Abstract
Health worker experience and community support may be higher in high HIV prevalence regions than low prevalence regions, leading to improved prevention of mother-to-child HIV transmission (PMTCT) programs. We evaluated 6-week and 9-month infant HIV transmission risk (TR) in a high prevalence region and nationally. Population-proportionate-to-size sampling was used to select 141 clinics in Kenya, and mobile teams surveyed mother-infant pairs attending 6-week and 9-month immunizations. HIV DNA testing was performed on HIV-exposed infants. Among 2521 mother-infant pairs surveyed nationally, 2423 (94.7%) reported HIV testing in pregnancy or prior diagnosis, of whom 200 (7.4%) were HIV-infected and 188 infants underwent HIV testing. TR was 8.8% (4.0-18.3%) in 6-week and 8.9% (3.2-22.2%) in 9-month cohorts including mothers with HIV diagnosed postpartum, of which 57% of infant infections were due to previously undiagnosed mothers. Of 276 HIV-exposed infants in the Nyanza survey, TR was 1.4% (0.4-5.3%) at 6-week and 5.1% (2.5-9.9%) at 9-months. Overall TR was lower in Nyanza, high HIV region, than nationally (3.3% vs. 7.2%, P=0.02). HIV non-disclosure to male partners and incomplete ARVs were associated with TR in both surveys [aOR=12.8 (3.0-54.3); aOR=5.6 (1.2-27.4); aOR=4.5 (1.0-20.0), aOR=2.5, (0.8-8.4), respectively]. TR was lower in a high HIV prevalence region which had better ARV completion and partner HIV disclosure, possibly due to programmatic efficiencies or community/peer/partner support. Most 9-month infections were among infants of mothers without prior HIV diagnosis. Strategies to detect incident or undiagnosed maternal infections will be important to achieve PMTCT.
Keywords: prevention of mother-to-child transmission of HIV; pediatric; surveillance; antiretroviral therapy, disclosure
Introduction
In 2013, an estimated 240,000 infants were born with HIV infection, more than 90% in sub-Saharan Africa (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2013). Effective prevention of mother-to-child transmission (PMTCT) of HIV programs requires maternal HIV testing, uptake of antiretroviral (ARV) drugs, early infant diagnosis of HIV and other postnatal services. Delays or missed steps in this PMTCT cascade compromise efforts to reduce pediatric HIV infections. While HIV testing has been broadly expanded and time to ARV initiation has been expedited with Option B+, non-adherence and poor retention in PMTCT programs remain problematic (Stringer et al., 2013; Tudor Car et al., 2011; Turan & Nyblade, 2013). Moreover, individual and community-wide efforts are needed to reduce barriers and improve utilization of PMTCT services (Ferguson et al., 2012). Areas of high HIV prevalence may have broader access to integrated services, more experience of health care providers and more maternal and community familiarity with HIV and PMTCT interventions, which may lead to improved programs and lower rates of infant HIV infection. Conversely, in settings with lower HIV prevalence, mothers may have less awareness or experience with HIV interventions, health providers may have limited experience in managing or counseling women, peer counselors may be less conversant with relevant issues, and systems may be more erratic if not frequently used, all leading to less effective programs (Tudor Car et al., 2011; Turan & Nyblade, 2013). To reach UNAIDS targets for elimination of infant HIV infection, it is important to define barriers to PMTCT implementation and to identify components or characteristics of effective PMTCT programs.
National evaluations of PMTCT programs include measures of maternal HIV testing, ARV use and rates of infant HIV among 6 week-old infants born to HIV-positive women. To date, programmatic data have rarely assessed infant HIV infection after 6 weeks of age. While peripartum and early breastmilk HIV transmission can be captured by the 6 week time point, there is still substantial risk of late postnatal HIV transmission that is largely unmeasured. We conducted an evaluation of the Kenya PMTCT program in 120 clinics dispersed nationally. We additionally oversampled Nyanza Province, a region with high HIV prevalence, to obtain more precise estimates of HIV transmission and to compare transmission in the high prevalence region with the national estimate. In addition, we assessed infant HIV at both 6 weeks and 9 months postpartum.
Methods
Study Design
We conducted two facility-based cross-sectional surveys of PMTCT effectiveness from June to December 2013. This 2-part evaluation assessed coverage and uptake of PMTCT services in the general population and uptake of HIV prevention interventions among HIV-positive women. The primary National PMTCT-MCH survey evaluated the effectiveness of the national PMTCT program among women attending 120 facilities in seven of eight provinces in Kenya. To increase statistical precision to assess factors associated with HIV-specific services, the secondary PMTCT-Nyanza survey purposively sampled HIV-infected women attending 30 facilities in Nyanza, the province with highest HIV prevalence.
Facility Selection
The sampling frame included all healthcare facilities providing routine antenatal and postnatal care, including PMTCT services, and childhood immunizations. Facility size was based on annual antenatal care (ANC) attendance and grouped as: small (<500 annual ANC visits), medium (500-999 annual ANC visits), and large (≥1,000 annual ANC visits) as reported to the National AIDS and STI Control Program. Annual ANC visits served as a proxy for the number of mother-infant pairs attending facilities for routine infant immunizations. The national PMTCT-MCH survey used probability proportionate to size (PPS) sampling to randomly sample 120 facilities from among the 540 medium and large facilities. The PMTCT-Nyanza survey included all large facilities in Nyanza province (n=30). Nine facilities in Nyanza were included in both surveys, thus a total of 141 facilities were sampled between both surveys. Small facilities and facilities located in North Eastern province were excluded due to logistical feasibility.
Study Population
All women bringing their infants for 6 week or 9 month infant immunizations were eligible to participate in the survey. Infants brought to the facility by someone other than their biological mother and mothers of infants not receiving 6 week or 9 month immunizations were excluded. The National PMTCT survey recruited all eligible mother-infant pairs attending selected facilities throughout Kenya during a fixed 5-day recruitment period, regardless of maternal HIV status. The PMTCT-Nyanza survey recruited all eligible HIV-positive mothers and their infants attending selected facilities in Nyanza during a fixed 10-day recruitment period. There was no overlap in mother-infant pairs between surveys; the nine overlapping facilities sampled mother-infant pairs at separate time points. Maternal HIV status was based on self-report and verified by the women’s Maternal & Child Health Booklet.
Data Collection
Trained mobile teams, composed of one nurse and one laboratory technician, were assigned to collect data from participating facilities. At most facilities, a nurse or counselor was available to assist study staff in identifying mother-infant pairs potentially eligible for participation. Study staff administered the survey using Open Data Kit on tablet computers. The survey instrument was adapted from previous surveys designed to measure PMTCT effectiveness (Kinuthia et al., 2011; World Health Organization, 2012) and field tested prior to implementation. Data was based on self-report and verified by the mother’s Maternal & Child Health Booklet, when applicable. The questionnaire covered uptake of ANC, maternal HIV testing, and use of ARVs among HIV-positive women, as well as, maternal and paternal demographics, household characteristics, and reproductive and family planning history. The infant module collected data on birth outcomes, immunizations, infant feeding method, and use of ARVs and HIV testing in HIV-exposed infants.
Maternal and Infant HIV Testing
Women whose HIV status was unknown and those who reported HIV-negative test during pregnancy or at delivery were offered rapid HIV-1 antibody testing, using Determine test kit (Abbott Laboratories, Chicago, Illinois). Women who self-reported being HIV seropositive were not retested. All HIV-positive mothers were offered infant HIV testing. Dry blood spot specimens for HIV DNA polymerase chain reaction (PCR) testing (Cobas Amplicor HIV Monitor Test, version 1.5; Roche Molecular Diagnostics, Pleasanton, CA) were collected from infants of consenting mothers and sent to the national KEMRI-CDC laboratories for PCR testing. Infant HIV PCR results were returned to the facility, whereby the facility provided the mothers their infant’s test results. Participants and/or mothers of infants testing HIV-positive were referred for support services and HIV care.
Ethical considerations
Written informed consent was obtained from all study participants, both to be interviewed and also to conduct HIV testing among participating mothers and HIV exposed infants. Ethical approval was obtained from the ethical review committees at the Kenya Medical Research Institute, the University of Washington, and the Associate Director for Science at the U.S. Centers for Disease Control and Prevention.
Statistical Analyses
Transmission risk (TR) at 6 weeks and 9 months was calculated by dividing the number of DNA-positive infants by infants at risk at each time point. Complete PMTCT was defined as receipt of ARVs [ARV prophylaxis or combination antiretroviral therapy (ART)] in pregnancy, at labor and delivery, and maternal and infant ARVs in the postpartum period. All estimates in the national PMTCT-MCH survey were weighted to account for complex survey design and clinic-level clustering; the PMTCT-Nyanza survey accounted for clustering at the clinic level. Crude comparisons between the two surveys were conducted after combining data from both surveys, assigning equal weights for participants in Nyanza survey, retaining generated weights for the national survey, and accounting for clustering at the clinic level. Rao-Scott chi-square tests were used to compare associations between maternal and infant characteristics and infant HIV infection, defined as HIV DNA-positive. Logistic regression models were used to determine odds ratios (OR) and 95% confidence intervals (CI) to examine independent correlates of infant HIV infection (vs. infant HIV-negative) and separately, compare TR across surveys. Covariates associated with infant HIV infection at the P≤0.05 level were included in multivariate analysis.
The sample was designed to provide estimates of PMTCT effectiveness and maternal HIV incidence with 95% confidence intervals of ±3% or less for 4% infant transmission or maternal HIV incidence based on HIV incidence of 4% per year and a design effect of 2. To attain required sample size for the national PMTCT-MCH Survey, study staff recruited eligible mother-infant pairs during a 5-day period. To attain required sample size of HIV-infected women in the Nyanza Survey, study staff recruited all eligible HIV-positive women and their infants attending clinic during a 10-day window. Because it takes more time to accrue HIV-positive women, the Nyanza Survey needed the additional 5 days to reach the required sample size.
Data were analyzed using STATA 13.1 svy commands (STATA Corporation, College Station, Texas).
Results
The national PMTCT-MCH survey included 2521 mother-infant pairs across 120 facilities. Of these, 2423 (94.7%; data weighted to account for survey design) women reported an HIV test during most recent pregnancy or prior HIV diagnosis, of whom 200 (7.4%) were HIV-infected. Of 200 infants born to mothers with known HIV diagnosis in pregnancy, 188 underwent HIV PCR DNA testing (Figure 1). Nine mothers of the remaining 12 infants refused HIV testing and three infants had indeterminate PCR results. The PMTCT-Nyanza survey included 298 HIV-infected mothers and their infants. Of these, 276 infants had defined HIV PCR DNA test result; 10 mothers refused infant testing and 12 had indeterminate results. Analyses for TR and correlates of transmission were restricted to 188 mother-infant pairs with negative or positive PCR results in the national PMTCT-MCH survey and separately, 276 mother-infant pairs with known PCR results in the PMTCT-Nyanza survey.
Figure 1.
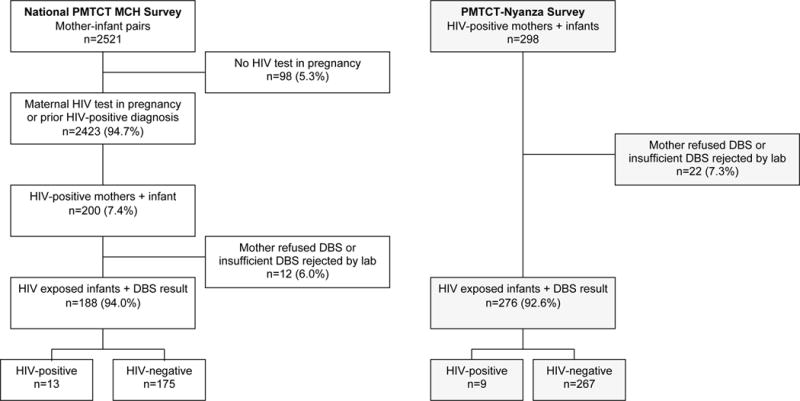
Flow chart describing enrollment of mother-infant pairs and infant HIV infection status in the national PMTCT-MCH Survey (left) and PMTCT-Nyanza Survey (right), Kenya. Proportions are weighted to account for study design. DBS, Dried blood spot; PMTCT, Prevention of mother-to-child transmission of HIV; MCH, Maternal and child health.
Sociodemographic and HIV-related characteristics
Across surveys, the mean age of participating HIV-infected mothers was 28 years and more than 80% of women were married or cohabiting (Table 1). Nationally, 40.4% of mothers had at least a secondary education and 24.4% reported piped water in the home. One-third (30.8%) of mothers in the Nyanza survey had beyond secondary education and 8.7% had piped water in the home. More than half of pregnancies were intended, 56% of women attended at least four ANC visits, and the mean gestational age at first ANC visit was 22.5 weeks (95% CI 21.3-23.6).
Table 1.
Maternal and infant characteristics by infant HIV status, among HIV-exposed infants attending 6 week and 9 month immunization visits
| NATIONAL PMTCT-MCH SURVEY | PMTCT-NYANZA SURVEY | |
|---|---|---|
| Weighted % or Mean (95% CI) (n=188) |
% or Mean (95% CI) (n=276) |
|
| Maternal characteristics | ||
| Maternal age, mean | 28.7 (27.6, 29.8) | 27.9 (27.3, 28.4) |
| Married or cohabiting | 82.4 (74.2, 88.4) | 84.4 (80.2, 87.9) |
| Education ≥ Secondary | 40.4 (31.7, 49.8) | 30.8 (25.0, 37.3) |
| Running water in home** | 24.4 (16.3, 34.9) | 8.7 (4.7, 15.4) |
| Primigravida | 12.3 (8.1, 18.2) | 10.5 (6.6, 16.4) |
| Number of living children, mean | 2.7 (2.5, 2.9) | 3.0 (2.7, 3.3) |
| Planned pregnancy | 57.6 (28.3, 66.5) | 55.1 (44.2, 65.6) |
| 4 or more ANC visits | 56.4 (47.5, 64.9) | 55.8 (49.2, 62.2) |
| Gestational age at 1st ANC (weeks), mean | 22.5 (21.3, 23.6) | 22.0 (21.0, 23.1) |
| Vaginal delivery | 89.4 (83.2, 93.6) | 91.3 (87.7, 93.9) |
| Preterm delivery (<37 weeks) | 32.9 (19.6, 49.5) | 21.6 (12.1, 35.5) |
| Non-facility delivery | 19.3 (12.7, 28.1) | 17.0 (10.4, 26.7) |
| HIV-specific characteristics | ||
| HIV diagnosis in pregnancy | 40.4 (31.2, 50.4) | 36.2 (29.1, 44.1) |
| Last CD4 count, cells/mm3** | ||
| ≥350 | 41.7 (32.1, 51.8) | 52.9 (44.3, 61.2) |
| <350 | 14.0 (8.3, 22.8) | 19.6 (15.0, 25.2) |
| Unknown | 44.3 (32.2, 57.1) | 27.5 (20.7, 35.6) |
| Partner HIV status** | ||
| HIV-negative | 29.7 (20.9, 40.3) | 17.2 (11.7, 24.6) |
| HIV-positive | 34.8 (25.6, 45.2) | 60.5 (51.8, 68.6) |
| Unknown | 35.6 (27.3, 44.7) | 22.3 (16.3, 29.7) |
| Disclosed HIV status to partner** | 76.6 (70.1, 82.1) | 86.6 (79.8, 91.4) |
| Infant characteristics | ||
| Female | 50.6 (44.2, 57.0) | 52.2 (47.8, 56.5) |
| Birth weight (kg), mean | 3.1 (3.0, 3.2) | 3.3 (3.2, 3.3) |
| Currently breastfeeding | 82.1 (72.6, 88.8) | 89.8 (84.0, 93.7) |
P-value <0.05; Data in the National PMTCT-MCH Survey are weighted to account for survey design and clinic-level clustering; data in the PMTCT-Nyanza Survey account for clinic-level clustering.
Forty percent of HIV-infected women in the PMTCT-MCH survey and 36.2% of those in the Nyanza survey were diagnosed with HIV during pregnancy. Half (55.7%) of HIV-infected mothers in the PMTCT-MCH survey knew their CD4 cell count as compared to 72.5% of those in the Nyanza survey. In the national survey, one-third (34.8%) of HIV-infected mothers reported an HIV-positive male partner, 35.6% did not know their male partners HIV status, and 76.6% had disclosed their HIV status to their male partner. A majority of HIV-infected mothers (60.5%) in Nyanza were in a seroconcordant relationship, 22.3% did not know the HIV status of their male partner, and 86.6% had disclosed their HIV status to their male partner (Table 1).
Mother-to-Child Transmission
Of the 188 HIV-exposed infants in the national PMTCT-MCH survey with determinate HIV-DNA results, overall, 13 were HIV DNA-positive (7.2%; 95% CI, 3.7-13.5%) [Figure 1]. HIV TR was 8.8% (4.0-18.3%) among 110 infants in the 6 week cohort and 4.8% (1.3-15.6%) among 78 infants in the 9 month cohort. Of 4 HIV-positive infants in the 9 month postpartum assessment who had prior 6 week results captured in their MCH booklet, 3 were HIV-positive and 1 infant was previously HIV-DNA negative at 6 weeks of age [data not shown].
In the PMTCT-Nyanza survey, overall HIV TR was 3.3% (95% CI: 1.7-6.1%). TR was 1.4% (0.4-5.3%) in the 6 week cohort and 5.1% (2.5-9.9%) in the 9 month cohort. Of 5 HIV-positive infants in the 9 month postpartum cohort with prior 6 week PCR results, 3 were HIV-positive and 2 were previously HIV-negative at 6 weeks of age [data not shown].
Overall HIV transmission from mother to infant was significantly lower in the PMTCT-Nyanza survey than the national PMTCT-MCH survey (3.3% vs. 7.2%, P=0.02).
Among mothers with prior HIV-negative or unknown status in the national survey, 11 were HIV seropositive at the 9 month visit. Including mothers with incident or newly diagnosed HIV since pregnancy, HIV TR at 9 month postpartum visit was 8.9% (3.2-22.2%), of which 57% of infant infections were due to previously undiagnosed mothers. The Nyanza oversample surveyed only known HIV-seropositive mothers, thus, there were no newly diagnosed mothers at 6 weeks or 9 months postpartum.
Correlates of infant HIV infection and uptake of PMTCT interventions
Nationally, mothers with unknown CD4 cell counts had a 6-fold higher risk of transmission (95% CI, 1.18-32.11) than mothers with known CD4 cell counts (Table 2). Non-disclosure of maternal HIV status to her male partner was associated with increased risk of infant HIV transmission (OR 12.8, 95% CI, 3.03-54.32). Female infants had a 5-fold higher risk of HIV infection (OR 4.99, 95% CI: 1.00-24.86) than male infants. There were no differences in maternal age, facility delivery, prematurity, infant birth weight or exposure to breastfeeding by infant HIV status (Table 2).
Table 2.
Correlates of infant HIV infection among HIV-exposed infants in the PMTCT-MCH and PMTCT-Nyanza Surveys, Kenya
| NATIONAL PMTCT-MCH SURVEY | PMTCT-NYANZA SURVEY | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Maternal Characteristics | ||||
| Maternal age, mean | 0.95 (0.88, 1.02) | 0.14 | 0.95 (0.79, 1.15) | 0.59 |
| Married or cohabiting | 4.15 (0.81, 21.39) | 0.09 | 0.63 (0.14, 2.85) | 0.54 |
| Education ≥ Secondary | 0.89 (0.13, 5.91) | 0.90 | 0.63 (0.15, 2.67) | 0.52 |
| Running water in home | 0.26 (0.05, 1.36) | 0.11 | – | – |
| Primigravida | 0.57 (0.11, 3.00) | 0.50 | 1.07 (0.10, 11.54) | 0.96 |
| Number of living children, mean | 0.93 (0.51, 1.71) | 0.81 | 1.27 (0.73, 2.19) | 0.39 |
| Planned pregnancy | 0.77 (0.16, 3.69) | 0.74 | 0.40 (0.10, 1.54) | 0.17 |
| 4 or more ANC visits | 0.92 (0.21, 4.10) | 0.91 | 0.38 (0.08, 1.76) | 0.21 |
| Mean gestational age at 1st ANC, weeks | 0.99 (0.92, 1.08) | 0.91 | 0.99 (0.89, 1.11) | 0.92 |
| Preterm delivery (<37 weeks) | 1.26 (0.30, 5.34) | 0.75 | 1.47 (0.34, 6.36) | 0.59 |
| Non-facility delivery | 1.24 (0.28, 5.51) | 0.78 | – | – |
| HIV diagnosis in pregnancy | 2.20 (0.42, 11.54) | 0.35 | 2.26 (0.63, 8.11) | 0.20 |
| Last CD4 count, cells/mm3 | ||||
| ≥350 | Reference | Reference | ||
| <350 | – | – | 0.53 (0.05, 5.86) | 0.60 |
| Unknown | 4.57 (0.81, 25.7) | 0.08 | 1.16 (0.28, 4.76) | 0.83 |
| Unknown CD4 cell count | 6.16 (1.18, 32.11) | 0.03 | 1.33 (0.34, 5.23) | 0.68 |
| Non-disclosure of HIV status to partner | 12.83 (3.02, 54.33) | 0.001 | 5.67 (1.17, 27.39) | 0.03 |
| Infant Characteristics | ||||
| Female | 4.99 (1.00, 24.86) | 0.05 | 0.73 (0.16, 3.30) | 0.67 |
| Birth weight <2.5 kg | 2.52 (0.37, 16.93) | 0.34 | 3.29 (0.22, 48.6) | 0.37 |
| Birth weight, mean | 0.79 (0.19, 3.26) | 0.74 | 0.34 (0.08, 1.42) | 0.13 |
| Currently breastfeeding | 2.05 (0.22, 19.19) | 0.53 | 0.90 (0.08, 9.82) | 0.93 |
Data in the National PMTCT-MCH Survey are weighted to account for survey design and clinic-level clustering; data in the PMTCT-Nyanza Survey account for clinic-level clustering; CI, Confidence interval; PMTCT, Prevention of mother-to-child transmission of HIV; MCH, Maternal and child health; OR, Odds ratio
Overall, 81.4% mothers in the PMTCT-MCH survey received antenatal ARVs; 63.4% were on ART and 18.0% reported receipt of ARVs. Most infants (93.5%) received ARV prophylaxis, of whom 95.0% received nevirapine. Mother-infant pairs receiving incomplete PMTCT had a 4-fold higher HIV TR (95% CI, 1.0-19.0) than mother-infant pairs receiving ARVs at all time-points (Table 3). There was a trend for increased TR among mothers who received no ARVs in pregnancy (P=0.061) and the postpartum period (P=0.058). Infant HIV infection was associated with no maternal ARVs at labor and delivery (OR 7.8; 95% CI, 1.6-37.3) and no infant ARV prophylaxis (OR 19.6; 95% CI, 3.0-126.6). After adjustment for nondisclosure of HIV status and infant sex, incomplete PMTCT interventions and lack of ARVs at labor/delivery and infant ARV prophylaxis remained significantly associated with infant HIV infection. After adjustment, there was a higher risk of transmission among women with no antenatal ARVs compared to women on ART (Adjusted OR [aOR] 6.1; 95% CI, 1.0-38.6, P=0.054).
Table 3.
Associations between PMTCT interventions and infant HIV infection among HIV-exposed infants
| NATIONAL PMTCT-MCH SURVEY | ||||||
|---|---|---|---|---|---|---|
| Weighted % (95% CI) | Unadjusted OR (95% CI) |
P | Adjusted OR b (95% CI) |
P | ||
| Outcomes | HIV+ infant (n=13) | HIV− infant (n=175) | ||||
| Complete EMTCT a | ||||||
|
| ||||||
| No | 72.6 (38.6, 91.8) | 37.8 (28.1, 48.5) | 4.4 (1.0, 19.0) | .049 | 4.5 (1.0, 20.0) | .051 |
|
| ||||||
| Yes | 27.4 (8.2, 61.4) | 62.2 (51.5, 71.9) | Referent | Referent | ||
|
| ||||||
| ARVs in pregnancy | ||||||
|
| ||||||
| No | 47.6 (13.3, 84.3) | 16.3 (10.2, 25.0) | 4.7 (0.9, 23.6) | .061 | 5.5 (1.0, 31.4) | .056 |
|
| ||||||
| Yes | 52.4 (15.7, 86.7) | 83.8 (75.0, 89.8) | Referent | Referent | ||
|
| ||||||
| ARVs during labor and delivery | ||||||
|
| ||||||
| No | 57.1 (18.1, 88.9) | 14.6 (8.8, 23.3) | 7.8 (1.6, 37.3) | .011 | 8.1 (1.4, 47.5) | .021 |
|
| ||||||
| Yes | 42.9 (11.1, 81.9) | 85.4 (76.7, 91.2) | Referent | Referent | ||
|
| ||||||
| ARVs in the postpartum period | ||||||
|
| ||||||
| No | 46.4 (11.9, 84.8) | 15.0 (9.3, 23.3) | 4.9 (0.9, 25.4) | .058 | 5.4 (0.8, 35.1) | .078 |
|
| ||||||
| Yes | 53.6 (15.2, 88.1) | 85.0 (76.7, 90.7) | Referent | Referent | ||
|
| ||||||
| Neonatal ARV prophylaxis | ||||||
|
| ||||||
| No | 43.0 (12.4, 80.0) | 3.7 (1.7, 8.1) | 19.6 (3.0, 126.6) | .002 | 11.3 (2.5, 51.5) | .002 |
|
| ||||||
| Yes | 57.0 (20.0, 87.6) | 96.3 (91.9, 98.4) | Referent | Referent | ||
| PMTCT-NYANZA SURVEY | ||||||
|---|---|---|---|---|---|---|
| Unweighted % (95% CI) | Unadjusted OR (95% CI) |
P | Adjusted OR c (95% CI) |
P | ||
| HIV+ infant (n=9) | HIV− infant (n=267) | |||||
| Complete EMTCT a | ||||||
|
| ||||||
| No | 55.6 (26.8, 81.1) | 26.2 (17.6, 37.2) | 3.5 (1.1, 11.7) | .041 | 2.5 (0.8, 8.4) | .120 |
|
| ||||||
| Yes | 44.4 (18.9, 73.3) | 73.8 (62.8, 82.4) | Referent | Referent | ||
|
| ||||||
| ARVs in pregnancy | ||||||
|
| ||||||
| No | 37.5 (13.9, 69.1) | 14.1 (8.4, 22.9) | 3.7 (0.9, 15.1) | .073 | 2.0 (0.6, 5.9) | .223 |
|
| ||||||
| Yes | 62.5 (30.0, 86.1) | 85.9 (77.1, 91.7) | Referent | Referent | ||
|
| ||||||
| ARVs during labor and delivery | ||||||
|
| ||||||
| No | 33.3 (12.0, 64.7) | 6.3 (3.5, 11.0) | 7.5 (1.8, 31.8) | .008 | 4.9 (1.7, 14.5) | .005 |
|
| ||||||
| Yes | 66.7 (35.3, 88.0) | 93.8 (89.0, 96.5) | Referent | Referent | ||
|
| ||||||
| ARVs in the postpartum period | ||||||
|
| ||||||
| No | 44.4 (18.9, 73.3) | 11.5 (7.0, 18.2) | 6.2 (1.7, 22.1) | .007 | 4.2 (1.3, 13.6) | .016 |
|
| ||||||
| Yes | 55.6 (26.8, 81.1) | 88.5 (81.2, 93.0) | Referent | Referent | ||
|
| ||||||
| Neonatal ARV prophylaxis | ||||||
|
| ||||||
| No | 33.3 (12.0, 64.7) | 0.8 (0.2, 3.0) | 66.2 (20.0, 219.4) | <.001 | 39.3 (7.9, 195.1) | <.001 |
|
| ||||||
| Yes | 66.7 (35.3, 88.0) | 99.3 (97.0, 99.8) | Referent | Referent | ||
Complete PMTCT defined as maternal ARVs in pregnancy, during delivery, and the postpartum period and infant ARVs;
Adjusted for maternal HIV disclosure to male partner and infant female sex;
Adjusted for maternal HIV disclosure to male partner
In the PMTCT-Nyanza survey, maternal HIV non-disclosure to male partners was associated with a 5-fold increased risk of infant HIV infection (95% CI, 1.17-27.39). Of those with known PMTCT uptake, 85.2% received ARVs during pregnancy, 92.8% at labor and delivery, and 87.4% in the postpartum period. Nearly all HIV-exposed infants (98.2%) received ARVs. Incomplete PMTCT was associated with 3-fold increased risk of infant HIV transmission (95 CI, 1.1-11.7). HIV TR was higher in mothers reporting no ARVs compared to mothers receiving ARVs at delivery (OR 7.5; 95% CI, 1.8-31.8) or postpartum (OR 6.2; 95% CI, 1.7-22.1). Lack of infant ARVs were associated with higher TR (OR 66.2; 95% CI, 20.0-219.4). In multivariate analysis adjusting for maternal HIV disclosure, TR remained higher among mothers with no ARVs at delivery and mothers and infants with no ARVs in the postpartum period (Table 3).
Discussion
This study estimated population-level PMTCT effectiveness using two surveys; one provided estimates of infant HIV transmission in a nationally representative sample and the second evaluated TR in a high HIV burden region in western Kenya. The HIV TR was significantly higher (7.2%) in the national survey than in the Nyanza survey (3.3%). This difference may be explained by differing ARV completion. In both surveys, over 80% of pregnant women and more than 90% of infants received ART or ARVs, however, over 80% of mother-infant pairs in Nyanza completed ARVs in contrast to only ~60% of mother-infant pairs nationally. Notably mothers in Nyanza had lower socioeconomic status and education than nationally, despite which their adherence to ART/ARVs was higher. Mothers in Nyanza were significantly more likely to report having had CD4 testing, to know their CD4 count, and have disclosed their HIV status to their partner. It is possible that community-level efforts to improve outreach and retention, have reduced barriers to uptake of PMTCT services more effectively in this high HIV prevalence region than in lower HIV prevalence regions (Nyandiko et al., 2010). The higher regional HIV prevalence may lead to more efficiency in clinics as more HIV-infected women present for care, health care workers are more experienced and there is availability of experienced peer counselors. Concurrently, it is plausible there is lower community-wide stigma and more community support to complete PMTCT (Turan & Nyblade, 2013). Analogously, high HIV prevalence countries like South Africa and Botswana have noted dramatic progress in PMTCT (Creek et al., 2008; Goga et al., 2015), and part of this progress may be driven by critical need of the high HIV burden, population-wide acceptance, and health system efficiencies as the proportion of HIV-infected mothers is high.
Maternal non-disclosure of HIV infection to male partners was associated with a significantly increased risk of infant HIV infection. Indeed, we also reported an association between non-disclosure and lower uptake of both maternal and infant ARVs for PMTCT (Kinuthia et al., 2015). These findings are consistent with studies reporting suboptimal uptake and adherence to PMTCT regimens in women who had not disclosed their HIV status (Ebuy, Yebyo, & Alemayehu, 2015; Jasseron et al., 2013; Kinuthia et al., 2011; Spangler, Onono, Bukusi, Cohen, & Turan, 2014) and add to the literature on disclosure and MTCT. Strategies to maintain confidentiality and facilitate disclosure to male partners are needed to maximize PMTCT effectiveness (Kinuthia et al., 2011; Schechter et al., 2014).
National estimates of 6 week and 9 month TR were 8.8% (4.0-18.3%) and 4.8% (1.3-15.6%), respectively. This is similar to the 2010 national PMTCT survey reporting 6 week TR of 7.7% (6.2-9.5%) in Kenya (Kiarie et al., 2012) and higher than observed in a register-based analysis of Kenyan HIV exposed infants which noted a TR of 4% at 6 weeks and 7% at 18 months (Ochanda et al., 2014). However, the latter study included only infants with complete data to 18-months of age, which is likely to underestimate TR because of excluding those lost to follow-up during longitudinal assessment. While we expect mean cumulative infant HIV TR to be higher at 9 months than at 6 weeks, differences in TR estimates at the two sampling times occur due to loss, referral of previously HIV diagnosed children to treatment clinics, and mortality. Modeling approaches that account for referral, loss, and mortality will be useful in the future to better estimate longitudinal HIV TR from sampling at 6 weeks and 9 months, two highly accessed maternal-child health visit time-points.
Incident and previously undiagnosed HIV infection during pregnancy and the postpartum period was ~1% nationally and was not estimated in the Nyanza survey. The risk of MTCT was 29% among women with incident infection during pregnancy/postpartum. This is consistent with studies reporting elevated transmission among women with incident versus chronic HIV infection (Drake, Wagner, Richardson, & John-Stewart, 2014; Gray et al., 2005; Moodley et al., 2011; Moodley, Esterhuizen, Pather, Chetty, & Ngaleka, 2009), and similar to a pooled MTCT rate of 23.6% among women seroconverting during and after pregnancy in African settings (Drake et al., 2014). High maternal viremia, low maternal antibodies, and absence of ARVs for PMTCT increase the risk of vertical transmission (Gray et al., 2005; Moodley et al., 2011; Moodley et al., 2009). Strategies including repeat testing and enhanced screening methods are needed to identify acute HIV infection during pregnancy and breastfeeding and further reduce MTCT. Prevention of maternal HIV infection in pregnancy is also a key priority.
This study has several strengths and limitations. This was an assessment of 141 clinics throughout Kenya – the scope of the sampling allowed nationally representative sampling and oversampling from a high prevalence region. In addition, our survey included 9 month visit assessment, which provided data on late postnatal HIV infection. Mother-infant pairs were selected based on attendance at 6 week and 9 month immunizations at participating health facilities. Although more than 90% of children in Kenya receive their 6 week immunizations and 87% receive their 9 month measles vaccination (Kenya National Bureau of Statistics, 2015), this sample does not include the subset of potentially most at-risk infants who do not attend MCH clinic. In addition, ~6-7% of women refused infant HIV testing, a group that may be at higher risk for HIV. Results were similar in sensitivity analyses including infants of unknown status as HIV-positive or HIV-negative. Utilization of PMTCT services was based on self-report and clinical record, when available. Maternal and paternal demographic data and partner HIV disclosure was based on maternal self-report and may be subject to social desirability bias. The national PMTCT-MCH survey used PPS sampling to select medium to large clinics in seven of the eight regions in Kenya; the North Eastern region was excluded due to security issues. However, its sparse population (an estimated 2% of women aged 15-49 years) (NASCOP, 2012), limits the impact of excluding this region. Both surveys excluded small clinics (<500 first ANC visits per year), and thus are not representative of small facilities. Facility size was based on all ANC visits and did not account for the number of HIV-infected women at each site. Finally, our sample size of HIV-infected mothers nationally was small, limiting precision of estimates.
In summary, in this pair of surveys, clinics in Nyanza appear to have attained the PMTCT target of <5% in 2013 even before wide implementation of Option B+. In contrast, the national MTCT estimates suggest the need for better coverage and completion of the PMTCT cascade. Lessons from high HIV prevalence settings, such as Nyanza, in terms of optimizing HIV disclosure and adherence of ARVs may be of value for lower prevalence settings. While resources are increasingly allocated to high HIV prevalence regions, our data suggest the need to consider additional community and facility-level support in low prevalence settings, where persistent gaps in PMTCT ARV adherence and completion may undermine the attainment of PMTCT goals.
Acknowledgments
Sources of support: This publication was made possible by support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through cooperative agreement [#U2GPS002047] from the U.S. Centers for Disease Control and Prevention (CDC), Division of Global HIV/AIDS (DGHA). C.J.M. was supported by the University of Washington STD/AIDS Research Training Fellowship (NIH NRSA T32AI007140) and NIH research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program – BIRCWH). Support for G.J.S. includes a NIH K24 grant (HD054314) and the University of Washington (UW) Global Center for Integrated Health of Women Adolescents and Children (Global WACh). Lastly, support by the NIH funded program, UW Center for AIDS Research (CFAR) (P30 AI027757).
Footnotes
Conflicts of interest: There are no conflicts of interest.
Disclaimers: The content is solely the responsibility of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
References
- Creek T, Tanuri A, Smith M, Seipone K, Smit M, Legwaila K, Shaffer N. Early diagnosis of human immunodeficiency virus in infants using polymerase chain reaction on dried blood spots in Botswana’s national program for prevention of mother-to-child transmission. Pediatr Infect Dis J. 2008;27(1):22–26. doi: 10.1097/INF.0b013e3181469050. [DOI] [PubMed] [Google Scholar]
- Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014;11(2):e1001608. doi: 10.1371/journal.pmed.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebuy H, Yebyo H, Alemayehu M. Level of adherence and predictors of adherence to the Option B+ PMTCT programme in Tigray, northern Ethiopia. Int J Infect Dis. 2015;33:123–129. doi: 10.1016/j.ijid.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong’ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17(5):564–580. doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- Goga AE, Dinh TH, Jackson DJ, Lombard C, Delaney KP, Puren A, South Africa, P.E.T. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health. 2015;69(3):240–248. doi: 10.1136/jech-2014-204535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Wawer MJ. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- Jasseron C, Mandelbrot L, Dollfus C, Trocme N, Tubiana R, Teglas JP, Warszawski J. Non-disclosure of a pregnant woman’s HIV status to her partner is associated with non-optimal prevention of mother-to-child transmission. AIDS Behav. 2013;17(2):488–497. doi: 10.1007/s10461-011-0084-y. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report. UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- Kenya National Bureau of Statistics. Kenya Demographic and Health Survey 2014. Nairobi, Kenya: Kenya National Bureau of Statistics; 2015. [Google Scholar]
- Kiarie JN, Ong’ ech J, Gachuno O, Muiruri P, Inwani I, Kinuthia J, Mutsotso W. National evaluation of PMTCT services: Kenya. 2012, March 5-8; Paper presented at the 19th Conference on Retroviruses and Opportunitistic Infections (CROI); Seattle, WA. [Google Scholar]
- Kinuthia J, Kiarie JN, Farquhar C, Richardson BA, Nduati R, Mbori-Ngacha D, John-Stewart G. Uptake of prevention of mother to child transmission interventions in Kenya: health systems are more influential than stigma. J Int AIDS Soc. 2011;14:61. doi: 10.1186/1758-2652-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuthia J, Singa B, McGrath C, Odeny B, Langat A, Katana A, John-Stewart G. Non-disclosure of maternal HIV status to partners is associated with less antiretroviral use for PMTCT; Paper presented at the International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Vancouver, Canada. 2015. [Google Scholar]
- Moodley D, Esterhuizen T, Reddy L, Moodley P, Singh B, Ngaleka L, Govender D. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis. 2011;203(9):1231–1234. doi: 10.1093/infdis/jir017. [DOI] [PubMed] [Google Scholar]
- Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23(10):1255–1259. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- NASCOP. Guidelines for Prevention of Mother to Child Transmission (PMTCT) of HIV/AIDS in Kenya. (4TH) 2012 [Google Scholar]
- Nyandiko WM, Otieno-Nyunya B, Musick B, Bucher-Yiannoutsos S, Akhaabi P, Lane K, Wools-Kaloustian K. Outcomes of HIV-exposed children in western Kenya: efficacy of prevention of mother to child transmission in a resource-constrained setting. J Acquir Immune Defic Syndr. 2010;54(1):42–50. doi: 10.1097/QAI.0b013e3181d8ad51. [DOI] [PubMed] [Google Scholar]
- Ochanda B, Schmitz M, Langat A, Mukui I, Mwangi A, Wafula R, Muttai H. HIV exposed infant cohort analysis: results from an innovative method for routinely monitoring longitudinal outcomes in HIV exposed infants, Kenya; 2014, July 20-25; Paper presented at the 20th International AIDS Conference; Melbourne, Australia. 2014. [Google Scholar]
- Schechter J, Bakor AB, Kone A, Robinson J, Lue K, Senturia K. Exploring loss to follow-up among women living with HIV in Prevention of Mother to Child Transmission programmes in Cote d’Ivoire. Glob Public Health. 2014;9(10):1139–1151. doi: 10.1080/17441692.2014.970659. [DOI] [PubMed] [Google Scholar]
- Spangler SA, Onono M, Bukusi EA, Cohen CR, Turan JM. HIV-positive status disclosure and use of essential PMTCT and maternal health services in rural Kenya. J Acquir Immune Defic Syndr. 2014;67(Suppl 4):S235–242. doi: 10.1097/QAI.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JS, Stinson K, Tih PM, Giganti MJ, Ekouevi DK, Creek TL, Coetzee D. Measuring coverage in MNCH: population HIV-free survival among children under two years of age in four African countries. PLoS Med. 2013;10(5):e1001424. doi: 10.1371/journal.pmed.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor Car L, van-Velthoven MH, Brusamento S, Elmoniry H, Car J, Majeed A, Atun R. Integrating prevention of mother-to-child HIV transmission (PMTCT) programmes with other health services for preventing HIV infection and improving HIV outcomes in developing countries. Cochrane Database Syst Rev. 2011;(6):CD008741. doi: 10.1002/14651858.CD008741.pub2. [DOI] [PubMed] [Google Scholar]
- Turan JM, Nyblade L. HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: a review of the evidence. AIDS Behav. 2013;17(7):2528–2539. doi: 10.1007/s10461-013-0446-8. [DOI] [PubMed] [Google Scholar]
- World Health Organization. A short guide on methods: measuring the impact of national PMTCT programmes: towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]


