Abstract
Pseudocirrhosis is an infrequently reported clinico-radiologic complication that primarily occurs in a subset of patients with a history of breast carcinoma metastatic to the liver that has been treated with systemic chemotherapy, particularly capecitabine, gemcitabine, trastuzumab, and/or paclitaxel. Even less common are cases of pseudocirrhosis secondary to other (i.e., non-breast) carcinomas. We describe a 43-year-old woman with a history of metastatic ovarian carcinoma treated several years prior with systemic chemotherapy who presented with progressive dysphagia and was found to have gastroesophageal junction adenocarcinoma and, incidentally, pseudocirrhosis.
Introduction
Pseudocirrhosis is a rare but important complication of metastatic carcinoma that can closely mimic cirrhosis.1,2 While it has previously been described primarily in patients with breast carcinoma metastatic to the liver, it is vital to consider as a clinical entity in patients with other types of metastatic malignancy to better guide diagnosis and treatment.
Case Report
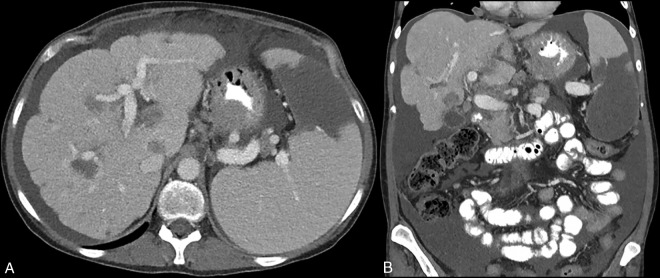
A 43-year old woman with a history of stage 3C serous epithelial ovarian carcinoma status post neoadjuvant chemotherapy (dose-dense carboplatin and paclitaxel) followed by total abdominal hysterectomy, bilateral salpingo-oophorectomy, and post-operative adjuvant chemotherapy was referred with weight loss and progressive dysphagia to solid foods. She had been in clinical remission for 3 years following debulking surgery and chemotherapy completion and was symptom-free up until 2 months prior. Physical examination was unremarkable, and laboratory investigation was notable only for microcytic anemia (11.1 g/dL). On esophagogastroduodenoscopy (EGD), there was an obstructing tumor starting 3 cm proximal to the gastroesophageal junction (GEJ) that had infiltrated into the gastric cardia, as seen on antegrade and retroflexed views (Figure 1); forceps biopsies were positive for carcinoma. Computed tomography demonstrated infiltrating GEJ carcinoma and hepatosplenic lesions concerning for metastases; in addition, the liver appeared segmentally atrophic and had a markedly lobular contour (Figure 2). Targeted liver biopsy revealed metastatic carcinoma without evidence of underlying cirrhosis, which indicated pseudocirrhosis related to prior paclitaxel therapy for advanced ovarian carcinoma as the most likely etiology of the patient’s incidental hepatic findings.
Figure 1.
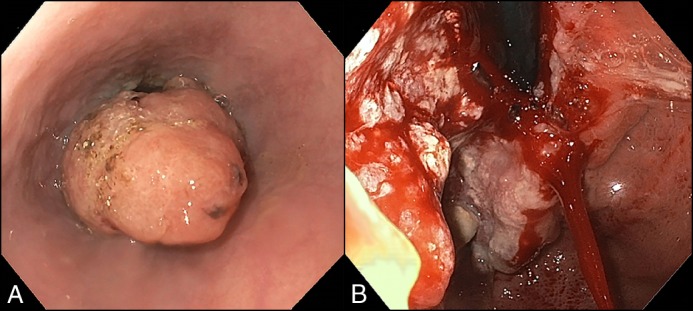
Endoscopic views of the gastroesophageal junction carcinoma. (A) Antegrade view demonstrating an ovular-spheroid mass occupying the majority of the distal esophageal lumen. (B) Retroflexed view revealing an ulcerated, infiltrative mass involving the gastric cardia.
Figure 2.
Computed tomography findings of pseudocirrhosis. (A) Axial view demonstrating markedly lobular hepatic contour as well as infiltrative gastroesophageal junction tumor and hepatosplenic lesions concerning for metastases. (B) Coronal view demonstrating segmental atrophy of the left hepatic lobe, further suggestive of pseudocirrhosis.
Although pseudocirrhosis has not been previously defined in ovarian (or primary GEJ) carcinoma, it is a known rare complication of metastatic (breast) cancer treated with this chemotherapeutic agent. Therefore, in light of this patient’s history of advanced ovarian carcinoma, no further testing or treatment for cirrhosis was pursued, particularly in light of the normal serum biochemistries and the absence of esophageal varices or portal hypertensive gastropathy on EGD or imaging. The patient underwent uneventful palliative endoscopic stenting of the GEJ with a self-expanding metallic stent and was referred to oncology for further care, which was to include carboplatin-based chemotherapy and possibly radiotherapy (Figure 3).
Figure 3.
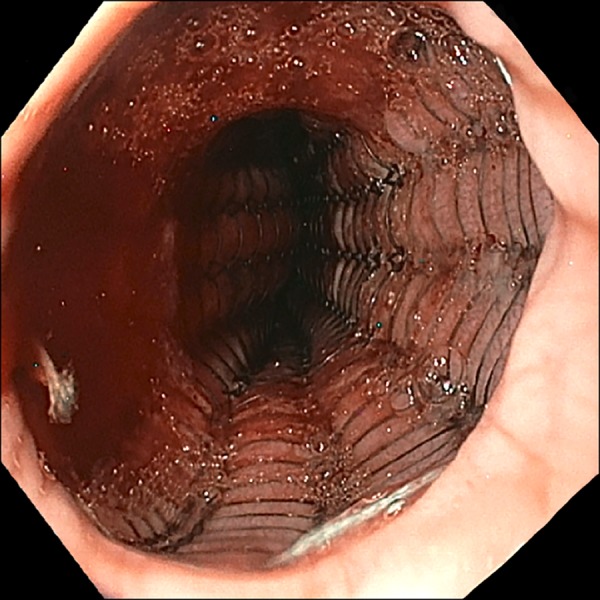
Endoscopic stenting of the gastroesophageal junction lesion using an Ultraflex nitinol 18 mm × 15 cm covered esophageal stent.
Discussion
Pseudocirrhosis was first reported nearly 40 years ago and is the term used to refer to the morphologic changes of the liver that mimic cirrhosis on imaging.1 It is a rare complication seen in patients with a history of advanced carcinoma, predominantly breast with hepatic metastases (micro or macro), and treatment with systemic chemotherapy, in particular capecitabine, gemcitabine, trastuzumab, and paclitaxel.2-5 Although the pathogenesis of pseudocirrhosis is uncertain, it is believed to be related to scarring and hepatic capsular retraction from the response of hepatic lesions to chemotherapy, focal fibrosis surrounding infiltrating hepatic metastases, and nodular regenerative hyperplasia following chemotherapy-induced hepatic ischemia and injury.2-6
To date, approximately 146 cases have been reported (based on a 2018 search of the PubMed database), most commonly in the context of metastatic breast carcinoma.2 To our knowledge, this patient represents the first case of ovarian carcinoma treatment-related pseudocirrhosis whose diagnosis was incidentally detected during workup of the newly discovered GEJ malignancy. As with this patient, most cases of pseudocirrhosis are asymptomatic, but a small proportion may develop pre-sinusoidal portal hypertension.2,6 The diagnosis of pseudocirrhosis is generally made by compatible clinical history, imaging features, and lack of evidence to suggest cirrhosis (e.g., on non-invasive measures or on liver biopsy, which may be performed but is not mandatory). No specific therapy is required for pseudocirrhosis, but in the subset of patients who have evidence of portal hypertension, appropriate screening (e.g., for varices, which our patient did not have on EGD or imaging) and treatment (e.g., diuretic therapy if there is ascites with a high serum-ascites albumin gradient that is not diet-controlled) is advised.2
In conclusion, pseudocirrhosis following chemotherapy for carcinoma metastatic to the liver is an important differential to consider not only in patients with a history of breast cancer, but also other metastatic carcinomas, including ovarian and possibly GEJ.
Disclosures
Author contributions: J. H. Tabibian wrote the manuscript and is the article guarantor. A. Zanazanian and A. Kalani edited the manuscript. A. Zanazanian and J. H. Tabibian provided the images.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Borja ER, Hori JM, Pugh RP. Metastatic carcinomatosis of the liver mimicking cirrhosis: Case report and review of the literature. Cancer. 1975;35:445–49. [DOI] [PubMed] [Google Scholar]
- 2.Adike A, Karlin N, Menias C, Carey EJ. Pseudocirrhosis: A case series and literature review. Case Rep Gastroenterol. 2016;10(2):381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CH, Chao TY. Education and imaging. Hepatobiliary and pancreatic: Pseudocirrhosis after chemotherapy. J Gastroenterol Hepatol. 2011;26(4):788.. [DOI] [PubMed] [Google Scholar]
- 4.Fennessy FM, Mortele KJ, Kluckert T, Gogate A, Ondategui-Parra S, Ros P, Silverman SG. Hepatic capsular retraction in metastatic carcinoma of the breast occurring with increase or decrease in size of subjacent metastasis. AJR Am J Roentgenol. 2004;182:651–55. [DOI] [PubMed] [Google Scholar]
- 5.Jeong WK, Choi S-Y, Kim J. Pseudocirrhosis as a complication after chemotherapy for hepatic metastasis from breast cancer. Clin Mol Hepatol. 2013;19:190–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jüngst C, Krämer J, Schneider G, Lammert F, Zimmer V. Subacute liver failure by pseudocirrhotic metastatic breast cancer infiltration. Ann Hepatol. 2013;12:834–36. [PubMed] [Google Scholar]