that supplement adolescents’ support structures, such as peer mentoring28 or mHealth29 approaches, may be promising for this group. Although not evaluated in our study, it is possible that as previously reported,26 negative interactions with clinic staff contributed to adolescents’ lower PMTCT uptake in our study. Addressing this barrier may be an important step in improving adolescent antenatal care. Strategies proposed to achieve this include training staff to interact sensitively with adolescent patients, allowing more time for appointments with adolescents, and consulting adolescents in the design of youth-friendly services.30,31
Our findings must be interpreted in light of the study’s limitations. Although this study was large and sampled 7 of 8 provinces in Kenya, the study was not designed for comparison of adult and adolescent mothers, so statistical power was limited for some of our analyses, in particular those restricted to HIV-positive women. Second, the data presented here are derived from self-reported responses to survey questions. However, women’s responses were verified in their MCH booklet if available, and analyses restricted to verified data showed similar results. More than 75% of women’s reported ANC attendance and maternal ARV use, but only 50% of reported infant ARV use, could be confirmed in their booklet. The findings related to infant ARV use should therefore be interpreted with some caution. Third, we collected data only on those women who presented for their infants’ vaccination visits, thus excluding women who fell out of the system completely. These women are likely younger and have lower rates of ANC attendance,32 so their inclusion might further strengthen the association reported here.
In conclusion, this study highlights the need for improved engagement of adolescent pregnant women in antenatal care and the PMTCT cascade, and calls for more detailed studies on challenges faced by these women to inform interventions that help overcome them.
Supplementary Material
Acknowledgments
Supported by President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of COAG#U2GPS002047, and the National Institutes of Health (T32 CA080416 to K.R., K24 HD054314 and P30 AI027757 to G.J.S., T32 AI007140 and K12HD052023 to C.J.M.), as well as the University of Washington Global Center for Integrated Health of Women Adolescents and Children.
The authors thank the women who participated in the study and the study staff.
Footnotes
Presented in part at the 8th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, July 19–22, 2015, Vancouver, BC, and at the 7th International Workshop on HIV Pediatrics, July 17–18, 2015, Vancouver, BC.
The authors have no conflicts of interest to disclose.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention or the Government of Kenya.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
References
- 1.Kenya National Bureau of Statistics. [Accessed August 15, 2015];Demographic and health survey: key indicators 2014. 2015 Available at: http://dhsprogram.com/pubs/pdf/PR55/PR55.pdf.
- 2.World Health Organization. [Accessed May 14, 2015];Early marriages, adolescent and young pregnancies. 2012 Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_13-en.pdf?ua=1.
- 3.UNAIDS. [Accessed May 14, 2015];The gap report. 2014 Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf.
- 4.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(suppl 2):S144–S153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 5.Philbin MM, Tanner AE, DuVal A, et al. Factors affecting linkage to care and engagement in care for newly diagnosed HIV-positive adolescents within fifteen adolescent medicine clinics in the United States. AIDS Behav. 2014;18:1501–1510. doi: 10.1007/s10461-013-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SH, Gerver SM, Fidler S, et al. Adherence to antiretroviral therapy in adolescents living with HIV. AIDS. 2014;28:1945–1956. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults–seven African countries, 2004–2013. MMWR Morb Mortal Wkly Rep. 2014;63:1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 8.Kasedde S, Luo C, McClure C, et al. Reducing HIV and AIDS in adolescents: opportunities and challenges. Curr HIV/AIDS Rep. 2013;10:159–168. doi: 10.1007/s11904-013-0159-7. [DOI] [PubMed] [Google Scholar]
- 9.Stringer E. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. 2008;86:57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. [Accessed July 27, 2015];2011 :1–48. Available at: http://www.unaids.org/sites/default/files/media_asset/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en_1.pdf.
- 11.Gourlay A, Wringe A, Todd J, et al. Factors associated with uptake of services to prevent mother-to-child transmission of HIV in a community cohort in rural Tanzania. Sex Transm Infect. 2015;91:520–527. doi: 10.1136/sextrans-2014-051907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirsten I, Sewangi J, Kunz A, et al. Adherence to combination prophylaxis for prevention of mother-to-child-transmission of HIV in Tanzania. PLoS One. 2011;6:e21020. doi: 10.1371/journal.pone.0021020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barigye H, Levin J, Maher D, et al. Operational evaluation of a service for prevention of mother-to-child transmission of HIV in rural Uganda: barriers to uptake of single-dose nevirapine and the role of birth reporting. Trop Med Int Health. 2010;15:1163–1171. doi: 10.1111/j.1365-3156.2010.02609.x. [DOI] [PubMed] [Google Scholar]
- 14.Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;19:1360–1366. doi: 10.1111/tmi.12369. [DOI] [PubMed] [Google Scholar]
- 15.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 16.Horwood C, Butler LM, Haskins L, et al. HIV-infected adolescent mothers and their infants: Low coverage of HIV services and high risk of HIV transmission in KwaZulu-Natal, South Africa. PLoS One. 2013;8:e74568. doi: 10.1371/journal.pone.0074568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatti G, Shaikh N, Eley B, et al. Adolescent and young pregnant women at increased risk of mother-to-child transmission of HIV and poorer maternal and infant health outcomes: a cohort study at public facilities in the Nelson Mandela Bay Metropolitan district, Eastern Cape, South Africa. S Afr Med J. 2014;104:874. doi: 10.7196/samj.8207. [DOI] [PubMed] [Google Scholar]
- 18.World health Organization. [Accessed September 6, 2015];Adolescent health. 2015 Available at: http://www.who.int/topics/adolescent_health/en/
- 19.Sherin KM, Sinacore JM, Li XQ, et al. HITS. A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508–512. [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake AL, Wagner A, Richardson B, et al. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001608. doi: 10.1371/journal.pmed.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.hlarlaithe MO, Grede N, de Pee S, et al. Economic and social factors are some of the most common barriers preventing women from accessing maternal and newborn child health (MNCH) and prevention of mother-to-child transmission (PMTCT) services: a literature review. AIDS Behav. 2014;18:516–530. doi: 10.1007/s10461-014-0756-5. [DOI] [PubMed] [Google Scholar]
- 23.Kasedde S, Kapogiannis BG, McClure C, et al. Executive summary: opportunities for action and impact to address HIV and AIDS in adolescents. J Acquir Immune Defic Syndr. 2014;66(suppl 2):S139–S143. doi: 10.1097/QAI.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 24.Gourlay A, Birdthistle I, Mburu G, et al. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodgson I, Plummer ML, Konopka SN, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS One. 2014;9:e111421. doi: 10.1371/journal.pone.0111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varga C, Brookes H. Factors influencing teen mothers’ enrollment and participation in prevention of mother-to-child HIV transmission services in Limpopo province, South Africa. Qual Health Res. 2008;18:786–802. doi: 10.1177/1049732308318449. [DOI] [PubMed] [Google Scholar]
- 27.Hill LM, Maman S, Groves AK, et al. Social support among HIV-positive and HIV-negative adolescents in Umlazi, South Africa: changes in family and partner relationships during pregnancy and the postpartum period. BMC Pregnancy Childbirth. 2015;15:117. doi: 10.1186/s12884-015-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg NE, van Lettow M, Tweya H, et al. Improving PMTCT uptake and retention services through novel approaches in peer-based family-supported care in the clinic and community: a 3-arm cluster randomized trial (PURE Malawi) J Acquir Immune Defic Syndr. 2014;67(suppl 2):S114–S119. doi: 10.1097/QAI.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mwapasa V, Pro G, Chinkhumba J, et al. Mother-infant pair clinic and SMS messaging as innovative strategies for improving access to and retention in eMTCT care and option B+ in Malawi: a cluster randomized control trial (the PRIME study) J Acquir Immune Defic Syndr. 2014;67(suppl 2):S120–S124. doi: 10.1097/QAI.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 30.Rukundo GZ, Abaasa C, Natukunda PB, et al. Antenatal services for pregnant teenagers in Mbarara Municipality, Southwestern Uganda: health workers and community leaders’ views. BMC Pregnancy Childbirth. 2015;15:351–355. doi: 10.1186/s12884-015-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hainsworth G, Hainsworth G, Engel DMC, et al. Scale-up of adolescent contraceptive services: lessons from a 5-country comparative analysis. J Acquir Immune defic Syndr. 2014;66(suppl 2):S200–S208. doi: 10.1097/QAI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 32.Mutua MK, Kimani-Murage E, Ettarh RR. Childhood vaccination in informal urban settlements in Nairobi, Kenya: who gets vaccinated? BMC Public Health. 2011;11:6. doi: 10.1186/1471-2458-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.