Abstract
A central feature of major depression (MDD) is heightened negative self‐focused thought (negative‐SFT). Neuroscientific research has identified abnormalities in a network of brain regions in MDD, including brain areas associated with SFT such as medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC). To our knowledge no studies have investigated the behavioral and neural correlates of negative‐SFT using a sentence completion task in a sample of individuals with varying depression histories and severities. We test the following hypotheses: (1) negative‐SFT will be associated with depression; and (2) depression and negative‐SFT will be related to resting‐state functional connectivity (rsFC) for brain regions implicated in SFT. Seventy‐nine women with varying depression histories and severities completed a sentence completion task and underwent resting‐state functional magnetic resonance imaging (rs‐fMRI). Standard seed‐based voxelwise rsFC was conducted for self‐network regions of interest: dorsomedial PFC (dmPFC) and pregenual ACC (pgACC). We performed linear regression analyses to examine the relationships among depression, negative‐SFT, and rsFC for the dmPFC and pgACC. Greater negative‐SFT was associated with depression history and severity. Greater negative‐SFT predicted increased rsFC between dmPFC and pgACC seeds and dorsolateral prefrontal (dlPFC) and parietal regions; depression group was also associated with increased pgACC‐dlPFC connectivity. These findings are consistent with previous literature reporting elevated negative‐SFT thought in MDD. Our rs‐fMRI results provide novel support linking negative‐SFT with increased rsFC between self‐network and frontoparietal network regions across different levels of depression. Broadly, these findings highlight a dimension of social‐affective functioning that may underlie MDD and other psychiatric conditions.
Keywords: cortical midline structures, functional connectivity, major depression, resting‐state fMRI, rumination, self‐focused thought
1. INTRODUCTION
A central feature of major depressive disorder (MDD) is elevated negative self‐focused thought (SFT) often consisting of repetitive thoughts focused on negative aspects about oneself, including feelings of worthlessness and self‐blame (American Psychiatric Association, 2013). Cognitive theories of depression have emphasized that individuals with MDD tend to interpret their experiences with a negative cognitive bias toward the self (Clark & Beck, 1999; Pyszczynski & Greenberg, 1987). Furthermore, studies have frequently associated depression with a bias toward negative‐SFT across several different measures. For example, using well‐validated self‐report questionnaires, greater rumination frequency (Joormann, Dkane, & Gotlib, 2006; Nolen‐Hoeksema, Wisco, & Lyubomirsky, 2008; Siegle, Moore, & Thase, 2004) and higher levels of self‐consciousness in relation to depression severity (Ingram & Smith, 1984; Smith & Greenberg, 1981) have been reported in MDD. When personality trait judgment tasks have been used to assess negative‐SFT in memory, MDD has consistently been associated with enhanced memory for one's own negative personality traits (Baños, Medina, & Pascual, 2001; Bradley & Mathews, 1983; Derry & Kuiper, 1981; Dobson & Shaw, 1987). Similarly, based on sentence completion task performance, individuals with MDD display higher proportions of negative‐SFT responses (Ingram, Lumry, Cruet, & Sieber, 1987). Rumination, a type of negative‐SFT that involves repetitive and often uncontrollable thinking about negative aspects of oneself, has been shown to reliably predict the onset of MDD, the duration of depressive symptoms, and susceptibility to relapse (Figueroa et al., 2015; Nolen‐Hoeksema et al., 2008). Together, these studies suggest that negative‐SFT may be a key social‐affective factor contributing to the development and maintenance of depression.
Neuroscientific research has identified abnormalities in a network of brain regions in MDD, including brain areas associated with SFT, such as the medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC; Berman et al., 2011; Davey, Harrison, Yücel, & Allen, 2012; Drevets, Price, & Furey, 2008; Greicius et al., 2007; Mayberg, 2003; Sheline et al., 2009). Further, task‐based functional neuroimaging studies in MDD suggest that aberrant activity in these self‐related brain regions, in particular the mPFC and ACC, may underlie negative‐SFT in MDD (Cooney, Joormann, Eugène, Dennis, & Gotlib, 2010; Grimm et al., 2009; Johnson, Nolen‐Hoeksema, Mitchell, & Levin, 2009; Lemogne et al., 2009; Nejad, Fossati, & Lemogne, 2013; Yoshimura et al., 2010). For example, using personality trait judgment paradigms, studies have shown that individuals with MDD have greater activity in mPFC and rostral ACC during self‐related thought conditions, especially while considering whether negative personality traits are self‐relevant (e.g., Lemogne et al., 2009; Yoshimura et al., 2010). Beyond task‐based neuroimaging, resting‐state functional connectivity (rsFC) findings have further implicated altered mPFC and ACC connectivity in MDD and negative‐SFT. For instance, depression severity and ruminative thought have been associated with heightened rsFC for the subgenual ACC and pregenual ACC (pgACC; Berman et al., 2011; Greicius et al., 2007; Zhu et al., 2012). Similar to findings in MDD, neuroimaging studies in healthy individuals indicate that negative‐SFT, such as rumination, is also associated with increased mPFC and ACC activity (e.g., Kross, Davidson, Weber, & Ochsner, 2009; Wagner et al., 2013) and connectivity (Berman et al., 2011). Thus, neuroimaging research to date suggests that negative‐SFT may be dimensionally related to connectivity of mPFC and ACC across individuals with and without MDD.
To our knowledge no studies have yet investigated the behavioral and neural correlates of negative‐SFT using a sentence completion task and resting‐state functional magnetic resonance imaging (rs‐fMRI) in a sample of individuals with varying depression histories and severities. In the current study, we tested the hypothesis that negative‐SFT would be associated with depression history and severity. We also investigated the relationships among depression, negative‐SFT, and rsFC for mPFC and ACC self‐related brain regions across the study sample.
2. MATERIALS AND METHODS
2.1. Participants
Participants included seventy‐nine women between the ages of 18 and 45 (mean age = 27.6 ± 7.0). All participants were screened for psychopathology using the Structured Clinical Interview for the DSM‐IV modified to assess DSM‐5 criteria (SCID‐I/P for DSM‐IV‐TR; First, Spitzer, Miriam, & Janet, 2002). Exclusion criteria included: lifetime history of psychosis or mania; current substance use disorder (i.e., within the last 6 months); significant risk for suicide; claustrophobia; daily nicotine use; self‐reported use of antidepressants/other psychotropic medications; hormonal contraceptive use; peri‐ or postmenopausal signs; highly irregular periods; recent pregnancy or breastfeeding (i.e., within the last 6 months); illicit drug use within 4 weeks of participation. Note, all eligible participants self‐reported that they had not used antidepressants or other psychotropic medications within a timeframe based on the half‐life of that particular drug (e.g., had not used fluoxetine for at least 30 days prior to participation). Many of the participants had previously taken antidepressant medications and reported a variety of reasons (e.g., side effects) for not currently taking medication. To confirm no illicit drug use, we performed urine drug tests during three of the seven study visits (diagnostic interview and two fMRI scans). We tested for marijuana, cocaine, opiate, methamphetamine, and amphetamine. We also asked participants about illicit drug use during every study session. It is important to also mention that participants did not receive psychotherapeutic treatment as part of this study nor was psychotherapy an exclusionary criterion.
Of 85 participants who were eligible, 80 completed neuroimaging sessions, with full data available for 79 participants (75% White, 16% Asian, 6% Black). Participants had different levels of depression history forming three separate groups: (1) no history of depression (n = 30; NoDep); (2) history of depression, but not currently depressed (n = 15; PastDep); and (3) currently depressed, meeting the diagnostic criteria for a DSM‐5 Depressive Disorder (n = 34; CurrentDep). Subjects with no history of depression (NoDep group) also had no history of any other psychological conditions, with the exception of one subject who received a diagnosis of Social Phobia in partial remission during the SCID interview. While we did not explicitly recruit women with anxiety disorders, presence of anxiety disorders was not an exclusionary criterion (Table 1). There were no significant group differences in demographic variables, but as expected there were significant differences in depression severity (Table 1).
Table 1.
Depression group characteristics
|
NoDep (n = 30) |
PastDep (n = 15) |
CurrentDep (n = 34) |
|
|---|---|---|---|
| Agea | 27.1 (7.6) | 28.0 (5.8) | 27.9 (7.1) |
| Education Levelb | |||
| High school diploma/equivalent | 0 | 1 | 0 |
| Some college, no degree | 12 | 4 | 10 |
| Associate's degree | 1 | 1 | 1 |
| Bachelor's degree | 7 | 6 | 11 |
| Master's degree | 8 | 3 | 10 |
| Doctoral degree | 2 | 0 | 2 |
| Raceb | |||
| White | 22 | 13 | 25 |
| Asian | 5 | 2 | 6 |
| African American | 3 | 0 | 1 |
| Unknown | 0 | 0 | 2 |
| BDI‐IIc | 0.9 (1.4) | 1.3 (1.8) | 19.5 (10.0)* |
| Depressive Episodesd | |||
| 0–1 | n/a | 5 | 5 |
| 2 | n/a | 4 | 4 |
| 3 | n/a | 3 | 4 |
| 4 | n/a | 2 | 6 |
| 5 | n/a | 0 | 1 |
| 6 | n/a | 0 | 3 |
| 7 | n/a | 0 | 1 |
| 9 or greater | n/a | 1 | 10 |
| Current major depressive episode duratione | n/a | n/a | 24.2 (56.0) |
| Current anxiety disorderf | 1 | 3 | 24 |
| RRSg | 35.8 (8.7) | 46.4 (9.4) | 62.8 (12.8) |
Participant age for the sample ranged from 18 to 45 years.
There were no significant group differences in education level (χ2(10) = 7.48, p > .6) or race (χ2(4) = 3.32, p > .5).
BDI‐II = Beck Depression Inventory‐II; BDI‐II scores ranged from 0 to 49; *expected group differences were found for depression severity (F 2,76 = 73.95, p < .001).
There was no significant difference between depression groups in number of depressive episodes in one's lifetime (χ2(9) = 9.01, p > .4).
Duration of major depressive episode is reported in average months.
Number of participants with a current anxiety disorder diagnosis based on SCID were different between groups (χ2(2) = 33.44, p < .001).
RRS = Ruminative Responses Scale; total RRS scores ranged from 24 to 82.
Participants were recruited from the Madison, WI area through flyers posted on community bulletin boards and websites, email advertisements to University of Wisconsin‐Madison faculty, staff, and students, and advertisements mailed to counseling centers and clinics. All participants provided written informed consent in accordance with the local IRB. Participants were paid for their participation.
2.2. Study procedures
The data reported herein are taken from a larger NIH R01‐funded study investigating the effects of cortisol on cognitive and neural function in depression. In the larger study, all participants took part in seven study visits, including two fMRI scans: one placebo scan and one hydrocortisone scan. Placebo and hydrocortisone administration was double‐blind and randomized across two fMRI scan sessions which were typically one week apart (5–61 days apart). Note: hydrocortisone was not given as a therapeutic agent; instead it was given to test for alterations in neurocognitive response to cortisol (i.e., hydrocortisone). One hour prior to each scan, participants received a pill containing either placebo or 20 mg hydrocortisone (order of drug administration randomized and double‐blind). Data reported here are taken from the placebo day fMRI scan. To account for potential differences due to scan order in the current study, we investigated the relationship between scan order and all behavioral and neural variables of interest.
2.3. Depression measure
Depression severity was measured with the Beck Depression Inventory‐II (BDI‐II) at each visit (Beck et al., 1961). We used the average of BDI‐II scores from the two fMRI scan visits. To reduce negative skew in the distribution of BDI‐II we applied a square root transformation of BDI‐II data (van Minnen, Wessel, Verhaak, & Smeenk, 2005; as in Roelofs et al., 2013). For tables and scatter plots, BDI‐II scores were back‐transformed to accurately reflect the BDI‐II score range.
2.4. Negative‐SFT measure
Negative‐SFT was measured using the sentence completion task (Exner, 1973). For the sentence completion task, 30 different sentence stems were given to participants to complete as they wished (e.g., “I think…”, or “My father…”). Each response was coded for focus and valence (Exner, 1973; Ingram & Smith, 1984). Reliability and validity for the sentence completion task as a measure of SFT has been reported in large samples of non‐psychiatric and psychiatric participants (Exner, 1973).
Sentence completion task responses were coded based on type of focus, including SFT and other‐focused (as in Exner, 1973). SFT responses referred to the self with little regard for other persons, while other‐focused responses discussed the characteristics, mental states, or actions of other people. Total focus scores corresponded to the sum of all responses for each response category. To examine negative‐SFT, each response was also coded in terms of the overall valence: positive, negative, or neutral (e.g., negative‐SFT, positive‐SFT; as in Ingram and Smith, 1984). See Table 2 for examples.
Table 2.
Examples of SCT responses for self‐focus by valence category
| SCT stem | Self‐negative | Self‐positive |
|---|---|---|
| I am | “pathetic.” | “happy with who I am.” |
| Others | “are disappointed in me.” | “think I am always happy and smiling.” |
Two raters trained in sentence completion coding and blind to depression group status coded responses in two steps. First, each rater separately coded responses for focus and valence for all participants. Interrater reliability was calculated for all ratings and adequate reliability was found for all response categories. Specifically, SFT, other‐focused, negative‐SFT, and positive‐SFT responses had intraclass correlation coefficients of .83, .82, .84, .90, respectively, which was within acceptable limits (Exner, 1973). Second, the raters discussed responses for each participant and agreed on final codes for each response (e.g., SFT and negative valence), which were used in the analyses. Based on our hypotheses for the current study and previous research using the same sentence completion task (Ingram et al., 1987; Ingram & Smith, 1984), only the proportion of negative‐SFT responses (number of negative‐SFT responses/total SFT responses) were used.
2.5. Data acquisition
All structural and functional magnetic resonance imaging (MRI) data were acquired using a 3T GE MRI scanner (Discovery MRI 750; GE Medical Systems, Waukesha, WI) equipped with an 8‐channel radio‐frequency coil array (GE Healthcare, Waukesha, WI). High‐resolution T1‐weighted structural images were acquired using a weighted BRAVO pulse sequence (TI: 450ms, TR/TE/flip angle (FA): 8.16 ms/3.2 ms/12°, matrix: 256 × 256 × 160, field of view (FOV): 215.6 mm, slice thickness: 1 mm, voxel size: 1 mm × 1 mm × 1 mm3, slices: 156). rs‐fMRI images were acquired using T2*‐weighted Echo Planar Imaging (EPI) sequence (TR/TE/FA: 2150 ms/22 ms/79°, matrix: 64 × 64, FOV: 22.4 cm, slice thickness: 3.5 mm, voxel size: 3.5 mm × 3.5 mm × 3.5 mm3, slices: 40 sagittal) using thinner slices and shorter echo time in order to minimize signal dropout in ventromedial prefrontal cortex. For the resting‐state scan (∼10 min), the participants were instructed to remain ‘‘calm, still, and awake’’ with their eyes open fixating on a cross back‐projected onto a screen via an LCD projector (Avotec, Stuart, FL).
2.6. Preprocessing and motion analysis for rs‐fMRI data
The rs‐fMRI data were processed using AFNI (Cox, 1996) and FSL (FMRIB Software Library; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). First, a rigid‐body volume registration was implemented to compensate for subjects’ motion (3dvolreg, fourth volume as the base image volume). Next, field map correction was performed using sagittal field maps (collected via a 3D SPGR sequence; TR/TE/FA: 5 ms/1.8 ms/7°, matrix: 192 × 128 × 44, FOV: 230 mm, slice thickness: 3.5 mm) to geometrically unwarp EPIs to reduce distortion caused by magnetic field inhomogeneities (IDEAL sequence; Reeder et al., 2005; and FMRIB Software Library; Woolrich et al., 2009). Next, the following preprocessing steps were performed: (1) slice‐time corrected EPI volumes (3dTshift, using first volume as a reference), (2) omitted first three volumes (3dcalc), (3) aligned EPI data to their respective T1‐weighted anatomical (align_epi_anat.py) and transformed to Talairach atlas space (Talairach and Tournoux, 1988; LPI) in a single interpolation to 2 × 2 × 2 mm3 voxels, (4) the 3D + time series were despiked (3dDespike), and (5) temporally filtered (band‐pass: .009 Hz < f < .08 Hz) and spatially smoothed with a 6‐mm full‐width half‐maximum (FWHM) Gaussian kernel (3dBandPass). Normalized T1 anatomical images were also segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using FAST in FSL (Zhang, Brady, & Smith, 2001) . White matter and CSF segments were used as masks to extract a representative time series from each tissue type.
We also examined motion for each subject, as individual differences in subject motion can contribute to resting‐state correlations (Power, Schlaggar, and Petersen, 2015). Five subjects (NoDep, n = 2; PastDep, n = 2; CurrentDep, n = 1) were excluded based on the following criteria: mean framewise motion displacement >3 mm (i.e., volume to volume movement across the time series), and/or total scan time <4 min after censoring all time points with framewise motion displacement >.2 mm and extreme timeseries displacement (i.e., time points where >10% of voxels were outliers; Power et al., 2015). These thresholds were selected to provide the most conservative criteria for motion correction (Power, Barnes, Snyder, Schlaggar, and Petersen, 2012; Yan et al., 2013). As in previous work (e.g., Ciric et al., 2017), average root‐mean‐squared (RMS) displacement was used as a summary measure of subject motion. Importantly, there were no significant associations between average RMS and depression group (F 2,71 = .53, p > .5), depression severity (r = .08, p > .5), or proportion of negative‐SFT responses (r = −.02, p > .9).
2.7. Functional connectivity analysis
We performed seed‐based voxelwise rsFC analyses (Biswal, Zerrin Yetkin, Haughton, and Hyde, 1995) for two mPFC seed regions of interest (ROIs) implicated in SFT (dmPFC: −2, 38, 16; pgACC: −2, 34, 2; both ROIs coordinates reported in MNI space; Murray, Debbané, Fox, Bzdok, & Eickhoff, 2015). For each participant, the mean resting‐state BOLD time series from each seed ROI was included in a GLM (3dDeconvolve) with nine regressors of no interest: (1–6) six motion parameters (three translations, three rotations) obtained from the rigid body alignment of EPI volumes and their six derivatives, (7) white matter time series, (8) ventricular (CSF) time series, and (9) a second‐order polynomial to model baseline signal and slow drift. To further control for subject motion within the GLM, volumes were censored for excessive motion, as described above.
To create the correlation maps for each seed ROI, we performed the following steps: (1) used GLM output to convert R 2 values to correlation coefficients (r), (2) used Fisher's r‐to‐z transform to convert r to z‐scores and corrected for degrees of freedom (Philippi, Motzkin, Pujara, & Koenigs, 2015). The resulting z‐score maps were then entered into the second‐level statistical analyses.
2.8. Statistical analyses
2.8.1. Behavioral data analyses
We performed two separate analyses, using group‐based and regression approaches, to examine the association between depression history or severity and negative‐SFT in the full sample (n = 79). For the group analysis, we performed one‐way ANOVAs with depression group as the independent variable (NoDep, PastDep, CurrentDep) and proportion of negative‐SFT responses as the dependent variable. For the regression analysis, we regressed the proportion of negative‐SFT responses onto depression severity.
To provide external validation of the relationship between negative‐SFT from the sentence completion task and repetitive thought, we also measured self‐reported rumination using the 22‐item Ruminative Responses Scale (RRS; Treynor, Gonzalez, & Nolen‐Hoeksema, 2003). Examples items from the RRS include: “Go someplace alone to think about your feelings”, “Think, what am I doing to deserve this”, and “Think about how alone you feel”. Participants rated their frequency of each item on a scale ranging from 1 (almost never) to 4 (almost always). The total RRS score corresponded to the sum of all responses. We performed a bivariate correlation between total RRS scores and the proportion of negative‐SFT responses.
2.8.2. rsFC data analyses
To examine the relationships among depression, negative‐SFT, and rsFC, we performed multivariate regression analyses (3dttest++ in AFNI) for dmPFC and pgACC seed ROIs for three separate models: (1) depression group, (2) depression severity, and (3) the proportion of negative‐SFT responses. Age was also included as a covariate in all models, as age was significantly associated with rsFC for dmPFC and pgACC seed ROIs (Supporting Information Table S1). All rsFC analyses were conducted using 74 participants, as five participants were excluded due to excessive motion. To correct for multiple comparisons, we implemented a family‐wise error (FWE) correction approach at the cluster level using a whole‐brain mask (3dClustSim in AFNI version updated August 2016; Carp, 2012; Forman et al., 1995) and applied cluster‐extent thresholding. To address the non‐Gaussian nature of fMRI data (Eklund, Nichols, & Knutsson, 2016), the autocorrelation function (‐acf) was used to calculate the FWHM for each subject (3dFWHMx in AFNI). The cluster‐extent threshold corresponded to the statistical probability ( = .05, or 5% chance) of identifying a random noise cluster at a predefined voxelwise threshold of p < .001 (uncorrected). Using this whole‐brain FWE cluster correction, a cluster‐corrected size of 137 voxels was significant at p FWE < .05 in the rsFC regression analyses. Regression results were overlaid on the normalized mean anatomical image.
To statistically account for potential differences in scan order (i.e., either placebo or hydrocortisone), we also performed follow‐up regression analyses for all significant rsFC results. We first extracted the average z‐score from all significant clusters identified for each subject, and then ran separate regressions (in R) to examine the association between the average z‐score for each significant cluster and the proportion of negative‐SFT responses, after controlling for scan order.
We also conducted supplemental moderation analyses for all significant rsFC results to determine whether depression history or severity moderated the association between negative‐SFT and rsFC. Moderation analyses were completed in SPSS (version 24; SPSS/IBM, Chicago, IL) using the macro PROCESS (Andrew F. Hayes, Ohio State University, Columbus, OH). Separate models with either depression history/depression severity or negative‐SFT as a moderator, were tested for all significant rsFC results: X = negative‐SFT or depression history, Y = average z‐scores from the significant cluster identified in rsFC analyses, and M = depression history/M = depression severity/M = negative‐SFT. To evaluate the significance of the moderation effect, standard non‐parametric bootstrapping procedures were performed with 5,000 samples (as in Hosking et al., 2017). All moderation models also included age as a covariate.
Lastly, we performed mediation analyses for all significant rsFC results from regression models with depression (either depression history or severity) to determine whether negative‐SFT mediated the relationship between depression and rsFC. Mediation analyses were completed in SPSS (version 24; SPSS/IBM, Chicago, IL) using the macro PROCESS (Andrew F. Hayes, Ohio State University, Columbus, OH). Separate models with negative‐SFT as a mediator were tested for all significant rsFC results from regression models with depression: X = depression history/X = depression severity, Y = average z‐scores from the significant cluster identified in rsFC analyses, and M = negative‐SFT. Given that depression history was a multicategorical independent variable (three groups: NoDep, PastDep, CurrentDep), when depression history was in the model we followed the procedures for mediation analyses with multicategorical independent variables (as in Hayes & Preacher, 2014), to examine the relative indirect and direct effects of each depression history group (PastDep or CurrentDep) relative to the control group (NoDep). Similar to the moderation analyses, standard non‐parametric bootstrapping procedures were performed for the mediation analyses with 5,000 samples. All mediation models also included age as a covariate.
3. RESULTS
3.1. Depression and negative‐SFT
Consistent with previous research on depression and negative‐SFT, there was a significant effect of depression group for negative‐SFT (F 2,76 = 6.82, p < .01, η2 = .15), with the CurrentDep group demonstrating a greater proportion of negative‐SFT responses than the NoDep group (t(62) = 3.68, p < .001, d = .93; Table 3). Although the CurrentDep group had more negative‐SFT responses than the PastDep group, the difference between the two groups was marginal (t(47) = 1.60, p = .11, d = .47). On average the PastDep group displayed a greater proportion of negative‐SFT responses than the NoDep group, however, the difference between the groups was not significant (t(43) = 1.34, p = .19, d = .41). Similar to the group analyses, using depression severity as a continuous variable, we found a significant positive relationship between depression severity and the proportion of negative‐SFT responses (B = 6.35, t(77) = 4.43, p < .001, ηp 2 = .20) (Supporting Information Figure S1). Follow‐up analyses revealed no significant correlations between scan order and depression history/severity or negative‐SFT (each p > .6). There was a significant correlation between negative‐SFT and rumination scores (r = .39, p < .01), providing some support for a relationship between negative‐SFT and repetitive thought in the current study.
Table 3.
Negative self‐focused responses
| NoDep (n = 30) | PastDep (n = 15) | CurrentDep (n = 34) | |
|---|---|---|---|
| Negative self‐focusa | .13 (.11) *** | .18 (.13) + | .25 (.15) ** |
| Positive self‐focusa | .33 (.17) | .34 (.20) | .27 (.17)– |
Proportion of negative and positive out of total self‐focused responses; proportion of negative self‐focus ranged between 0 and .57 and proportion of positive self‐focus ranged between 0 and .76.
Hypothesized relationships are in italics. – p > .1, + p = .1, *p < .05, **p < .01, ***p < .001.
3.2. rsFC, depression, and negative‐SFT
In line with prior rsFC studies in MDD, there was a significant association between depression group and rsFC for the pgACC. Depression group was related to increased rsFC between pgACC and dorsolateral prefrontal cortex (dlPFC), postcentral gyrus extending to supplementary motor area (SMA), and superior temporal gyrus (Figure 1); the CurrentDep group revealed significantly greater rsFC than both the PastDep and NoDep groups (Table 4). In contrast to depression group results, there were no significant associations between depression severity and rsFC for the dmPFC or pgACC seed ROIs.
Figure 1.
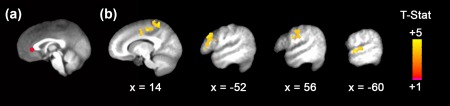
Depression group was associated with connectivity between pgACC and prefrontal, parietal, and temporal cortex. (a) pgACC seed ROI; (b) Images from left to right: depression group was associated with greater connectivity between pgACC and right medial postcentral gyrus (x = 14), left middle frontal gyrus extending to dorsolateral PFC (dlPFC; x = −52); right postcentral gyrus (x = 56), and right superior temporal gyrus (x = –60). The seed ROI and all results are displayed on the group average structural MRI in MNI‐space. All results survived whole‐brain cluster correction (p FWE < .05, p = .001 uncorrected) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Regression results using connectivity for seeds ROIs and depression group
| Seed ROI | Cluster location | MNI coordinates (x, y, z) | Cluster size (voxels) | t Value |
Average connectivitya NoDep (n = 28) |
Average connectivity PastDep (n = 13) |
Average connectivity CurrentDep (n = 33) |
|---|---|---|---|---|---|---|---|
| pgACC | R. postcentral gyrus extending to SMAb | 14, −50, 65 | 1,065 | 4.92 | 0.09 (1.9) | 0.58 (2.5) | 2.76 (2.3)* |
| L. middle frontal gyrus extending to dlPFCb | −53, 22, 34 | 469 | 4.82 | −0.76 (2.2) | −0.27 (2.8) | 2.18 (2.8)* | |
| R. postcentral gyrus | 66, −20, 41 | 443 | 4.60 | 0.10 (2.2) | −0.68 (2.9) | 2.86 (2.5)* | |
| L. superior temporal gyrus (BA 22) | −61, −6, 0 | 153 | 4.77 | 0.60 (3.1) | 0.90 (3.3) | 3.89 (3.1)* |
All regression results were significant after controlling for age (p FWE = .05, uncorrected p = .001).
Average connectivity scores correspond to z‐scores; means and standard deviations are reported for each depression group.
SMA = supplementary motor area, dlPFC = dorsolateral prefrontal cortex.
*The CurrentDep group had significantly greater connectivity than both the PastDep and NoDep groups (each p < .05).
As predicted, negative‐SFT was significantly related to enhanced rsFC for both the dmPFC and pgACC seed ROIs (Table 5). For the dmPFC, greater negative‐SFT was associated with enhanced rsFC with the left inferior parietal lobule (IPL; Figure 2a). Similarly, for the pgACC, greater negative‐SFT was associated with increased rsFC with several regions including the dlPFC, precuneus extending to middle temporal gyrus, inferior parietal cortex, and paracentral lobule extending to SMA (Figure 2b). Importantly, follow‐up analyses indicated that there were no significant effects of scan order for any of the rsFC findings (each p > .6).
Table 5.
Regression results using connectivity for seeds ROIs and negative self‐focus
| Seed ROI | Cluster location | MNI coordinates (x, y, z) | Average raw connectivity value* | Cluster size (voxels) | t Value |
|---|---|---|---|---|---|
| dmPFC | L. inferior parietal lobule | −40, −61, 45 | 1.49 | 414 | 4.79 |
| pgACC | L. precuneus | −16, −60, 49 | 1.74 | 4,429 | 5.71 |
| R. precuneus extending to middle temporal gyrus (BA 39) | 30, −78, 39 | 0.79 | 3,263 | 6.02 | |
| L. middle frontal gyrus extending to dlPFC | −42, 5, 52 | 0.87 | 1,819 | 5.59 | |
| R. middle frontal gyrus extending to dlPFC | 42, 21, 29 | 1.05 | 1,813 | 5.07 | |
| R. paracentral lobule extending to SMA (BA 6) | 4, −34, 59 | 1.47 | 1,160 | 4.79 |
All regression results were significant after controlling for age (p FWE = .05, uncorrected p = .001). *Raw connectivity values correspond to average z‐scores across the sample. dlPFC = dorsolateral prefrontal cortex; SMA = supplementary motor area.
Figure 2.
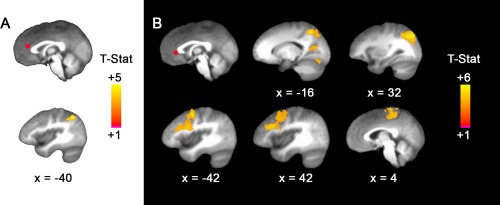
Negative self‐focused thought was associated with connectivity between mPFC regions and parietal and temporal cortex. (a) Top: dmPFC seed ROI; Bottom: Higher proportion of negative self‐focused responses was associated with greater connectivity between dmPFC and left inferior parietal lobule; (b) Top images from left to right: pgACC seed ROI; higher proportion of negative self‐focused responses was associated with greater connectivity between pgACC and left precuneus (x = –16), right precuneus extending to middle temporal gyrus (x = 32); Bottom images from left to right: higher proportion of negative self‐focus was associated with greater connectivity between pgACC and left middle frontal gyrus extending to dorsolateral PFC (dlPFC; x = –42), right middle frontal gyrus extending to dlPFC (x = 42), and right paracentral lobule extending to SMA (BA 6; x = 4). The seed ROIs and all results are displayed on the group average structural MRI in MNI‐space. All results survived whole‐brain cluster correction (p FWE < .05, p = .001 uncorrected) [Color figure can be viewed at http://wileyonlinelibrary.com]
In supplemental moderation analyses, the relationships between depression group and rsFC for the pgACC were not moderated by negative‐SFT (each p > .5). Similarly, the associations between negative‐SFT and rsFC for dmPFC and pgACC were not moderated by either depression group or severity (each p > .2). Finally, in the mediation analyses with depression group as a multicategorical independent variable (Supporting Information Figure S2), negative‐SFT did significantly mediate the relationship between depression group and rsFC for pgACC. Relative to the NoDep group, the CurrentDep group had greater rsFC between pgACC and dlPFC as well as pgACC and postcentral gyrus/SMA as a result of the mediating effect of negative‐SFT on rsFC (Supporting Information Tables S2 and 3). Negative‐SFT was not a significant mediator for the PastDep group (Supporting Information Tables S2–5).
4. DISCUSSION
To our knowledge, this is the first study to use a sentence completion task and rs‐fMRI to examine the behavioral and neural correlates of negative‐SFT in a sample of individuals with varying depression history and severity. Our results supported our hypotheses, demonstrating significant relationships between negative‐SFT and depression, as well as between depression, negative‐SFT, and rsFC of self‐related brain regions. Behaviorally, negative‐SFT varied by depression group, with the currently depressed group exhibiting greater negative‐SFT than the no depression history group. Interestingly, there was no significant difference in negative‐SFT between the currently depressed and past depression groups. In the regression analysis, negative‐SFT was associated with higher depression severity across the sample. Neurally, negative‐SFT was significantly related to increased rsFC between the dmPFC and pgACC seeds and dlPFC and medial and lateral parietal regions. Depression group was also associated with significantly increased rsFC between pgACC and dlPFC, as well as other parietal and temporal regions. For the currently depressed group, negative‐SFT significantly mediated the relationship between depression group and rsFC between pgACC and dlPFC as well as pgACC and postcentral gyrus/SMA. Below, we discuss each of these major findings in turn.
Our observation that currently depressed individuals had greater negative‐SFT than individuals with no depression history closely parallels two previous behavioral studies using the sentence completion task in MDD (Ingram et al., 1987) and subclinical depression (Ingram & Smith, 1984). The present results are also consistent with a large body of clinical and behavioral research linking depression and depression severity with elevated negative‐SFT across different self‐report and behavioral measures (Baños et al., 2001; Bradley & Mathews, 1983; Clark & Beck, 1999; Derry & Kuiper, 1981; Dobson & Shaw, 1987; Ingram & Smith, 1984; Joormann et al., 2006; Nolen‐Hoeksema et al., 2008; Siegle et al., 2004; Smith & Greenberg, 1981). Based on extant research, it has further been proposed that patterns of negative‐SFT, such as rumination or negative cognitive styles, may serve as a vulnerability factor for the development and recurrence of depression (Alloy et al., 2006; Burcusa & Iacono, 2007; McLaughlin & Nolen‐Hoeksema, 2011; Watkins, 2015). Consistent with this hypothesis, in the present study negative‐SFT was elevated in both the current and past depression groups, with no significant difference in levels of negative‐SFT between the two groups. Lastly, we found a significant correlation between negative‐SFT on the sentence completion task and self‐reported rumination, providing some external validation for a relationship between negative‐SFT and repetitive thought (Watkins, 2008).
Regarding the rsFC analyses, negative‐SFT was associated with enhanced connectivity between dmPFC and left IPL, two of the main brain regions of the default mode network (DMN). These results extend prior task‐based and rs‐fMRI studies which associate MDD with hyperactivity and connectivity of the DMN (Kaiser, Andrews‐Hanna, Wager, & Pizzagalli, 2015; Sheline et al., 2009). Based on research implicating the DMN in SFT (Buckner, Andrews‐Hanna, & Schacter, 2008; Gusnard, Akbudak, Shulman, & Raichle, 2001; Qin & Northoff, 2011; Whitfield‐Gabrieli et al., 2011), researchers have proposed that DMN hyperconnectivity may be related to elevated negative‐SFT in depression (Berman et al., 2011; Kaiser et al., 2015; Philippi & Koenigs, 2014; Sheline et al., 2009; Whitfield‐Gabrieli & Ford, 2012). Indeed, previous rs‐fMRI research has identified significant correlations between elevated DMN connectivity and greater rumination in both subclinical and MDD populations (Berman et al., 2011; Hamilton et al., 2011; Zhu et al., 2012), which is consistent with our findings. Note, the left IPL cluster identified in our study also extends into the anterior inferior parietal lobule, which is part of the frontoparietal network (FPN; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008), suggesting that negative‐SFT in depression may also be associated with altered dmPFC‐FPN connectivity.
There is growing evidence for altered connectivity between mPFC regions and dlPFC in MDD. For example, a meta‐analysis of 25 seed‐based rsFC studies in MDD found consistently heightened connectivity between mPFC, including pgACC, and dlPFC in MDD (Kaiser et al., 2015). Similarly, in a task‐based fMRI study, individuals with MDD exhibited greater connectivity between pgACC and dlPFC while engaging in self‐related thought as compared with healthy controls (Lemogne et al., 2009). Together, these studies are consistent with our findings associating current depression and negative‐SFT with greater connectivity between pgACC and dlPFC. Our results may also be relevant to literature reporting dlPFC dysfunction in MDD (Koenigs & Grafman, 2009; Mayberg, 2003). For example, individuals with MDD tend to display abnormal dlPFC activity during tasks involving cognitive control (e.g., Dichter, Felder, & Smoski, 2009), negative emotion regulation (e.g., Heller et al., 2013), and rumination (Cooney et al., 2010). However, further research using both task‐based and rs‐fMRI in MDD will be required to establish the relationship between functional activity of dlPFC and resting‐state connectivity with dlPFC in depression.
The results linking negative‐SFT with greater rsFC between pgACC and lateral prefrontal and posterior parietal regions are also consistent with network perspectives of depression (Drevets et al., 2008; Mayberg, 2003; Whitfield‐Gabrieli & Ford, 2012). Specifically, our findings mirror rsFC studies in MDD, which reliably demonstrate increased rsFC between the DMN, including pgACC, and the FPN, including the dlPFC and posterior parietal cortex (Kaiser et al., 2015). Our results are also consistent a functional near‐infrared spectroscopy study showing increased rsFC of the FPN in late life depression (Rosenbaum et al., 2016). Whereas the DMN is implicated in SFT or internally focused attention (Buckner et al., 2008), the FPN is associated with cognitive control and externally focused attention (Fox et al., 2005; Seeley et al., 2007). The DMN and FPN are typically negatively correlated at rest (Fox et al., 2005), flexibly coupled during autobiographical memory tasks (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010), and increased anti‐correlation between these networks is associated with better working memory performance (Hampson, Driesen, Roth, Gore, & Constable, 2010). Thus, one possible interpretation of increased DMN‐FPN connectivity in MDD is that FPN regions are over‐recruited to accommodate the elevated negative‐SFT occurring in depression at the cost of paying attention to the external world (Kaiser et al., 2015). Interestingly, this neurobiological explanation aligns with theoretical accounts of heightened SFT in depression, which emphasize an impaired ability to turn attention to external stimuli when the situation requires it (Ingram, 1990).
The findings relating negative‐SFT and current depression with increased rsFC between pgACC and postcentral gyrus/SMA may be relevant to the regulation of negative emotion in MDD. Although the SMA is known to play an important role in motor planning and motor imagery (Goldberg, 1985), recent research indicates that the SMA may also be involved in the regulation of emotion (Kohn et al., 2014), in particular of negative emotions (e.g., Rodigari & Oliveri, 2014). For example, a recent transcranial magnetic stimulation study found that repetitive stimulation to the SMA increased the perceived valence of negative emotional stimuli in healthy individuals (Rodigari & Oliveri, 2014). Neuroimaging research has revealed abnormal structure and function of SMA in depression (Liu et al., 2012; Zhang et al., 2016). Thus, dysfunction of SMA may contribute to enhanced negative emotions in MDD, including related to oneself.
There are some limitations to the present study that should be noted. First, while we investigated negative‐SF using a validated SCT (Exner, 1973), it is possible that the type of SF recruited by this task was more automatic as opposed to controlled. Researchers have suggested that these different types of SF may rely on partially different neural correlates (Lemogne et al., 2009). Future research will therefore be needed to examine the neural correlates of both automatic and controlled SF tasks in depression. Second, we examined the relationship between negative‐SFT and rs‐fMRI, so it is unclear whether the same brain regions and networks would be recruited during fMRI tasks that explicitly engage negative‐SFT in individuals with depression. However, task‐based fMRI studies to date suggest that similar patterns of connectivity, in particular of mPFC and ACC regions, are found during paradigms where individuals with depression actively engage in negative‐SFT (Nejad et al., 2013 for review). Third, only female participants were included in the present study, thus it remains unknown whether these results would generalize to a male population with depression. Further research could investigate whether the neural and behavioral correlates of negative‐SFT in depression differ in men versus women. Fourth, we did not collect information about participation in psychotherapy for depression in the present study. Psychotherapeutic treatment for depression has demonstrated effects on neurobiology and behavior (e.g., Crowther et al., 2015; McGrath et al., 2013), including specifically related to negative‐SFT (Yoshimura et al., 2014; Yoshimura et al., 2017). Therefore, future research will be required to further examine the effects of psychotherapy on rsFC of dmPFC and pgACC regions and negative‐SFT for individuals with varying depression histories and severities. Fifth, because we did not specifically recruit for anxiety disorders in the present study, we did not have adequate statistical power to test whether clinically significant anxiety moderates the effects of negative‐SFT on rsFC. Finally, consistent with a dimensional approach to psychopathology (Insel et al., 2010), our study recruited individuals with a broad range of severity of depressive symptoms, which made it somewhat difficult to estimate the duration and total number of depressive episodes. This limits comparison with past studies adopting a categorical approach focused on MDD and more severe forms of depression. However, this dimensional approach is also a strength of the current study in that we included mild and moderate forms of depression in addition to MDD.
Given the high risk for recurrence in MDD (Burcusa & Iacono, 2007), these findings may have important clinical implications for the development of targeted treatments aimed at reducing negative‐SFT and restoring network connectivity in MDD. Preliminary studies using therapies that target negative‐SFT, such as rumination‐focused cognitive behavioral therapy, provide some support for the efficacy of such an approach (Watkins, 2015). More broadly, these findings highlight a dimension of social‐affective function that might underlie not only MDD but also other psychiatric conditions, such as post‐traumatic stress disorder (Bryant & Guthrie, 2007; McLaughlin & Nolen‐Hoeksema, 2011; Philippi & Koenigs, 2014). Consistent with a dimensional perspective of mental health (Insel et al., 2010; Widiger & Edmundson, 2014), future research will be necessary to determine the precise relationships among negative‐SFT and severity of symptoms across a range of psychiatric disorders.
Clinical studies have also begun to investigate the neural mechanisms of therapeutic change before and after successful treatment of MDD. Across different therapeutic interventions, reduced depressive symptoms were associated with normalization of DMN connectivity at rest (Li et al., 2013; Liston et al., 2014), and reduced activity and connectivity of the mPFC and pgACC while engaging in negative‐SFT (Yoshimura et al., 2014; Yoshimura et al., 2017). Moreover, treatment studies using transcranial magnetic stimulation for depression have shown normalization of rsFC between mPFC and dlPFC (e.g., Liston et al., 2014). An important question for future research is whether pretreatment levels of negative‐SFT would predict changes in rsFC within the DMN or between DMN and FPN following treatment. The use sentence completion tasks to measure treatment outcomes could provide another more cost‐effective alternative to tracking treatment success through neuroimaging, above and beyond levels of depression.
CONCLUSION
In summary, we replicated previous research in MDD, revealing an association between negative‐SFT and depression history and severity. We also demonstrated novel results linking negative‐SFT with rsFC within and between neural networks involved in internally and externally focused attention. These findings highlight a key dimension of social‐affective functioning that may cut across different psychiatric disorders.
CONFLICTS OF INTEREST
None.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
This study was supported by a National Institute of Mental Health grant awarded to HA (R01MH094478). EW is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (KL2TR001109). We thank the participants for making this research possible. We also recognize Amy Lang and Channi Ernstoff for their assistance in coding the data for this study.
Philippi CL, Cornejo MD, Frost CP, et al. Neural and behavioral correlates of negative self‐focused thought associated with depression. Hum Brain Mapp. 2018;39:2246–2257. 10.1002/hbm.24003
Funding information National Institute of Mental Health, Grant Number: R01MH094478; National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number: KL2TR001109
REFERENCES
- Alloy, L. B. , Abramson, L. Y. , Whitehouse, W. G. , Hogan, M. E. , Panzarella, C. , & Rose, D. T. (2006). Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology, 115(1), 145–156. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM‐5. Washington, D.C: American Psychiatric Association. [Google Scholar]
- Baños, R. , Medina, P. , & Pascual, J. (2001). Explicit and implicit memory biases in depression and panic disorder. Behaviour Research and Therapy, 39(1), 61–74. [DOI] [PubMed] [Google Scholar]
- Berman, M. G. , Peltier, S. , Nee, D. E. , Kross, E. , Deldin, P. J. , & Jonides, J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Zerrin Yetkin, F. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Bradley, B. , & Mathews, A. (1983). Negative self‐schemata in clinical depression. British Journal of Clinical Psychology, 22(3), 173–181. [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. , & Guthrie, R. M. (2007). Maladaptive self‐appraisals before trauma exposure predict posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 75(5), 812–815. [DOI] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Burcusa, S. L. , & Iacono, W. G. (2007). Risk for recurrence in depression. Clinical Psychology Review, 27(8), 959–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp, J. (2012). The secret lives of experiments: Methods reporting in the fMRI literature. NeuroImage, 63(1), 289–300. [DOI] [PubMed] [Google Scholar]
- Ciric, R. , Wolf, D. H. , Power, J. D. , Roalf, D. R. , Baum, G. L. , Ruparel, K. , … Satterthwaite, T. D. (2017). Benchmarking of participant‐level confound regression strategies for the control of motion artifact in studies of functional connectivity. Clean FMRI Time Ser Mitigating Noise Adv Acquis Correct Strateg, 154, 174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, D. A. , & Beck, A. T. (1999). Scientific foundations of cognitive theory and therapy of depression. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Cooney, R. E. , Joormann, J. , Eugène, F. , Dennis, E. L. , & Gotlib, I. H. (2010). Neural correlates of rumination in depression. Cognitive, Affective &Amp; Behavioral Neuroscience, 10(4), 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Crowther, A. , Smoski, M. J. , Minkel, J. , Moore, T. , Gibbs, D. , Petty, C. , … Dichter, G. S. (2015). Resting‐state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(7), 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey, C. G. , Harrison, B. J. , Yücel, M. , & Allen, N. B. (2012). Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychological Medicine, 42(10), 2071–2081. [DOI] [PubMed] [Google Scholar]
- Derry, P. A. , & Kuiper, N. A. (1981). Schematic processing and self‐reference in clinical depression. Journal of Abnormal Psychology, 90(4), 286. [DOI] [PubMed] [Google Scholar]
- Dichter, G. S. , Felder, J. N. , & Smoski, M. J. (2009). Affective context interferes with cognitive control in unipolar depression: An fMRI investigation. Journal of Affective Disorders, 114(1–3), 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, K. S. , & Shaw, B. F. (1987). Specificity and stability of self‐referent encoding in clinical depression. Journal of Abnormal Psychology, 96(1), 34. [DOI] [PubMed] [Google Scholar]
- Drevets, W. C. , Price, J. L. , & Furey, M. L. (2008). Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure & Function, 213(1–2), 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T. E. , & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner, J. E. (1973). The self focus sentence completion: A study of egocentricity. Journal of Personality Assessment, 37(5), 437–455. [DOI] [PubMed] [Google Scholar]
- Figueroa, C. A. , Ruhé, H. G. , Koeter, M. W. , Spinhoven, P. , Van der Does, W. , Bockting, C. L. , & Schene, A. H. (2015). Cognitive reactivity versus dysfunctional cognitions and the prediction of relapse in recurrent major depressive disorder. Journal of Clinical Psychiatry, 76, e1306–e1312. [DOI] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Miriam, G. , & Janet, B. W. (2002). Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Forman, S. D. , Cohen, J. D. , Fitzgerald, M. , Eddy, W. F. , Mintun, M. A. , & Noll, D. C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magnetic Resonance in Medicine, 33(5), 636–647. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, G. (1985). Supplementary motor area structure and function: Review and hypotheses. Behavioral and Brain Sciences, 8(04), 567–588. [Google Scholar]
- Greicius, M. D. , Flores, B. H. , Menon, V. , Glover, G. H. , Solvason, H. B. , Kenna, H. , … Schatzberg, A. F. (2007). Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Neurocircuitry Neuroplasty Abnormal Mood Anxiety Disorder, 62(5), 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, S. , Ernst, J. , Boesiger, P. , Schuepbach, D. , Hell, D. , Boeker, H. , & Northoff, G. (2009). Increased self‐focus in major depressive disorder is related to neural abnormalities in subcortical‐cortical midline structures. Human Brain Mapping, 30(8), 2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard, D. A. , Akbudak, E. , Shulman, G. L. , & Raichle, M. E. (2001). Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(7), 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, J. P. , Furman, D. J. , Chang, C. , Thomason, M. E. , Dennis, E. , & Gotlib, I. H. (2011). Default‐mode and task‐positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry, 70(4), 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, M. , Driesen, N. , Roth, J. K. , Gore, J. C. , & Constable, R. T. (2010). Functional connectivity between task‐positive and task‐negative brain areas and its relation to working memory performance. Proceedings of the International School on Magnetic Resonance and Brain Function, 28(8), 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. , & Preacher, K. J. (2014). Statistical mediation analysis with a multicategorical independent variable. The British Journal of Mathematical and Statistical Psychology, 67(3), 451–470. [DOI] [PubMed] [Google Scholar]
- Heller, A. S. , Johnstone, T. , Peterson, M. J. , Kolden, G. G. , Kalin, N. H. , & Davidson, R. J. (2013). Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry, 70(11), 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking, J. G. , Kastman, E. K. , Dorfman, H. M. , Samanez‐Larkin, G. R. , Baskin‐Sommers, A. , Kiehl, K. A. , … Buckholtz, J. W. (2017). Disrupted prefrontal regulation of striatal subjective value signals in psychopathy. Neuron, 95(1), 221–231.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, R. E. (1990). Self‐focused attention in clinical disorders: Review and a conceptual model. Psychology Bulletin, 107(2), 156. [DOI] [PubMed] [Google Scholar]
- Ingram, R. E. , Lumry, A. E. , Cruet, D. , & Sieber, W. (1987). Attentional processes in depressive disorders. Cognitive Therapy and Research, 11(3), 351–360. [Google Scholar]
- Ingram, R. E. , & Smith, T. W. (1984). Depression and internal versus external focus of attention. Cognitive Therapy and Research, 8(2), 139–151. [Google Scholar]
- Insel, T. , Cuthbert, B. , Garvey, M. , Heinssen, R. , Pine, D. S. , Quinn, K. , … Wang, P. (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. [DOI] [PubMed] [Google Scholar]
- Johnson, M. K. , Nolen‐Hoeksema, S. , Mitchell, K. J. , & Levin, Y. (2009). Medial cortex activity, self‐reflection and depression. Social Cognitive and Affective Neuroscience, 4(4), 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann, J. , Dkane, M. , & Gotlib, I. H. (2006). Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy, 37(3), 269–280. [DOI] [PubMed] [Google Scholar]
- Kaiser, R. , Andrews‐Hanna, J. , Wager, T. , & Pizzagalli, D. (2015). Large‐scale network dysfunction in major depressive disorder: A meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry, 72(6), 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs, M. , & Grafman, J. (2009). The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research, 201(2), 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, N. , Eickhoff, S. B. , Scheller, M. , Laird, A. R. , Fox, P. T. , & Habel, U. (2014). Neural network of cognitive emotion regulation – An ALE meta‐analysis and MACM analysis. NeuroImage, 87, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross, E. , Davidson, M. , Weber, J. , & Ochsner, K. (2009). Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Trauma Neurodevelopment Neuroplasty: Implications for Later Anxiety, 65(5), 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne, C. , Le Bastard, G. , Mayberg, H. , Volle, E. , Bergouignan, L. , Lehéricy, S. , … Fossati, P. (2009). In search of the depressive self: Extended medial prefrontal network during self‐referential processing in major depression. Social Cognitive and Affective Neuroscience, 4(3), 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. , Liu, L. , Friston, K. J. , Shen, H. , Wang, L. , Zeng, L.‐L. , & Hu, D. (2013). A treatment‐resistant default mode subnetwork in major depression. Biological Psychiatry, 74(1), 48–54. [DOI] [PubMed] [Google Scholar]
- Liston, C. , Chen, A. C. , Zebley, B. D. , Drysdale, A. T. , Gordon, R. , Leuchter, B. , … Dubin, M. J. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological Psychiatry, 76(7), 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Hu, M. , Wang, S. , Guo, W. , Zhao, J. , Li, J. , … Chen, H. (2012). Abnormal regional spontaneous neural activity in first‐episode, treatment‐naive patients with late‐life depression: A resting‐state fMRI study. New Drugs Abuse, 39(2), 326–331. [DOI] [PubMed] [Google Scholar]
- Mayberg, H. S. (2003). Positron emission tomography imaging in depression: A neural systems perspective. Neuroimaging Clinics of North America, 13(4), 805–815. [DOI] [PubMed] [Google Scholar]
- McGrath, C. L. , Kelley, M. E. , Holtzheimer, P. E. , Dunlop, B. W. , Craighead, W. E. , Franco, A. R. , … Mayberg, H. S. III , (2013). Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry, 70(8), 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A. , & Nolen‐Hoeksema, S. (2011). Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy, 49(3), 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Minnen, A. , Wessel, I. , Verhaak, C. , & Smeenk, J. (2005). The relationship between autobiographical memory specificity and depressed mood following a stressful life event: A prospective study. The British Journal of Clinical Psychology, 44(Pt 3), 405–415. [DOI] [PubMed] [Google Scholar]
- Murray, R. J. , Debbané, M. , Fox, P. T. , Bzdok, D. , & Eickhoff, S. B. (2015). Functional connectivity mapping of regions associated with self‐ and other‐processing. Human Brain Mapping, 36(4), 1304–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad, A. B. , Fossati, P. , & Lemogne, C. (2013). Self‐referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience, 7, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen‐Hoeksema, S. , Wisco, B. E. , & Lyubomirsky, S. (2008). Rethinking rumination. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 3(5), 400–424. [DOI] [PubMed] [Google Scholar]
- Philippi, C. L. , & Koenigs, M. (2014). The neuropsychology of self‐reflection in psychiatric illness. Journal of Psychiatric Research, 54, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi, C. L. , Motzkin, J. C. , Pujara, M. S. , & Koenigs, M. (2015). Subclinical depression severity is associated with distinct patterns of functional connectivity for subregions of anterior cingulate cortex. Journal of Psychiatric Research, 71, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Schlaggar, B. L. , & Petersen, S. E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyszczynski, T. , & Greenberg, J. (1987). Self‐regulatory perseveration and the depressive self‐focusing style: A self‐awareness theory of reactive depression. Psychological Bulletin, 102(1), 122–138. [PubMed] [Google Scholar]
- Qin, P. , & Northoff, G. (2011). How is our self related to midline regions and the default‐mode network? NeuroImage, 57(3), 1221–1233. [DOI] [PubMed] [Google Scholar]
- Reeder, S. B. , Pineda, A. R. , Wen, Z. , Shimakawa, A. , Yu, H. , Brittain, J. H. , … Pelc, N. J. (2005). Iterative decomposition of water and fat with echo asymmetry and least‐squares estimation (IDEAL): Application with fast spin‐echo imaging. Magnetic Resonance in Medicine, 54(3), 636–644. [DOI] [PubMed] [Google Scholar]
- Rodigari, A. , & Oliveri, M. (2014). Disrupting SMA activity modulates explicit and implicit emotional responses: An rTMS study. Neuroscience Letters, 579, 30–34. [DOI] [PubMed] [Google Scholar]
- Roelofs, J. , van Breukelen, G. , de Graaf, L. E. , Beck, A. T. , Arntz, A. , & Huibers, M. J. H. (2013). Norms for the beck depression inventory (BDI‐II) in a large Dutch community sample. Journal of Psychopathology and Behavioral Assessment, 35(1), 93–98. [Google Scholar]
- Rosenbaum, D. , Hagen, K. , Deppermann, S. , Kroczek, A. M. , Haeussinger, F. B. , Heinzel, S. , … Ehlis, A.‐C. (2016). State‐dependent altered connectivity in late‐life depression: A functional near‐infrared spectroscopy study. Neurobiology of Aging, 39, 57–68. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline, Y. I. , Barch, D. M. , Price, J. L. , Rundle, M. M. , Vaishnavi, S. N. , Snyder, A. Z. , … Raichle, M. E. (2009). The default mode network and self‐referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle, G. J. , Moore, P. M. , & Thase, M. E. (2004). Rumination: One construct, many features in healthy individuals, depressed individuals, and individuals with lupus. Cognitive Therapy and Research, 28(5), 645–668. [Google Scholar]
- Smith, T. W. , & Greenberg, J. (1981). Depression and self‐focused attention. Motivation and Emotion, 5(4), 323–331. [Google Scholar]
- Spreng, R. N. , Stevens, W. D. , Chamberlain, J. P. , Gilmore, A. W. , & Schacter, D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. NeuroImage, 53(1), 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach, J. , Tournoux, P. (1988). Co‐planar stereotaxic atlas of the human brain. Thieme, New York.
- Treynor, W. , Gonzalez, R. , & Nolen‐Hoeksema, S. (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27, 247–259. [Google Scholar]
- Vincent, J. L. , Kahn, I. , Snyder, A. Z. , Raichle, M. E. , & Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, G. , Koch, K. , Schachtzabel, C. , Peikert, G. , Schultz, C. C. , Reichenbach, J. R. , … Schlösser, R. G. (2013). Self‐referential processing influences functional activation during cognitive control: An fMRI study. Social Cognitive and Affective Neuroscience, 8(7), 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, E. (2015). Psychological treatment of depressive rumination. Depression, 4, 32–36. [Google Scholar]
- Watkins, E. R. (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134(2), 163–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Ford, J. M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , Moran, J. M. , Nieto‐Castañón, A. , Triantafyllou, C. , Saxe, R. , & Gabrieli, J. D. E. (2011). Associations and dissociations between default and self‐reference networks in the human brain. NeuroImage, 55(1), 225–232. [DOI] [PubMed] [Google Scholar]
- Widiger, T. A. , & Edmundson, M. (2014). Diagnoses, dimensions, and DSM‐5 In: Barlow DH, (Ed). The Oxford Handbook of Clinical Psychology: Updated Edition. New York, NY: Oxford University Press; pp 256–280. [Google Scholar]
- Woolrich, M. W. , Jbabdi, S. , Patenaude, B. , Chappell, M. , Makni, S. , Behrens, T. , … Smith, S. M. (2009). Bayesian analysis of neuroimaging data in FSL. Math Brain Imaging, 45(1), S173–S186. [DOI] [PubMed] [Google Scholar]
- Yan, C.‐G. , Cheung, B. , Kelly, C. , Colcombe, S. , Craddock, R. C. , Di Martino, A. , … Milham, M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, S. , Okamoto, Y. , Matsunaga, M. , Onoda, K. , Okada, G. , Kunisato, Y. , … Yamawaki, S. (2017). Cognitive behavioral therapy changes functional connectivity between medial prefrontal and anterior cingulate cortices. Journal of Affective Disorders, 208, 610–614. [DOI] [PubMed] [Google Scholar]
- Yoshimura, S. , Okamoto, Y. , Onoda, K. , Matsunaga, M. , Okada, G. , Kunisato, Y. , … Yamawaki, S. (2014). Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self‐referential processing. Social Cognitive and Affective Neuroscience, 9(4), 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, S. , Okamoto, Y. , Onoda, K. , Matsunaga, M. , Ueda, K. , Suzuki, S. , & Yamawaki, S. HIGETO. (2010). Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. Journal of Affective Disorders, 122, 76–85. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Li, L. , Wu, M. , Chen, Z. , Hu, X. , Chen, Y. , … Gong, Q. (2016). Brain gray matter alterations in first episodes of depression: A meta‐analysis of whole‐brain studies. Neuroscience and Biobehavioral Reviews, 60, 43–50. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Brady, M. , & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Wang, X. , Xiao, J. , Liao, J. , Zhong, M. , Wang, W. , & Yao, S. (2012). Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode, treatment‐naive major depression patients. Neural Circuitry Mood, 71(7), 611–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


