Abstract
Decellularization allows the production of extracellular matrix (ECM) scaffolds. Here we describe the use of combined positive pressure and negative pressure to drive decellularization reagents into the vasculature and airways, respectively, of structurally intact lungs in order to remove cells and cellular material leaving an intact ECM scaffold.
Keywords: Decellularization, Lung, Negative pressure bioreactor, Organ scaffold
1 Introduction
Organ decellularization involves the removal of cells and cellular material from an organ leaving the extracellular matrix (ECM) scaffold. The resulting scaffold is composed of many different molecules including collagen, elastin, fibronectin, and proteoglycans [1]. Such scaffolds can be used to study the sub-structure of the organ or can be recellularized for a variety of investigational or clinical applications [2, 3].
The lung is a heterogeneous organ at both the structural and cellular level. It contains over 60 different cell types and structurally can be grossly divided into parenchyma, blood vessels, conducting airways, and alveolar airways [4]. The alveolar tissue, which makes up approximately 65% of the total lung tissue area, is highly cellular with only 12%–15% being ECM [5]. In contrast, the non--alveolar tissue is much less cellular, with approximately 50% of the tissue being ECM.
Several methods of lung decellularization have been described [1, 6, 7]. The various methods differ in which reagent combinations are used, which portal of entry is used to deliver the reagents (airway, vasculature, or both), and how the reagents are applied (positive vs negative pressure). We have found that utilizing both airways and vasculature, under negative and positive pressure, respectively, allows for the best penetration of the decellularization reagents. Different reagent combinations provide different degrees of decellularization and have different effects on the ECM [8]. An intact ECM is important to the functional structure of the lung, and some components of the ECM such as glycosaminoglycans also seem to play a role in directing cell differentiation and are therefore vitally important when attempting to recellularize a scaffold [1]. This protocol prepares lung tissue for recellularization and thus is designed to optimize removal of cells and functional cellular material while minimizing effects on the ECM. Collagen, the most abundant molecule in lung ECM, remains relatively intact after decellularization with this protocol [9]. Other components of the ECM such as laminin, elastin, and glycosaminoglycan’s remain structurally intact, but at lower levels than non-decellularized lungs due to some of these molecules being removed by the decellularization process [1, 9]. DNA is completely eliminated by this process, at least as determined using conventional detection methods.
2 Materials
2.1 Reagents
Prepare reagents in 5–10 L carboys with spouts. Prepare all reagents with deionized water. Sterilely filter all reagents through 0.22 µm filter after mixing.
Deionized Water (DI H2O)
Triton: 0.1% v/v Triton X--100
Sodium Deoxycholate: 2 % w/v (See Note 1)
Sodium Chloride: 1 M NaCl
DNase: 30 µg/ml DNase, 1.3 mM MgSO4, 2 mM CaCl2. (See Note 2)
Phosphate Buffered Saline (PBS)
2.2 Pumps and Controller
Pump Controller Device: Device which can be programmed to control rate and direction of flow based on pressure measurements and a time schedule. Controllers are available from several manufactures. (We use Harvard Apparatus Catalog # 132500)
Two peristaltic pumps: Capable of pumping approximately 5–100 ml/min. (We use Harvard Apparatus Catalog # 132400)
Pressure transducer (Working range between 0 and 500 mmhg)
Four low resistance check valves (one-way valves) with barb connectors to attach tubing
2.3 Bioreactor
Air-tight chamber with tubing attachments in the lid. Sufficient size to contain lung without touching walls of chamber. This can be custom built. (See Note 3)
2.4 Ventilator Reservoir
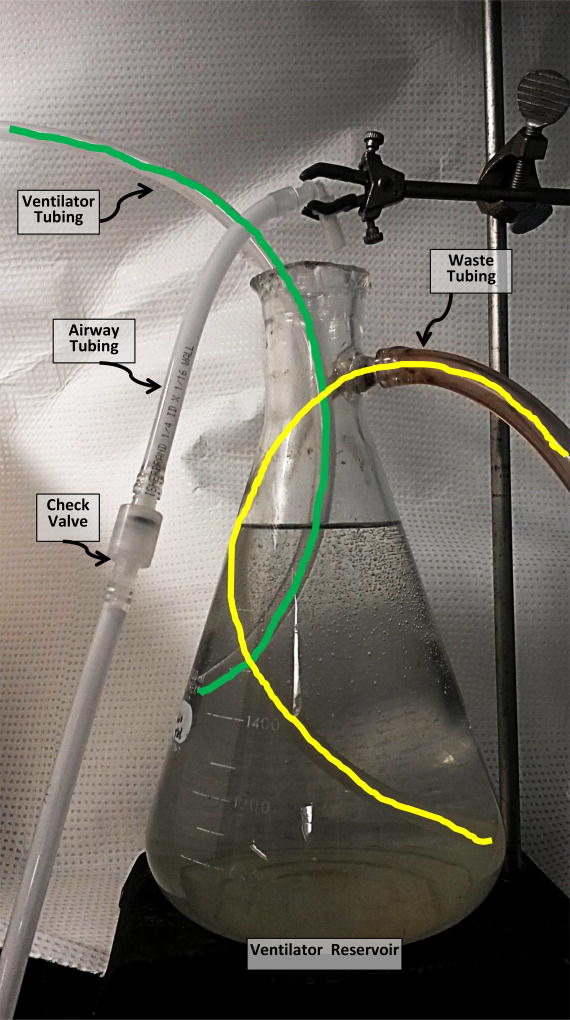
Erlenmeyer flask with spout, 1L capacity. Attach tube from interior of spout to bottom of the flask. (Fig. 1)
Fig. 1.
Photo of Ventilator Reservoir. Lines are drawn over tubing in this image to allow the path to be followed inside the flask.
2.5 Principles of Bioreactor system function
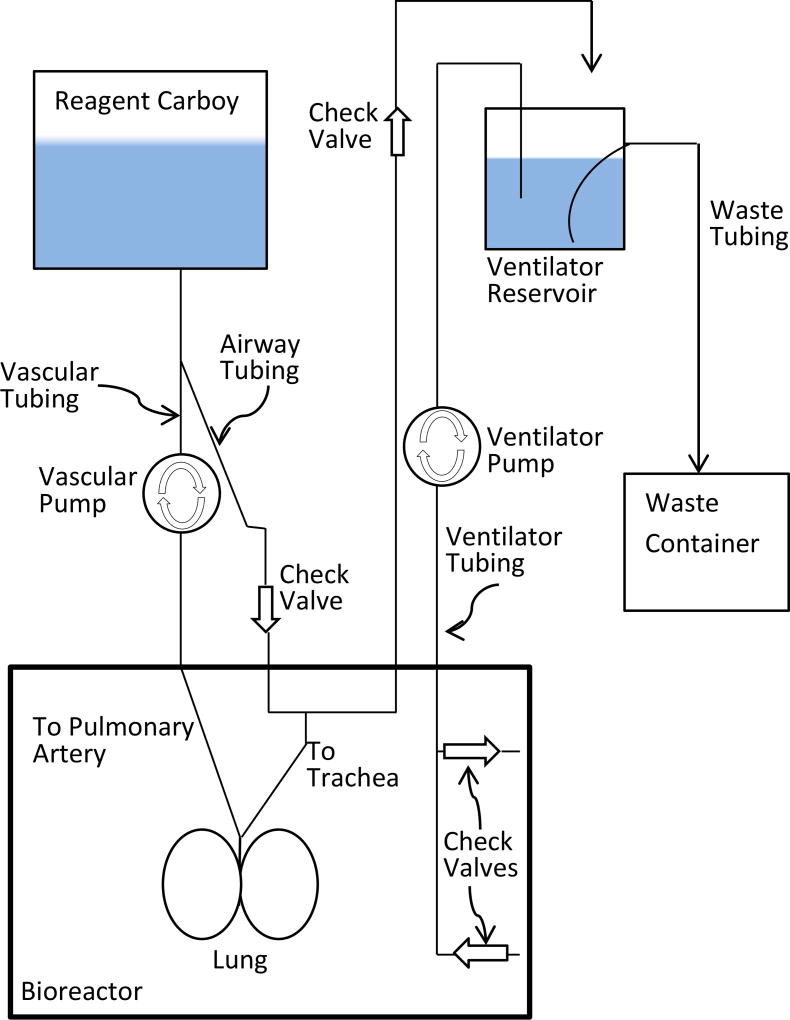
2.5.1 Vascular
The vascular Pump uses positive pressure to pump reagent from the Reagent Carboy into the pulmonary artery. The reagent passes through the vasculature and drains out of the pulmonary vein.
2.5.2 Airway
The airway is infused with reagent which is drawn into the trachea from the Reagent Carboy by negative pressure as fluid is removed from the bioreactor by the Ventilator Pump. The reagent is then pushed into the Ventilator Reservoir by positive pressure as fluid is returned to the Bioreactor from the Ventilator Reservoir. A Check Valve between the Reagent Carboy and the trachea ensures that no reagent refluxes from the airway back to the Reagent Carboy. Likewise, a Check Valve between the trachea and the Ventilator Reservoir ensures no air or waste reagent is drawn back into the lung. The distal end of the Airway Tubing is suspended above the Ventilator Reservoir to allow visualization of fluid flow.
2.5.3 Ventilator system
The Ventilator Pump alternately moves fluid back and forth between the Bioreactor and the Ventilator Reservoir. Check valves at the proximal end of the Ventilator Tubing help to remove debris from the lower portion of the bioreactor and return fluid to the upper portion. The distal end of the Ventilator Tubing is positioned halfway between the spout and the bottom of the Ventilator Reservoir. This ensures that the Ventilator Tubing will be deep enough to remain submerged when fluid is drawn from the Ventilator Reservoir, but not so deep that debris is drawn in from the bottom. The Waste Tubing allows excess fluid to drain from the Ventilation Reservoir. The interior portion of the Waste Tubing should extend to the bottom of the Ventilator Reservoir to allow debris to flow to the Waste Container.
3 Methods
Attach tubing to the decellularization reagent carboy and run pumps to prime tubing with reagent and expel all air.
Fill ventilator reservoir with DI H2O. Ensure that the end of the ventilator tubing is properly positioned in the ventilator reservoir. (See Note 4)
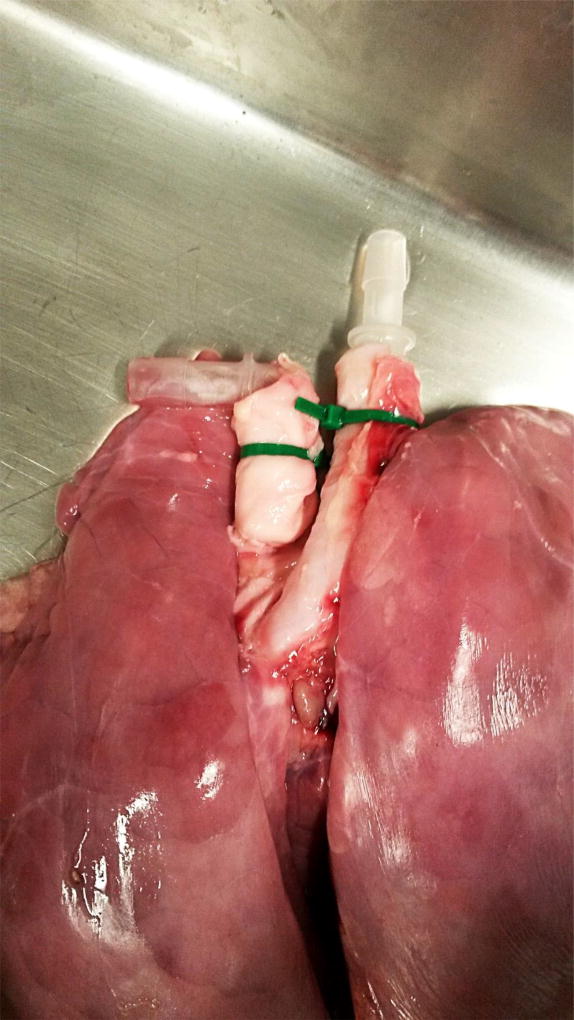
Insert a barb connector into the trachea and place a zip-tie over the tissue and barb connector to secure lung to the connector. (Fig. 2)
Insert a barb connector into pulmonary artery and place a zip-tie over the tissue and barb connector to secure lung to the connector. (Fig. 2)
Attach lung to bioreactor tubing using the barb connectors (Figs. 3 and 4).
Fill bioreactor with DI H2O. Fill bioreactor to the brim, minimizing airspace as much as possible.
Close bioreactor. Ensure lid is air-tight.
Loosen the lid of the reagent carboy to prevent a vacuum from forming when fluid is removed.
Zero the pressure transducer.
Set the vascular pump to maintain a continuous pressure of 15 mmHg with a minimum flow of 5 ml/min.
- Set ventilator pump as follows:
- Expel approximately 100 ml (See Note 5) of fluid from the bioreactor over 1 to 2 minutes. This will generate negative pressure in the bioreactor and draw fluid from the carboy into the airway.
- Pause for 10 min
- Pump 70 ml (See Note 6) into the bioreactor over 1 to 2 minutes
- Pause for 1 min
- Repeat starting from step a
Allow the bioreactor to run for the duration listed in Table 1.
After the time has elapsed for a particular decellularization reagent, pause the pumps.
Disconnect the tubing from the reagent carboy and reconnect to the next reagent carboy.
Restart the pumps. Continue to transition from one carboy to the next, referencing Table 1 for run durations, until the lung has been treated with all of the decellularization reagents. (See Note 7)
The process can be further automated by the use of a computer-controlled manifold to automatically switch between the decellularization reagents at the appropriate times without manually disconnecting the tubing from one reagent carboy and reconnecting to another.
Store the decellularized lung in an isotonic solution such as PBS or NaCl, with 50–100 I.U./mL penicillin and 50–100 µg/mL streptomycin.
Fig. 2.
Lung with barb connectors in the Pulmonary Artery (left) and Trachea (right)
Fig. 3.
Bioreactor assembly
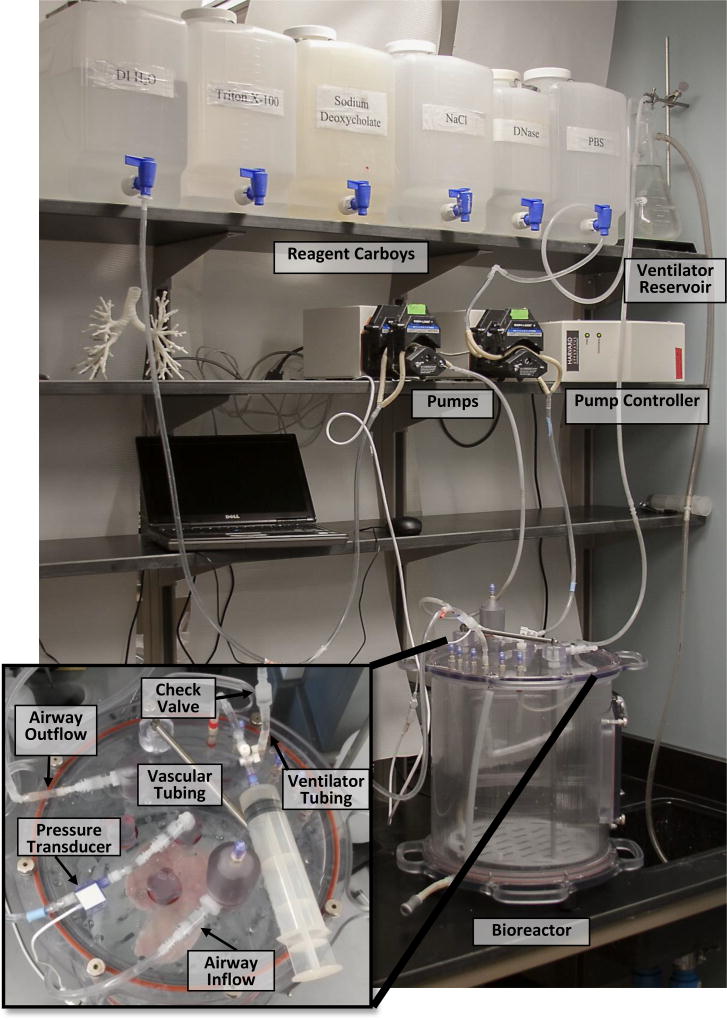
Fig. 4.
Photo of decellularization system with close-up of the top of the bioreactor showing the connectors
Table 1.
Decellularization Reagent Run Times
| Decellularization Reagent | Run Times (In Hours) |
|---|---|
| DI H2O | 2 |
| Triton | 6 |
| Sodium Deoxycholate | 6 |
| NaCl | 2 |
| DNase | 2 |
| PBS | 5 |
4 Notes
-
Sodium Deoxycholate is a lung irritant. Wear a mask when weighing Sodium Deoxycholate and avoid generating dust.
The fine powder can be difficult to dissolve. We recommend preparing a 4–8% stock solution on a heated stir-plate which can then be diluted in the carboy as needed.
Do not reconstitute DNase until a few hours prior to using. DNase begins to degrade within a few hours of reconstitution. Once reconstituted, degradation can be slowed by storing between 2 and 8 °C until ready to use.
Our bioreactors are cylinders constructed of translucent acrylic plastic. We use a 24 cm diameter × 27 cm tall bioreactor for lungs from pigs under approximately 20kg, or individual lung lobes. We use a 34 cm diameter × 36 cm tall bioreactor for lungs from larger pigs.
The distal end of the ventilator tubing is positioned halfway between the spout and the bottom of the Ventilator Reservoir. This ensures that the Ventilator Tubing will be deep enough to remain submerged when fluid is drawn from the Ventilator Reservoir, but not so deep that debris is drawn in from the bottom.
The volume expelled is the tidal volume of the lungs and may need to be adjusted based on the size of the lungs.
The volume returned to the bioreactor is less than the volume expelled to account for the volume of the reagent being continuously added through the vasculature.
If the decellularization process needs to be separated into multiple days, remove the lung from the bioreactor and store at between 2 and 8 °C in an isotonic solution such as PBS or NaCl, with 50–100 I.U./mL penicillin and 50–100 µg/mL streptomycin. Once the lung is in the storage container, use a damp paper towel to cover any portions of the lung exposed to air to prevent those areas from drying out.
References
- 1.Price AP, England KA, Matson AM, et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–91. doi: 10.1089/ten.TEA.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crabbé A, Liu Y, Sarker SF, et al. Recellularization of decellularized lung scaffolds is enhanced by dynamic suspension culture. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0126846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols JE, La Francesca S, Vega SP, et al. Giving new life to old lungs: methods to produce and assess whole human paediatric bioengineered lungs. J Tissue Eng Regen Med. 2016 doi: 10.1002/term.2113. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 4.Franks TJ, Colby TV, Travis WD, et al. Resident Cellular Components of the Human Lung: Current Knowledge and Goals for Research on Cell Phenotyping and Function. Proc Am Thorac Soc. 2008;5:763–766. doi: 10.1513/pats.200803-025HR. [DOI] [PubMed] [Google Scholar]
- 5.Stone KC, Mercer RR, Freeman BA, et al. Distribution of Lung Cell Numbers and Volumes between Alveolar and Nonalveolar Tissue. Am Rev Respir Dis. 1992;146:454–456. doi: 10.1164/ajrccm/146.2.454. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchiya T, Mendez J, Calle EA, et al. Ventilation-Based Decellularization System of the Lung. Biores Open Access. 2016;5:118–126. doi: 10.1089/biores.2016.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raredon MSB, Rocco KA, Gheorghe CP, et al. Biomimetic Culture Reactor for Whole-Lung Engineering. Biores Open Access. 2016;5:72–83. doi: 10.1089/biores.2016.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He M, Callanan A. Comparison of Methods for Whole-Organ Decellularization in Tissue Engineering of Bioartificial Organs. Tissue Eng Part B Rev. 2013;19:194–208. doi: 10.1089/ten.teb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price AP, Godin LM, Domek A, et al. Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Eng Part C Methods. 2015;21:94–103. doi: 10.1089/ten.TEC.2013.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]