Abstract
Epidemiologic studies examining the association between hepatitis C virus (HCV) and chronic kidney disease (CKD) have yielded conflicting findings. Analyzing data from a Taiwanese cohort, Lai and colleagues report a novel finding that the odds of CKD were nearly 3-fold higher in HCV-infected persons with genotype 2 compared with genotype 1. HCV genotype distributions differ in regions around the world. Can genotypic differences in CKD risk explain some of the heterogeneity in prior studies?
Keywords: hepatitis C virus, chronic kidney disease, HCV RNA
The pathophysiology of chronic hepatitis C virus (HCV) infection – immune complexes and cryoglobulinemia, frequent extrahepatic manifestations, and an established link to membranoproliferative glomerulonephritis [1] – provides strong support for HCV as a cause of chronic kidney disease (CKD). However, glomerulonephritis is a relatively uncommon cause of CKD in the general population and the epidemiologic link between HCV and CKD has been debated.
Numerous cross-sectional and longitudinal studies have examined the HCV-CKD association, and most, but not all [2], have reported associations between HCV seropositivity and increased CKD risk. Recent meta-analyses of general-population [3] and HIV-positive [4] cohorts reported 45% and 64% increased risks of developing CKD (stage 3 or higher), respectively, in HCV seropositive versus seronegative individuals.
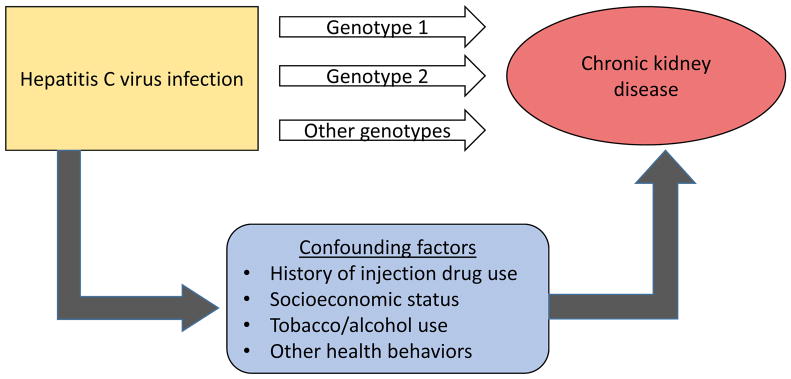
However, there are reasons to suspect that HCV-CKD associations from observational studies may be overstated. Particularly in the US and Europe, HCV infection is strongly linked to injection drug use. Injection of opioids and use of cocaine (by injection or non-injection route) have been plausibly linked to acute kidney injury and CKD. As drug use is illegal and stigmatizing, it is often underreported and difficult to capture accurately. Beyond the direct effects of drug use on the kidneys, drug use is associated with additional socioeconomic and behavioral factors that may contribute to CKD risk. For example, in a large study of US veterans, the relative hazard of ESRD associated with HCV declined from 3-fold in a model adjusting for demographic factors and comorbidity to less than 2-fold when basic socioeconomic factors (marital status, income, adherence with medical care) were added to the model [5]. It is difficult to fully account for the potential confounding effects of drug use and related factors in the observed association between HCV and CKD, particularly in large administrative databases (Figure 1).
Figure.
Disentangling the relationship between hepatitis C virus and chronic kidney disease.
Some groups have attempted to sidestep the issue of socio-behavioral confounding by exploiting the natural phenomenon of spontaneous HCV clearance, which has been strongly linked to IL28B polymorphisms. Approximately 20% of individuals who are infected with HCV spontaneously clear the infection. Individuals who clear an initial HCV infection are also likely to clear subsequent exposures. Such individuals generally remain seropositive for HCV but have undetectable levels of HCV RNA is serum. If one assumes that the mechanisms underpinning spontaneous HCV clearance are not associated with CKD or with socioeconomic and behavioral factors linked to HCV exposure, then HCV seropositive non-viremic individuals (cleared infection) may be the ideal comparison group to determine if chronic HCV infection per se is associated with CKD risk.
This approach has led to conflicting findings. For example, a study that included data from over 40,000 US veterans [6], found that HCV seropositive individuals had a highly statistically significant 30% increased risk of developing CKD. However, among seropositive persons, viremic individuals were at no higher risk compared with non-viremic individuals (adjusted hazard ratio [HR] 0.92; 95% confidence interval [CI] 0.85, 1.00). In contrast, a more recent study that included data from over 1 million US veterans [5], found that, compared with HCV seronegative individuals, viremic persons were at significantly increased risk for developing CKD and ESRD, while HCV seropositive non-viremic individuals were not.
In cohort studies of HIV-positive individuals, similarly conflicting conclusions have emerged regarding the independent association of HCV viremia and CKD. A large North American HIV cohort reported an overall increased risk of CKD among HCV seropositive persons, but no risk difference by HCV viremia status [7]. In contrast, a European HIV cohort [8] and a secondary analysis of data from two international clinical trials [9] reported similar CKD risk in HCV seronegative individuals and HCV seropositive non-viremic individuals, but increased CKD risk in viremic persons, with evidence of a dose-response relationship with HCV RNA magnitude.
In this issue of Kidney International, a manuscript by Lai and colleagues examines the cross-sectional association between HCV and CKD in a Taiwanese cohort of 13,805 middle-aged participants recruited in 1991–1992 [10]. CKD was defined as either estimated glomerular filtration rate <60 mL/min/1.73m2 or grade 1 or higher proteinuria (dipstick test) that was present at both the baseline visit and at a follow-up visit conducted a median of 16 months later. The prevalence of CKD was 3.3% in the sample. Among 660 participants who were HCV seropositive (4.8%), 431 had detectable HCV RNA (65%), consistent with chronic infection. Compared with HCV seronegative participants, the prevalence of CKD was not significantly higher in HCV seropositive non-viremic individuals (odds ratio [OR] 1.32; 95% confidence interval [CI] 0.68, 2.56), but was significantly higher in viremic individuals (OR 1.74; 95% CI 1.08, 2.82). Additionally, the authors found a statistically significant dose-response relationship between HCV RNA magnitude and CKD prevalence. These findings support results from prior studies [5, 8, 9] that suggest HCV infection itself (and not unmeasured confounders) is the principal determinant of the observed association between HCV and CKD.
In a novel finding, Lai and colleagues reported evidence of heterogeneity in CKD associations according to HCV genotype. In participants with genotype data, approximately half were infected with genotype 1 and half were infected with genotype 2. In an analysis including 393 viremic individuals with known genotype, those with genotype-2 had nearly 3-fold higher odds of CKD than those with genotype-1 (OR 2.98; 95% CI 1.77, 5.03).
This finding is intriguing, because it hints at a possible explanation for some of the variability in the existing literature. Most epidemiologic studies examining the association between HCV and CKD were conducted in the US or in East Asia and were based on HCV serostatus, with limited or no data on viremia or genotype. In the US approximately 70% of HCV infections are genotype 1, with only approximately 15% caused by genotype 2. In contrast, genotype 2 is more common in East Asia. A recent meta-analysis [3] reported that the observed association between HCV and CKD in longitudinal studies was substantially stronger in the 4 East Asian studies (HR 1.69; 95% CI 1.44, 1.98) than in the 6 US studies (HR 1.15; 95% CI 1.02, 1.31) that were included in the meta-analysis. Moreover, between-study heterogeneity was higher in the US stratum than in the East Asian stratum.
While the finding of genotypic differences in the association between HCV and CKD reported by Lai and coworkers is provocative, confirmation is needed before fully embracing this result. First, the Lai study was relatively small, with genotype comparisons derived from a sample of fewer than 400 participants. Second, two other studies - albeit also relatively small - found no difference in the associations of genotype 1 and non-genotype-1 HCV with CKD [8, 9]. Finally, a pathophysiologic basis for HCV genotypic difference in CKD remains to be elucidated.
Acknowledgments
Dr. Lucas is supported by the National Institute on Drug Abuse (K24 DA035684 and R01 DA026770) and by the Johns Hopkins University Center for AIDS Research (P30 AI094189). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. The New England journal of medicine. 1993;328(7):465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 2.Asrani SK, Buchanan P, Pinsky B, Rey LR, Schnitzler M, Kanwal F. Lack of association between hepatitis C infection and chronic kidney disease. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2010;8(1):79–84. doi: 10.1016/j.cgh.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Wang P, Yang C, Jiang W, Wei X, Mu X, Li X, Mi J, Tian G. A systematic review and meta-analysis: Does hepatitis C virus infection predispose to the development of chronic kidney disease? Oncotarget. 2017;8(6):10692–10702. doi: 10.18632/oncotarget.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabrizi F, Dixit V, Martin P, Messa P. Hepatitis C virus increases the risk of kidney disease among HIV-positive patients: Systematic review and meta-analysis. Journal of medical virology. 2016;88(3):487–497. doi: 10.1002/jmv.24353. [DOI] [PubMed] [Google Scholar]
- 5.Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, Kovesdy CP. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61(5):1495–1502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butt AA, Wang X, Fried LF. HCV infection and the incidence of CKD. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;57(3):396–402. doi: 10.1053/j.ajkd.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Lucas GM, Jing Y, Sulkowski M, Abraham AG, Estrella MM, Atta MG, Fine DM, Klein MB, Silverberg MJ, Gill MJ, et al. Hepatitis C Viremia and the Risk of Chronic Kidney Disease in HIV-Infected Individuals. The Journal of infectious diseases. 2013;208(8):1240–1249. doi: 10.1093/infdis/jit373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters L, Grint D, Lundgren JD, Rockstroh JK, Soriano V, Reiss P, Grzeszczuk A, Sambatakou H, Mocroft A, Kirk O, et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS. 2012;26(15):1917–1926. doi: 10.1097/QAD.0b013e3283574e71. [DOI] [PubMed] [Google Scholar]
- 9.Mocroft A, Neuhaus J, Peters L, Ryom L, Bickel M, Grint D, Koirala J, Szymczak A, Lundgren J, Ross MJ, et al. Hepatitis B and C co-infection are independent predictors of progressive kidney disease in HIV-positive, antiretroviral-treated adults. Plos One. 2012;7(7):e40245. doi: 10.1371/journal.pone.0040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai TS, Lee MH, Yang HI, et al. Hepatitis C viral load, genotype and increased risk of chronic kidney disease: REVEAL-HCV Study. Kidney International. 2017 doi: 10.1002/hep.29192. XXX(X):XX–XX. [DOI] [PubMed] [Google Scholar]