SUMMARY
Penicillins and cephalosporins can cause a similar spectrum of allergic reactions at a similar rate.
Cross-reactive allergy between penicillins and cephalosporins is rare, as is cross-reaction within the cephalosporin group. Patients should therefore not be labelled ‘cephalosporin-allergic’.
Cross-reactive allergy may occur between cephalosporins (and penicillins) which share similar side chains.
Generally, a history of a penicillin allergy should not rule out the use of cephalosporins, and a history of a specific cephalosporin allergy should not rule out the use of other cephalosporins.
Specialist advice or further investigations may be required when the index reaction was anaphylaxis or a severe cutaneous adverse reaction, or when the antibiotics in question share common side chains.
When recording a drug allergy in the patient’s records, it is important to identify the specific drug suspected (or confirmed), along with the date and nature of the adverse reaction. Records need to be updated after a negative drug challenge.
Introduction
To label an individual with a ‘cephalosporin allergy’ is misleading. Given the structural diversity of the cephalosporin family, hypersensitivity is seldom a class effect but is much more likely to relate to the individual drug. Cross-reactivity within the family is very limited and is more likely to relate to the side chain than the core structure.1 A greater awareness of this in clinical practice would lead to the availability of alternative cephalosporins and prevent unnecessary use of other classes of broad-spectrum antibiotics.
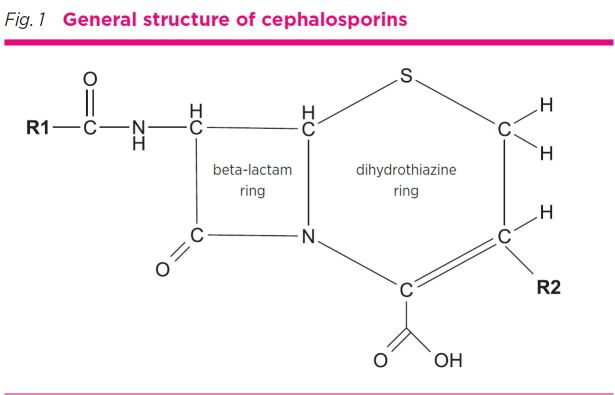
Cephalosporins were first introduced in the 1960s,2 and are one of the most commonly used first-line antibiotics.3 They have a beta-lactam ring linked to a six-member dihydrothiazine ring4 with additional side chains at the R1 and R2 location (Fig. 1). Cephalosporins are commonly classified by their ‘generations’ (first to fifth) which relates to the order of their development (not their efficacy) and has relevance to antibacterial spectrum and beta-lactamase resistance. Their chemical structure tends to become more complex with successive generations. This classification has limited relevance to allergy and allergic cross-reactivity.
Fig. 1.
General structure of cephalosporins
Cephalosporins cause allergic reactions with a similar spectrum and incidence to that of other antibiotics, such as penicillins.5 Reactions include type I hypersensitivity (urticaria, angioedema, anaphylaxis), and type IV hypersensitivity (maculopapular exanthem, severe cutaneous adverse reactions such as Stevens-Johnson syndrome, toxic epidermal necrolysis or acute generalised exanthematous pustulosis or organ hypersensitivity).
Structural chemistry and allergy
Immunological reactivity to small molecules such as antibiotics depends on the formation of haptens. These are stable covalent complexes of the drug with larger carrier molecules such as serum or membrane proteins. For penicillin, this occurs when the beta-lactam ring spontaneously opens to form penicilloyl which binds to lysine residues on host proteins.6
Beta-lactam ring
Cephalosporins and penicillins share the four-atom beta-lactam ring structure. In penicillins the beta-lactam ring is linked to a five-member thiazolidine ring whereas in cephalosporins it is linked to the dihydrothiazine ring (see Fig. 1 and Fig. 2).
Fig. 2.
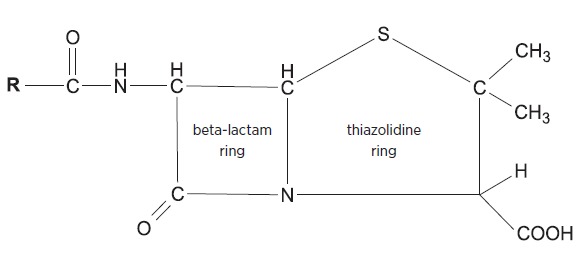
General structure of penicillins
It was previously thought that people allergic to penicillins had a high likelihood of allergy to any cephalosporins (reportedly up to 23.9%).7 More recent studies have demonstrated cross-reactivity rates as low as 1%.8
The common beta-lactam ring is the putative reason for potential cross-reactivity between penicillins and cephalosporins. However, there is in fact little theoretical basis for this. Penicillins are chemically reactive due to a high degree of tension between the beta-lactam ring and the thiazolidine ring, whereas the cephalosporin beta-lactam ring forms a more stable structure with its dihydrothiazine ring. This makes haptenisation of proteins with cephalosporins a slower and less efficient process. Also, when the cephalosporin beta-lactam ring is disrupted to form a cephalosporyl determinant, this structure is unstable and fragments rapidly so it is not antigenic.9
Cross-reactive side chains
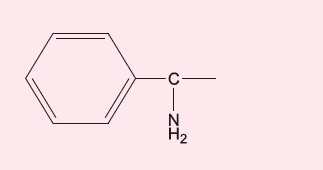
Studies have revealed that the side chains of beta-lactam antibiotics are important antigenic determinants in allergy (Table). For example, if someone reacts to the amino side chain of amoxicillin rather than the beta-lactam core structure, they are likely to have a cross-reactive allergy to ampicillin which shares a very similar side chain, but not to benzylpenicillin or other penicillins.10
Table. Cephalosporins and penicillins grouped by R1 side chain similarity.
| R1 side chain | Cephalosporin | Penicillin |
|---|---|---|
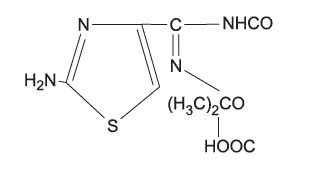
|
cefaclor, cefalexin | ampicillin, amoxicillin |
|
cefoxitin, cefalotin | - |
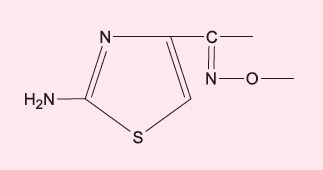
|
cefotaxime, ceftriaxone, cefepime | - |
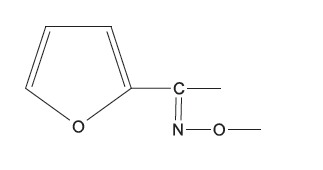
|
cefuroxime | - |
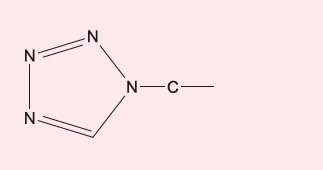
|
cefazolin | - |
|
ceftazidime | aztreonam |
|
ceftaroline | - |
|
cefotetan | - |
Antigenic determinants for cephalosporin hypersensitivity have only recently become better defined. The cephalosporin R2 side chain is usually lost after the opening of the beta-lactam ring, so is less likely to cause allergy (Fig. 1). It is thought that the R1 side chain determines the specificity of immunological reactions to cephalosporins.11 For this reason, cross-reactive allergy across the whole cephalosporin family is seldom if ever seen.
The R1 side chain as an antigenic determinant appears to explain the cross-reactivity that can be seen between certain beta-lactam antibiotics, as well as within the cephalosporin family. For example, aminopenicillins such as ampicillin and amoxicillin have similar R1 side chains to the aminocephalosporins cefalexin and cefaclor, and patients with sensitisation to the amino side chain have a risk of cross-reactive allergy between amoxicillin and cefalexin but can tolerate other (non-amino) penicillins and cephalosporins without this side chain.
Predicting cross-reactivity
Of the cephalosporins currently available in Australia, similar or identical side chains can be found within the same generation, such as in the third-generation cephalosporins cefotaxime and ceftriaxone, or across generations, such as in cefalexin (first generation) and cefaclor (second generation), and in cefalotin (first generation) and cefoxitin (second generation) (Table). However, predicting cross-reactivity among the cephalosporins remains challenging and reactivity may be due to the entire cephalosporin molecule and not just the R1 side chain (Table).1 A special case is the well-known phenomenon of cefaclor serum sickness-like reaction, occurring most commonly in childhood, which is not cross-reactive with other cephalosporins or penicillins (see Box).12-16
Box. Serum sickness-like reactions with cefaclor.
Cefaclor is associated with serum sickness-like reactions in children and sometimes adults. This is characterised by rash, fever, arthralgia, arthritis and lymphadenopathy, but serum complement concentrations are not reduced and immune complexes have not been identified. The mechanism is thought to be due to the genetically determined biotransformation of the drug to produce lymphocytotoxic metabolites.12
Patients who suffer this reaction may acquire a ‘cephalosporin allergy’ label. However, this is incorrect because, although patients may have a recurrence on rechallenge with cefaclor, in vitro studies have shown a lack of cross-reactivity with similar molecules12,13 and patients have been shown to tolerate other cephalosporins.14,15,16
Investigations
Blood tests (immunoassays) for specific IgE antibodies (sIgE) (formerly known as RAST) to penicillin, amoxicillin and cefaclor are available but have very limited sensitivity. The positive predictive value is high but the negative predictive value is low, therefore a negative blood test does not rule out allergy. Tests are not available for the majority of cephalosporins.17 The basophil activation test may have more diagnostic accuracy,18 but is currently only available in research laboratories.19
Skin prick, intradermal (early or delayed) and patch testing are more sensitive than immunoassays, however their negative predictive values are not established due to a lack of sufficiently powered studies.20 Several cephalosporins are not available in a solution suitable for skin testing due to poor solubility, and the diagnostic value of extemporaneously prepared solutions has not been established. Skin-test sensitivity to cephalosporins can decrease over time21 which complicates interpretation. If the skin test is positive to the index drug, then a negative skin test to a related drug might help to exclude cross-reactive allergy. However, this would need to be confirmed by oral or parenteral challenge.
Challenge testing
Challenge testing should only be done at specialist discretion. This involves the deliberate administration of a cephalosporin, usually in graded dosage. It should be carried out under expert supervision in a centre with facilities to manage acute allergic reactions. It is the gold standard test for patients with a history of allergy to a cephalosporin.
Testing with a drug putatively linked to a previous reaction (homologous challenge) is warranted when there is an indication to use the drug, if there is significant uncertainty about the history, or if the reaction occurred in the distant past. In low-risk cases (mild reactions, history suggesting index reaction intolerance rather than allergy), oral rechallenge without prior skin testing can be considered to facilitate delabelling.
A history of a severe delayed-type 4 hypersensitivity reaction (Stevens-Johnson syndrome/toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms) is considered a permanent contraindication to challenge testing since the T-cell immunological memory is likely to persist.22 A history of immediate allergy and even anaphylaxis is not an absolute contraindication to (cautious) challenge since type 1 allergy frequently resolves over several years21,23 and a negative challenge clears the drug for future use.
When the index drug is known, and is found positive on sIgE blood test, skin prick or intradermal testing, then the challenge is done with an alternative cephalosporin with a different R1 side chain (heterologous challenge) as this may show the absence of cross-reactive allergy. In the event of severe anaphylaxis to a specific cephalosporin, the specialist may opt to challenge with an alternative beta-lactam, despite negative in vitro and in vivo testing (Fig. 3). For a patient labelled with ‘cephalosporin allergy’ in which the index cephalosporin is not known, a cautious challenge may be warranted with the cephalosporin that is most likely to be useful.
Fig. 3.
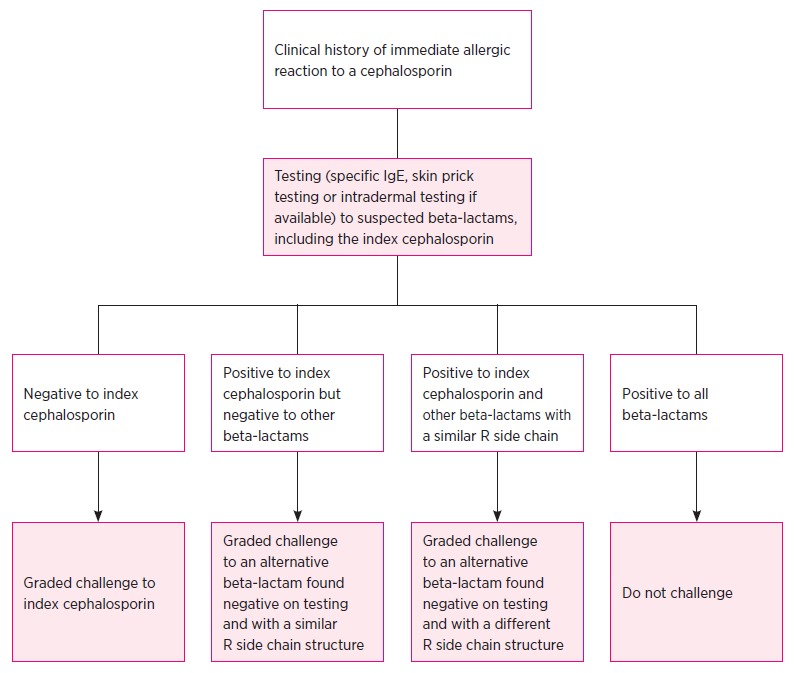
Decision tree for patients with a history of an immediate (anaphylactic) reaction to a cephalosporin
Recording a patient’s allergy
Clinical history is of paramount importance when recording a reaction. This should include the indication for the antibiotic used, comorbidities, and concurrent drugs. A detailed description of the reaction is essential, including the date and the actual name of the drug rather than the family or class of drug. Electronic health records may facilitate recording of such details.
The term ‘cephalosporin allergy’ should not be used. It is inaccurate and indicates a contraindication to the entire class of cephalosporins. Concepts of drug allergy have changed and we now know that such a blanket contraindication is usually inappropriate.
Recommendations
In general:
a history of penicillin allergy should not rule out the use of cephalosporins
a history of allergy to a specific cephalosporin should not rule out the use of other cephalosporins.
Exceptions include when:
the index reaction was anaphylaxis or a severe cutaneous adverse reaction
the antibiotics in question share common side chains.
In these circumstances, specialist advice or investigation is recommended.
Footnotes
CPD - This article has a continuing professional development activity for pharmacists available at https://learn.nps.org.au
Conflict of interest: none declared
REFERENCES
- 1.Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Zaffiro A, Caruso C, et al. IgE-mediated hypersensitivity to cephalosporins: Cross-reactivity and tolerability of alternative cephalosporins. J Allergy Clin Immunol 2015;136:685-691.e3. 10.1016/j.jaci.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 2.Abraham EP. A glimpse of the early history of the cephalosporins. Rev Infect Dis 1979;1:99-105. 10.1093/clinids/1.1.99 [DOI] [PubMed] [Google Scholar]
- 3.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742-50. 10.1016/S1473-3099(14)70780-7 [DOI] [PubMed] [Google Scholar]
- 4.Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol 2010;126:994-9. 10.1016/j.jaci.2010.06.052 [DOI] [PubMed] [Google Scholar]
- 5.Macy E, Poon K-Y T. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med 2009;122:778.e1-7. 10.1016/j.amjmed.2009.01.034 [DOI] [PubMed] [Google Scholar]
- 6.Torres M, Mayorga C, Blanca M. Urticaria and anaphylaxis due to betalactams (penicillins and cephalosporins). In: Pichler WJ, editor. Drug hypersensitivity. Basel: Karger AG; 2007. p. 190-203. [Google Scholar]
- 7.Atanasković-Marković M, Velicković TC, Gavrović-Jankulović M, Vucković O, Nestorović B. Immediate allergic reactions to cephalosporins and penicillins and their cross-reactivity in children. Pediatr Allergy Immunol 2005;16:341-7. 10.1111/j.1399-3038.2005.00280.x [DOI] [PubMed] [Google Scholar]
- 8.Campagna JD, Bond MC, Schabelman E, Hayes BD. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med 2012;42:612-20. 10.1016/j.jemermed.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 9.Ariza A, Mayorga C, Fernandez TD, Barbero N, Martín-Serrano A, Pérez-Sala D, et al. Hypersensitivity reactions to β-lactams: relevance of hapten-protein conjugates. J Investig Allergol Clin Immunol 2015;25:12-25. Available from: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25898690&dopt=Abstract [PubMed] [Google Scholar]
- 10.Pichichero ME, Zagursky R. Penicillin and cephalosporin allergy. Ann Allergy Asthma Immunol 2014;112:404-12. 10.1016/j.anai.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 11.Antunez C, Blanca-Lopez N, Torres MJ, Mayorga C, Perez-Inestrosa E, Montañez MI, et al. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J Allergy Clin Immunol 2006;117:404-10. 10.1016/j.jaci.2005.10.032 [DOI] [PubMed] [Google Scholar]
- 12.Kearns GL, Wheeler JG, Childress SH, Letzig LG. Serum sickness-like reactions to cefaclor: role of hepatic metabolism and individual susceptibility. J Pediatr 1994;125:805-11. 10.1016/S0022-3476(06)80187-3 [DOI] [PubMed] [Google Scholar]
- 13.Kearns GL, Wheeler JG, Rieder MJ, Reid J. Serum sickness-like reaction to cefaclor: lack of in vitro cross-reactivity with loracarbef. Clin Pharmacol Ther 1998;63:686-93. 10.1016/S0009-9236(98)90093-5 [DOI] [PubMed] [Google Scholar]
- 14.King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health 2003;39:677-81. 10.1046/j.1440-1754.2003.00267.x [DOI] [PubMed] [Google Scholar]
- 15.Vial T, Pont J, Pham E, Rabilloud M, Descotes J. Cefaclor-associated serum sickness-like disease: eight cases and review of the literature. Ann Pharmacother 1992;26:910-4. 10.1177/106002809202600708 [DOI] [PubMed] [Google Scholar]
- 16.Reynolds RD. Cefaclor and serum sickness-like reaction. JAMA 1996;276:950-1. 10.1001/jama.1996.03540120028017 [DOI] [PubMed] [Google Scholar]
- 17.Fontaine C, Mayorga C, Bousquet PJ, Arnoux B, Torres MJ, Blanca M, et al. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy 2007;62:47-52. 10.1111/j.1398-9995.2006.01268.x [DOI] [PubMed] [Google Scholar]
- 18.Vareeckal-Joseph S, Le A, Heddle R, Wiese M, Hissaria P. The role of basophil activation test in the diagnosis of type 1 hypersensitivity reaction mediated by betalactam antibiotics. Intern Med J 2016;46 Suppl 4:24. 10.1111/imj.62_13197 [DOI] [Google Scholar]
- 19.Mayorga C, Sanz ML, Gamboa PM, García BE, Caballero MT, García JM, et al. Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC . In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol 2010;20:103-9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20461964 [PubMed] [Google Scholar]
- 20.Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol 2013;45:131-42. 10.1007/s12016-013-8367-x [DOI] [PubMed] [Google Scholar]
- 21.Romano A, Gaeta F, Valluzzi RL, Zaffiro A, Caruso C, Quaratino D. Natural evolution of skin-test sensitivity in patients with IgE-mediated hypersensitivity to cephalosporins. Allergy 2014;69:806-9. 10.1111/all.12390 [DOI] [PubMed] [Google Scholar]
- 22.Pinho A, Marta A, Coutinho I, Gonçalo M. Long-term reproducibility of positive patch test reactions in patients with non-immediate cutaneous adverse drug reactions to antibiotics. Contact Dermat 2017;76:204-9. 10.1111/cod.12720 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg A, Confino-Cohen R. Skin testing and oral penicillin challenge in patients with a history of remote penicillin allergy. Ann Allergy Asthma Immunol 2008;100:37-43. 10.1016/S1081-1206(10)60402-4 [DOI] [PubMed] [Google Scholar]
FURTHER READING
- Katelaris CH, Smith WB. ‘Iodine allergy’ label is misleading. Aust Prescr 2009;32:125-8. 10.18773/austprescr.2009.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Katelaris CH. ‘Sulfur allergy’ label is misleading. Aust Prescr 2008;31:8-10. 10.18773/austprescr.2008.006 [DOI] [PMC free article] [PubMed] [Google Scholar]