Abstract
Introduction
Responding adaptively to one’s social environment is a key factor predicting the course of major depressive disorder (MDD). Socially rejecting events can exacerbate, whereas socially accepting events can ameliorate depressive symptoms. The neural responses to rejection and acceptance in MDD are relatively unexplored.
Methods
We used functional magnetic resonance imaging (fMRI) to measure neural responses to romantic rejection and acceptance in women diagnosed with current MDD (n=19) and a matched group of healthy controls (HCs) (n=19). During fMRI, participants received rejecting, accepting, and neutral feedback from self-selected potential romantic partners.
Results
In women with MDD but not HCs, rejection significantly increased activity in the right anterior insula relative to neutral feedback. Greater activation during rejection was found in the dorsal anterior cingulate cortex in MDD compared to HCs. Women with MDD reported stronger emotional responses than HCs to both rejection and acceptance. In addition, left and right nucleus accumbens (NAcc) activity mediated the relationship between trait reward responsiveness and increased ratings of feeling “happy and accepted” following acceptance in HCs, but not the MDD group.
Discussion
Women with MDD were behaviorally and neurally hyperresponsive to rejection. Although both groups were behaviorally responsive to acceptance, in MDD this was dissociated from NAcc activity. These findings highlight abnormal behavioral and neural responses to social cues in MDD, with implications for disease prognosis and the development of novel and sensitive biomarkers for MDD focused on neural pathways for social-affective processing.
Limitations
Conclusions may be limited to depressed women in a romantic context.
Introduction
Being disliked by others can lead to low self-esteem (Leary and Baumeister, 2000) —a significant causal factor for major depressive disorder (MDD) (Sowislo and Orth, 2013). Socially rejecting events such as childhood parental rejection, adolescent peer victimization, and unwanted romantic breakups have been shown to be among the strongest predictors of MDD compared to other types of life stressors (Copeland et al., 2013; Kendler et al., 2003; Monroe et al., 1999; Rapee, 1997; Slavich et al., 2009). Once MDD develops, increased sensitivity to rejection can exacerbate symptoms (Boyce et al., 1992; Joiner and Coyne, 1999). Conversely, social support ameliorates stressors such as childhood maltreatment and peer rejection (Kaufman et al., 2004; Zimmer–Gembeck et al., 2007) and has positive effects on depressive symptoms (George et al., 1989). Thus, the quality of one’s social environment influences not only the development, but the course of MDD. The current study investigated the neural responses to social rejection and acceptance in women with current MDD compared to healthy controls (HC) using functional magnetic resonance imaging (fMRI). The goal of this study was to better understand how patients with MDD function in the social environment, with implications for disease prognosis and novel treatment strategies that may focus on increasing social resilience and competency.
Despite the importance of the social environment on the course of MDD, only a limited number of studies have examined the neural responses to social rejection or acceptance in current MDD. Using positron emission tomography (PET), we previously found that participants with MDD had an overall reduced release of endogenous opioids in response to rejection and acceptance, suggesting a disrupted ability to recover from rejection, and a lack of sustained pleasure from acceptance (Hsu et al., 2015). Three studies have used fMRI to examine blood-oxygen-level dependent (BOLD) signal in response to rejection and acceptance in MDD. In the first study, young participants (age 15–24 years) were given feedback during scanning that they were liked by peers, based on “first impressions” of the participant’s photograph. Compared to HCs, participants with current MDD exhibited greater BOLD activity in the amygdala in response to the positive feedback (Davey et al., 2011). In the second study, also in young participants (age 11–17 years), greater BOLD activity was found in participants with MDD compared to HCs in the amygdala, subgenual anterior cingulate cortex (sgACC), anterior insula (AI), and nucleus accumbens (NAcc) during the rejection in an online chatroom interaction task (Silk et al., 2014). Whereas the first study examined only acceptance and found greater amygdala activity in MDD (Davey et al., 2011), the second study examined both rejection and acceptance, but found greater amygdala activity in MDD (specifically, attenuated deactivation compared to HCs) only during rejection (Silk et al., 2014). Both of these studies examined the responses of young participants to social feedback from peers. The third study examined medicated MDD adults and compared their neural responses to increasing levels of social exclusion with those of HCs (Kumar et al, 2017). Using Cyberball, a virtual game in which participants are excluded by anonymous others, the authors found that participants with MDD showed greater activity in the amygdala, insula, and ventrolateral prefrontal cortex (vlPFC) during exclusion. Using a novel fMRI task, the present study examined explicit and targeted rejection and acceptance in adult MDD.
The current study focused on women with MDD and an age-matched sample of HC women (age 18–55 years) who were scanned with fMRI while receiving rejecting or accepting feedback from potential romantic partners. We focused on women only in this study because they are more likely to be diagnosed with MDD (Weissman et al., 1996) and have longer and more frequent relapse episodes (Oquendo et al., 2013). In addition, failed interpersonal relationships and low social support have been shown to be more predictive of MDD in women compared to men (Kendler et al., 2005; Kendler and Gardner, 2014). We chose to use a “romantic” feedback paradigm because opposite-sex feedback has been consistently shown to result in greater neural responses, suggesting greater salience, compared to same-sex feedback (Davey et al., 2010; Somerville et al., 2010; Bolling et al., 2012; Silk et al., 2014), assuming that participants in these studies were mostly heterosexual. Furthermore, to increase the salience of the feedback in the current study, we developed a paradigm in which a participant personally selected highly desired preferred-gender dating partners, which were then used in that participant’s individual fMRI task (similar to our previous PET studies: (Hsu et al., 2015, 2013)).
Based on previous fMRI studies in MDD (Davey et al, 2011; Kumar et al, 2017; Silk et al, 2014), we hypothesize that rejection will result in greater activity in the sgACC, AI, amygdala, vlPFC, and NAcc compared to HCs. We also hypothesize greater activity in response to rejection in the dorsal anterior cingulate cortex (dACC), a region consistently activated using rejection/exclusion paradigms (Eisenberger, 2012). In response to acceptance, we hypothesize that the NAcc, a primary reward region sensitive to social reward (Gossen et al, 2014), will be more active in HCs compared to participants with MDD, and mediate subjective feelings of happiness.
Materials and Methods
Participants
Twenty women diagnosed with current MDD and 20 age-matched HC women were recruited from the community through local advertisements (mean age in years±s.d.; MDD=29.6±11.0; HC=29.8±11.1). The M.I.N.I International Neuropsychiatric Interview (Sheehan et al., 1998) was used to diagnose for current MDD and to screen HCs for current or past history of psychiatric disorders. Participants with MDD had mild to moderate depression severity (17-item Hamilton Depression Rating Scale (Hamilton, 1960), 14.8±3.0, range 10–21), with an average age at onset of first episode 18. 38±7.37 years (range 9–38). Of the 20 participants with MDD, 14 had experienced more than 5 distinct episodes of depression prior to the study. Fifteen participants met criteria for Recurrent MDD, and 9 met criteria for MDD with melancholic features. Seven participants with MDD had a family history of depression. Four participants with MDD were on SSRI monotherapy at the time of the study but still met criteria for a current depressive episode (HAMD-17, 12.75±3.0). All other participants were free of psychotropic medications for at least two months. For a table of symptom expression for the depressive episode at the time of the study, see SI Table 1. One participant with MDD was excluded from analyses due to an imaging artifact persistent in all four runs, causing broad signal dropout in the striatum. One HC was excluded due to movement (maximum frame displacement (FD) > 3 in half or more runs; FD was calculated for each participant run (Power et al., 2014)). The final sample included 19 MDD and 19 HC participants (see SI Table 2 for full demographics). All protocols were approved by the Institutional Review Board of the University of Michigan Medical School, and written informed consent was obtained.
Social Feedback Task
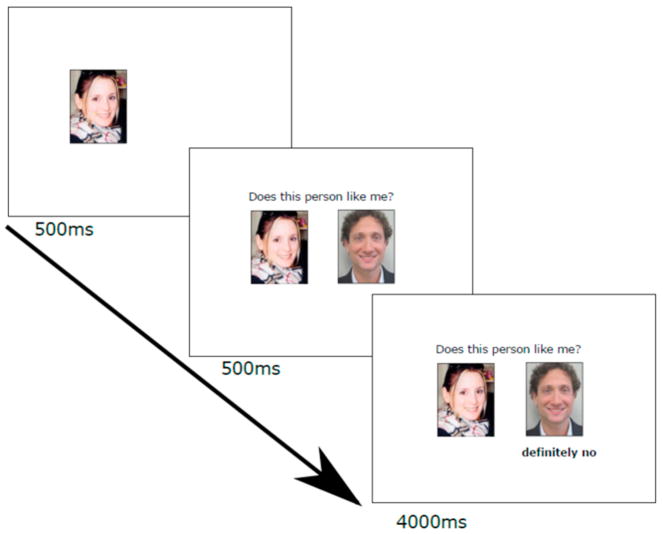
The Social Feedback Task used in our PET studies (Hsu et al., 2015, 2013) was adapted for this fMRI study and stimuli were presented using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Participants viewed fictional dating profiles of their preferred gender and rated each profile on how much they would like the potential partner and how much they expected to be liked back. To increase the saliency of the feedback, only the highest rated profiles were presented during fMRI. During scanning, participants received three types of feedback: rejection (Rej; “very likely no” and “definitely no”), acceptance (Acc; “very likely yes” and “definitely yes”), and neutral (Neu; “not completed”), in a blocked design (Figure 1). Each block consisted of four 5-second trials of the same feedback type, with a 10 to 14 second jittered interval between blocks. Each participant completed 4 functional scan runs, each containing 6 pseudorandomized blocks (2 blocks each of Rej, Acc, and Neu). Each run was 3 minutes, 12 seconds plus approximately 30 seconds shim time between runs, for a total of approximately 15 minutes.
Figure 1.
Task design. Each trial began with the participant’s own headshot for 500ms, the addition of the question “Does this person like me?” along with picture from a highly-rated profile (500ms), followed by the feedback (4000ms). A rejection trial is shown. Rejection trials contained the feedback “very likely no” or “definitely no”, acceptance trials contained the feedback “very likely yes” or “definitely yes”, and neutral trials contained the feedback “not completed” to indicate that this person had not yet completed his rating of the participant. Four trials of the same feedback type were presented in one block, and cross-hair fixations (10–14s) were presented between blocks.
To assess emotional changes, following the scan session participants were reminded of the feedback they received during the scan in three blocks (18 trials of one feedback type per block). After each block participants completed state versions of the Rosenberg Self-Esteem scale (Rosenberg, 1965), the Desire for Social Interaction scale (Hsu et al., 2013), and indicated how “happy”, “sad”, “rejected”, and “accepted” they felt. Responses to 5-point Likert-type scales were recorded using a 5-button response box. Similar to our previous studies (Hsu et al., 2015, 2013) the scores for “sad” and “rejected”, and “happy” and “accepted” were averaged for analysis. The Social Feedback Task did not involve deception, however participants were asked to immerse themselves in the experience and imagine that the feedback was real (Hsu et al., 2015, 2013) (see SI 3).
Scores for trait reward responsiveness (BAS-RR, (Carver and White, 1994)), the ability to experience pleasure in anticipation of reward, were obtained from a subscale of the behavioral activation system prior to fMRI scanning. State emotions were analyzed using mixed two-way ANOVAs, with group (MDD, HC) and feedback type (Rej, Acc, Neu) as between- and within- group factors, respectively.
fMRI Data Acquisition
Whole-brain functional images were obtained by using a T2*-weighted pulse sequence in a 3.0 Tesla GE Signa 9.0 scanner (Milwaukee, WI, USA) with a standard frequency coil. To reduce signal dropout in subcortical and around sinus regions, single-shot combined spiral in/out sequence was used (Glover and Law, 2001) (repetition time, TR, 2000ms; echo time, TE, 30ms; flip angle, 90°; field of view, FoV, 20cm × 20cm, 64 × 64 matrix; in-plane resolution, 3.13 × 3.13mm; slice thickness, 4mm). A high-resolution T1-weighted pulse sequence was acquired to provide anatomical localization (3D spoiled gradient recalled echo; TR, 12ms; TE, 5ms; TI, 500ms; flip angle, 15°; FoV, 26cm × 26cm, 256 × 256 matrix; in-plane resolution, 1.02 × 1.02mm; slice thickness, 1.2mm). Head motion was minimized using foam pads and a forehead strap.
Analysis
Functional 4D images were preprocessed using a standard pipeline in FMRIB Software Library (FSL) and included slice time correction, realignment, spatial smoothing (5 mm full-width at half maximum) using a Gaussian kernel, coregistration, and normalization to MNI standard space (Montreal Neurological Institute, Quebec, CA). Participant scans were screened for movement with a threshold of 3mm translation and 3° rotation. T-maps were calculated for each participant for the primary contrasts Rej>Neu and Acc>Neu. For each contrast, group-level random effects analyses were performed using Statistical Parametric Mapping v.8 (SPM8; Wellcome Institute of Cognitive Neurology, London, UK). A whole-brain gray matter mask was applied (Wager, 2018), and voxel-wise whole-brain one-sample (HCs only; MDDs only) and two-sample (HCs vs. MDDs) t-tests (FWE-corrected P<.05) were performed. All fMRI analyses reported here use voxel-based comparisons with familywise error corrections. Following whole-brain t-tests, contrasts were further explored using regions of interest (ROIs) small-volume correction (SVC) in SPM8 using the same primary thresholds.
ROI masks were anatomically defined using the Harvard Brain Atlas (“The Whole Brain Atlas,” 2017) probability masks thresholded at .25 confidence and binarized, and included the dACC, sgACC, AI, amygdala, vlPFC, and NAcc, for a total of 12 bilateral masks. The dACC masks were bounded between y=[0, 36] and the AI masks were bounded at y=8, based on a previous study that examined neural responses to social exclusion (Way et al., 2009). Averaged BOLD signal across all voxels within each ROI were extracted for both the Rej>Neu and Acc>Neu contrasts, using the MarsBar region of interest toolbox (v.0.38) for SPM8. Extracted ROI data were screened for normality, outliers, and unequal variances, and analyzed using R (Foundation for Statistical Computing, Vienna, Austria).
The extracted ROI values were tested as correlates of state emotional changes using Pearson’s correlations. Our hypothesized model for ROI activity as a mediator of significant state-trait correlations was assessed using Bayesian mediation analyses. Given our relatively small sample size, we took this approach because Bayesian methods do not rely as heavily on sample size or distribution normality (Lee and Song, 2004). Additionally, Bayesian mediation has been shown to outperform frequentist methods of increasing power (bootstrapping) while minimizing Type I error rates (Koopman et al., 2015). Analyses were done using a Bayesian hypothesis test for mediation described by Nuijten and colleagues (Nuijten et al., 2014), using the R package BayesMed. Parameters were estimated (Yuan and MacKinnon, 2009) via a Markov chain Monte Carlo (MCMC) algorithm computed through Just Another Gibbs Sampler (JAGS) set with a burn-in of 1000 steps and 10,000 iterations of collected samples for each relationship. Posterior densities for each parameter were plotted and evaluated for normality and to examine credibility intervals, means, and medians for each parameter distribution (α, β, τ′, and the indirect effect of α*β). Bayes Factors (BF10) describing the likelihood of data fitting the order-restricted hypothesized direction of each relationship contrasted with the null hypothesis and an unrestricted model, are estimated. Using this analysis technique, a BF10>1 indicates support in favor of the hypothesized effect (alternative hypothesis) (Nuijten et al, 2014).
Results
Behavior
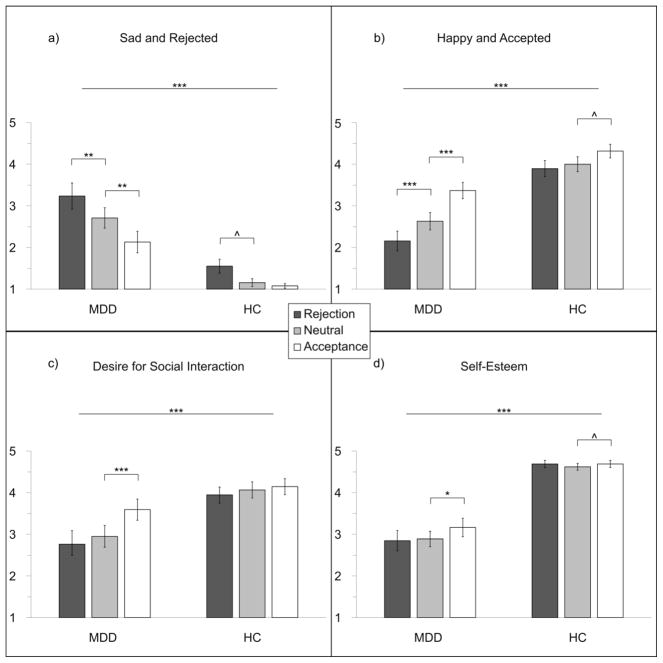
The Social Feedback Task influenced emotional states across both groups (Figure 2). The type of feedback received (Rej, Acc, or Neu) had a significant main effect on ratings of “sad and rejected” (F2,72=19.03, P<.001), ratings of “happy and accepted” (F2,72=25.48, P<.001), self-esteem (F2,72=5.13, P=.008) and desire to for social interaction (F2,72=11.13, P<.001). There was a significant main effect of group on all four state measures. Compared to HCs, participants with MDD scored higher on “sad and rejected” (F1,36=57.93, P<.001) and lower on “happy and accepted” (F1,36=30.50, P<.001), self-esteem (F1,36=57.93, P<.001), and desire for social interaction (F1,36=9.04, P<.005).
Figure 2.
Emotional states reported following different types of feedback, by group: a) Mean ratings (± standard error) of “sad and rejected” increased following rejection and decreased following acceptance in participants with MDD; b) mean ratings of “happy and accepted” decreased following rejection and increased following acceptance in participants with MDD; c) Participants with MDD had a significantly greater desire for social interaction following acceptance; d) Participants with MDD had significantly increased self-esteem following acceptance. All four state measures had a significant effect of group, and a significant interaction between group and feedback type. HCs had an increase in “sad and rejected” (P = .028) following rejection, and an increase in self-esteem (P = .028) and “happy and accepted” (P = .024) following acceptance that did not survive correction for multiple comparisons (corrected P = .0125). ^P<.05, *P<.0125, **P<.01, ***P<.001
There was a significant group-by-feedback interaction on all four state measures (“sad and rejected”, (F2,72=3.36, P=.04); “happy and accepted”, (F2,72=5.81, P=.005); self-esteem, (F2,72=3.64, P=.03); desire for social interaction, (F2,72=4.82, P=.01)). The interaction effects were largely driven by participants with MDD responding more strongly to rejection and acceptance. Following these significant omnibus tests, within-subject comparisons in each group were conducted to examine the effects of feedback type. Participants with MDD reported feeling significantly more “sad and rejected” (t18=3.04, P=.004) and less “happy and accepted” (t18=4.03, P<.001) following rejection compared to neutral (Figure 2). Participants with MDD also had significantly greater responses to acceptance compared to neutral on all four state measures (feeling less “sad and rejected”, t18=3.45, P=.001; more “happy and accepted”, t18=5.08, P<.001; more self-esteem, t18=2.52, P=.011; more desire for social interaction, t18=3.94, P<.001). HCs reported feeling more “sad and rejected” (t18=2.00, P=.030) following rejection, and greater self-esteem (t18=2.05, P=.028) and “happy and accepted” (t18=2.12, P=.024) following acceptance.
fMRI
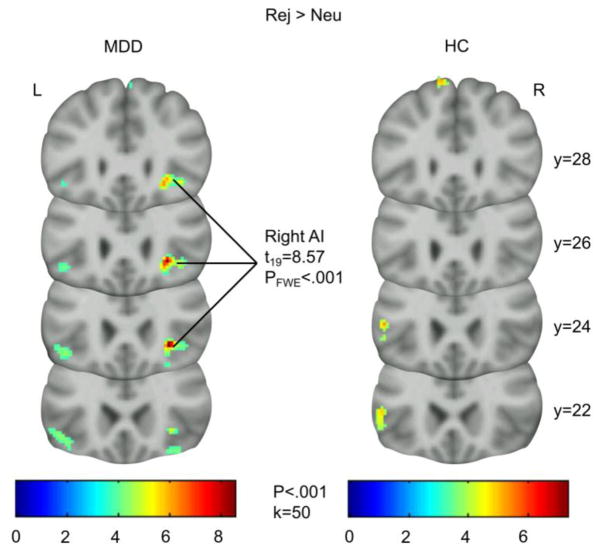
Whole-brain analyses show a strong BOLD signal in the right AI during rejection (Rej>Neu; Figure 3) in the MDD group with FWE correction (x, y, z = 28, 24, −4; t18=8.57, PFWE-whole-brain)<.001). Examination of this peak using a viewing threshold of P=.001 showed that this cluster spread anteriorly, into the right vlPFC. No other peaks were significant at the whole-brain level with a threshold PFWE-whole-brain<.05. Using our ROI mask, small-volume correction (PFWE-SVC<.05) on group comparisons revealed significantly greater activity in the left dACC in the MDD group (MDD>HC, Rej>Neu: x, y, z = −6, 20, 24; t18=4.6, PFWE-SVC=.032). Data extracted from this cluster (threshold P<.001) showed that this difference was due to decreased activation in the HC group (Rej>Neu: −.0845) and increased activation in the MDD group (Rej>Neu: .1757). There was no other significant activity between or within groups for any contrast at whole-brain PFWE<.05, however the results of post-hoc analyses can be found in SI Table 3.
Figure 3.
Significant activity (PFWE-whole-brain < .001, voxel-wise analysis) in right AI during Rej>Neu trials in participants with MDD, but not HCs. AI, anterior insula; MDD, major depressive disorder group; HC, healthy control group; Rej>Neu, rejection - neutral contrast.
Bayesian Modeling
Extracted ROI values were not found to be related to state behavior outcomes in the Rej>Neu contrast in either group. BAS-RR was significantly correlated with feeling “happy and accepted” following acceptance in HCs (r=.62, P=.004), but not in the MDD group (r=.07, P=.77). In HCs, “happy and accepted” was also significantly correlated with the anatomically-based ROI values for left (r=.81, P<.001) and right (r=.72, P<.001) NAcc, but were unrelated in the MDD group (r’s=.02 and .00, P’s=.93 and .99). Fisher’s r-to-Z transformations followed by one-tailed Z-tests showed that r’s between groups were significantly different (BAS-RR and feeling “happy and accepted”: Z=1.85, P=.03; feeling “happy and accepted” and left NAcc: Z=3.13, P=.0009; feeling “happy and accepted” and right NAcc: Z=2.57, P=.005). Exploratory paired t-tests on extracted values confirmed no between-groups differences in left (t=.23, P=.82) nor right (t=.15, P=.88) NAcc activity during Acc>Neu.
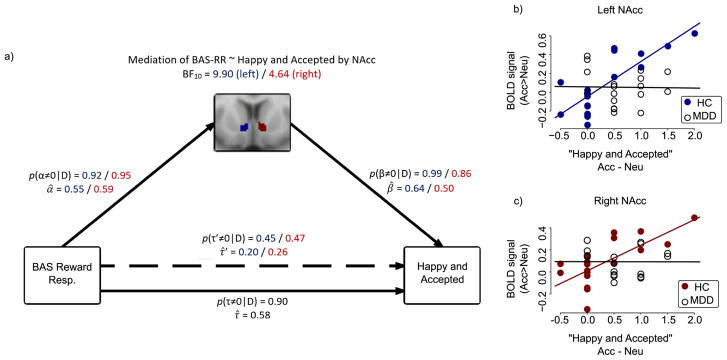
A Bayesian correlation analysis of the relationship between BAS-RR and “happy and accepted” was performed as the basis for a mediation model, and a substantial (Jeffreys, 1961) (τ=.58, BF10=17.4) relationship was found between BAS-RR and “happy and accepted” in HCs. To reflect our hypotheses about these relationships, we assigned paths α, τ′, and β to greater than zero (indicating positive relationships) and found substantial evidence of mediation by the left (BF10=9.91) and right (BF10=4.64) NAcc (Figure 4), such that there was no longer a relationship between BAS-RR and “happy and accepted” once accounting for the left (BF10=.11) and right (BF10=.10) NAcc (indicating complete mediation). Although participants with MDD did not significantly differ from HCs on BAS-RR scores (P=.07), BAS-RR was not predictive of feeling “happy and accepted” in this group (τ=.07, BF10=.22). Furthermore, the extracted NAcc values in the MDD group did not correlate with any of the state behavioral outcomes.
Figure 4.
(a) Strong evidence for mediation by both left and right NAcc of the relationship between BAS-RR and state “happy and accepted” changes during Acc>Neu in HCs. The posterior probability for α (correlation between the independent variable to the mediator) is computed using MCMC parameter estimates, followed by the posterior probability for path β (a partial correlation between the mediator and the dependent variable, controlling for the independent variable). Evidence for a correlation between the independent and dependent variables, controlling for the mediator, is represented by path τ′. The probability for a mediation effect of the mediator on the path between the independent and dependent variable is computed by multiplying the posterior probabilities α and β. Bayes Factor (BF) greater than 1 indicates evidence in favor of the model. Numbers in blue correspond to the model including the left NAcc, and numbers in red correspond to the right NAcc. BF10 is the Bayes Factor for the mediation model as a whole; p(α≠0|D) is the probability that the relationship α exists, given the data; p(β≠0|D) is the probability that the relationship β exists, given the data and controlling for the effects of α; p(τ≠0|D) is the probability that the relationship τ exists, given the data and before accounting for the mediator; p(τ′≠0|D) is the probability that the relationship τ exists, given the data and after accounting for the mediator; α̂, β̂, τ̂′, and τ̂ represent the mean coefficients for their respective paths. (b) Correlations between left NAcc activity and state “happy and accepted” changes during Acc>Neu in HC group (Pearson’s r = .81, P < .001) and MDD group (r = −.02, P = .93); (c) Correlations between right NAcc activity and state “happy and accepted” changes during Acc>Neu in HC group (r = .72, P >.001) and MDD group (r = −.004, P = .99). Participants indicated how “happy” and “accepted” they felt on a 1–5 Likert-type scale following Acc and Neu blocks. Scores for each item were averaged together within each condition. Values reported here are the averaged scores during Acc minus the averaged scores during Neu.
Discussion
To our knowledge, this is the first study to investigate how adult women with MDD respond to romantic rejection and acceptance using fMRI. Behavioral and fMRI data showed that women with MDD were sensitive to both rejection and acceptance, and strongly recruited neural regions associated with emotional salience (AI and dACC) during rejection. These results are consistent with earlier findings in which BOLD response in the AI during rejection was increased in a sample of adolescents with MDD (Silk et al., 2014), and in adults with MDD during social exclusion (Kumar et al, 2017). We also extended our earlier PET findings in which the endogenous opioid response in the NAcc to acceptance was associated with social motivational changes in HCs, but not in participants with MDD, despite a greater emotional response to acceptance in the MDD group (Hsu et al., 2015), by modeling the role of this region as a mediator of a known trait-state relationship (Carver and White, 1994). Consistent with our previous study, the current results suggest that women with MDD are hyperresponsive to both rejection and acceptance, however the neural representation of acceptance may be particularly altered in this group.
Sensitivity to rejection is a significant predictor of depression (Chango et al., 2012), especially in women (Ayduk et al., 2001), causing increased self-directed hostile cognition (Breines and Ayduk, 2015) and maladaptive rumination (Pearson et al., 2011). Here, women with MDD showed stronger emotional responses to rejection than HCs, and robust activity in the AI was found in women with MDD (PFWE-whole-brain<.001) but not in HCs. This finding is consistent with studies in which adolescents with MDD vs. HCs showed increased AI activation during peer rejection (Silk et al., 2014), and adults with MDD vs. HCs showed increased insula activation during social exclusion (Kumar et al, 2017). In addition, we demonstrated for the first time that women with current MDD vs. HCs showed significantly greater activity in the dACC in response to rejection. Only one other study reported greater dACC activity in MDD following rejection, however this was in adolescents and the difference was subtle – found only 7 to 9 seconds after the feedback (Silk et al, 2014). It is possible that increased dACC activity in adult MDD may reflect increased recruitment of this region with increasing age for evaluating threatening stimuli (Hung et al, 2012).
Although both groups responded emotionally to social acceptance, a significant group by feedback type interaction effect on emotional ratings suggests that these changes were more pronounced in the MDD group compared to HCs. Previous studies did not assess mood change specific to acceptance (Silk et al, 2014) or showed that acceptance was less rewarding compared to HCs, although a baseline measurement was not reported (Davey et al, 2011). By comparing responses to acceptance relative to the neutral condition, the present study showed increased mood, self-esteem, and desire for social interaction in MDD. This agrees with our previous PET study in which participants with MDD responded more positively than HCs to acceptance relative to baseline (Hsu et al., 2015). This suggests that in our Social Feedback Task, participants with MDD had the capacity to experience pleasure from social reward. Other studies using humor (Sherdell et al., 2012) or sucrose (Dichter et al., 2010) as rewards have also shown that participants with MDD have similar hedonic responses as HCs. However, our neuroimaging results suggest that the neural representation of these apparent hedonic responses in the MDD group may differ from HCs in important ways.
To investigate the role of reward-related neural activity on the relationship between trait reward-responsiveness and state happiness following social reward, we tested a mediation model that included extracted NAcc values. In HCs, but not in the MDD group, NAcc activity was strongly predictive of how “happy and accepted” participants felt following acceptance. Previous work from our lab has shown that the improved mood following acceptance in participants with MDD was short-lived – quickly returning to baseline levels (Hsu et al., 2015). This is also consistent with a study that revealed that sustained BOLD activity in the NAcc was directly related to the ability to sustain positive affect, and that participants with MDD are able to maintain neither BOLD activity nor positive affect for more than a few moments (Heller et al., 2009). Thus, anhedonia in MDD may be a consequence of disturbances in the temporal fluctuation of striatal activity and emotional responses to reward, rather than simply an inability to experience pleasure. Participants with MDD showed no differences in NAcc activity compared to HCs. It is possible that our blocked design did not allow us to detect subtle fluctuations in BOLD activity over time that may correspond to the more extreme emotional responses in the MDD group. Similarly, using an event-related design, one study found that positive social feedback increased amygdala activity in MDD compared to HCs, suggesting heightened sensitivity to social evaluation in MDD (Davey et al., 2011). We did not detect amygdala activation during Acc>Neu in either group, potentially due to a lack of sustained amygdala activity during the 19-second feedback block used in the present study. Future studies will require real-time measurements in NAcc and other regions to directly assess whether these regions play a role in the inability to sustain positive mood following acceptance, and/or are activated during the initial sensitivity to social feedback.
The reward responsiveness subscale of the BAS (BAS-RR) was designed to capture the subjective hedonic quality of rewards and has been directly related to happiness following reward (Carver and White, 1994), as well as ventral striatal/NAcc activity in response to reward (Simon et al., 2010). This is the first study to show a direct relationship between these three variables. In HCs, the relationship between BAS-RR and state happiness with reward originally identified by Carver & White (Carver and White, 1994) was replicated in our study using social acceptance as reward, and here we showed that this relationship was completely mediated by the NAcc. Although the MDD group had an emotional response to acceptance and did not differ from HCs on BAS-RR, no relationship between the BAS-RR and “happy and accepted” could be identified in this group. Furthermore, unlike in HCs, average NAcc BOLD responses to Acc>Neu was neither predicted by BAS-RR nor indicative of positive emotional state outcomes. In their detailed review, Rizvi and colleagues suggest that anhedonia in MDD may reflect a dysfunction of reward anticipation or motivation (Rizvi et al., 2016), rather than experience. Consistent with this hypothesis, our results showed that acceptance was emotionally rewarding for the MDD group, however this was not associated with ventral striatal reward processing, which may be reflective of reward anticipation or motivation.
Although HCs showed emotional responses to rejection, they did not show expected increases in the dACC or AI. Our task is different from the Cyberball task, which consistently activates the dACC (Eisenberger, 2012). In contrast, a meta-analysis of studies using romantic rejection did not find dACC activation (Cacioppo et al, 2013), similar to our finding (although this meta-analysis found only one activated voxel in the dACC from Cyberball studies). Furthermore, the dACC appears to play a role in “expectancy violation” during rejection (Somerville et al, 2006). Since our task presented four trials of the same feedback type in each block, expectancy violation and dACC activity may have only occurred during the first trial when the feedback type was unpredictable, however this analysis would require more trials in an event-related design. Nevertheless, greater dACC activity found in MDD may play a unique adaptive role that requires further study. In one study, women with a past history of MDD showed increased dACC activity during repeated negative evaluation, which was associated with reduced depressive symptoms at a 6-month follow-up (Dedovic et al, 2016). It is not clear why activation in the AI was not detected in HCs, however our modest sample size, relatively large anatomically-based ROIs, and focus on women may have been contributing factors.
We did not find significant activity during Acc>Neu in either group, however activation was detected in the mPFC in HCs at a whole-brain uncorrected threshold of P<.001 (SI Table 3). This is similar to the mPFC activation found during positive social feedback relative to a control condition in healthy adolescents and young adults (also at an uncorrected threshold of P<.001) (Davey et al, 2010). Another study found significant mPFC activation during social acceptance across healthy and depressed adolescents (Silk et al, 2014), and another found significant ventral ACC activation in healthy young adults (Somerville et al, 2006), however the effects in both studies were relative to a rejection condition. In our analyses of acceptance relative to a neutral condition, we did not detect significant activity, however future studies may use larger sample sizes and/or additional ROIs based on peak activations from our task (see SI Table 3 for voxel-wise peaks of activation using whole-brain uncorrected threshold of P<.001).
Since our task did not use deception, group differences in response to social feedback may have also reflected differences in the ability to simulate social experiences. Both groups reported that the task felt “real,” and that their responses to positive feedback were similar to a real-life situation, however when asked about negative feedback, MDD participants reported a greater similarity to real-life situations compared to HC participants (see SI 3). This effect is consistent with the large body of literature showing that MDD is characterized by an increased elaboration of negative information (Gotlib and Joormann, 2010), and increased negative mental imagery (Holmes et al, 2016). Thus, MDD participants may be have been better able to elaborate on and imagine the rejecting feedback, making it feel more “real,” resulting in increased behavioral and neural responses. Future studies will need to examine the role of these cognitive mechanisms in in MDD in response to simulated vs. “real” social feedback.
In summary, the present study found abnormal emotional and neural responses to romantic rejection and acceptance in adult women with MDD, using a novel, ecologically-relevant task. Our findings are consistent with previous studies showing increased activity in the AI and vlPFC during rejection in MDD (Kumar et al, 2017; Silk et al, 2014), and for the first time showed increased activity in the dACC, a region consistently activated using rejection/exclusion paradigms in adults with MDD (Eisenberger, 2012). In response to acceptance, the present study uniquely showed increased mood, self-esteem, and desire for social interaction in MDD, however these behavioral changes were dissociated from NAcc activity. Previous studies of acceptance have not reported this behavioral or neural effect (Davey et al, 2011; Silk et al, 2014), although the study samples of these earlier studies were younger and of mixed gender, compared to the present study in adult women.
Limitations
We found preliminary evidence that women with MDD were hyperresponsive to rejection and acceptance compared to HCs. It is possible that HCs exhibited ceiling and floor effects in emotional state changes compared to participants with MDD, however the strong correlations and mediation effect in HCs indicate that the changes observed in this group displayed a meaningful range of responses, which were directly related to changes in neural activity. Our sample also consisted of women with only mild to moderate MDD severity, but nevertheless we found a robust effect of rejection on women with MDD. The generalizability of these findings may also be limited to MDD in women, and in the context of romantic social interactions. Additional studies would be required to replicate these results or compare them to men with MDD, and in other social contexts.
Concluding remarks
MDD symptoms can be worsened by perceived rejection (Boyce et al., 1992; Joiner and Coyne, 1999), and improved by social support (George et al., 1989). Understanding the mechanism by which patients with MDD process social cues may lead to novel treatment strategies focused on increasing social resilience and competency, particularly for those who are sensitive to their social environment. Indeed, overreacting to rejection cues could have deleterious effects on social relationships (Smart-Richman and Leary, 2009), further reducing social support and contributing to a downward spiral in MDD. Interestingly, our sample of women with MDD had mild to moderate symptoms, despite showing strong behavioral and neural responses to rejection, and enhanced emotional responses to acceptance. This suggests that the social environment may play a large role in MDD prognosis, such that a negative social event may cause a transition to severe MDD, or that a positive social event may accelerate remission. The strong neural responses to rejection that we observed in mild-to-moderate MDD may also serve as a sensitive biomarker for those at risk for worsened symptoms in their current social environment. On the other hand, future studies with more precise temporal evaluation of the neural responses to acceptance may identify those with MDD who would benefit the most from social support and interpersonal therapy, potentially leading to faster recovery.
Supplementary Material
Highlights.
Women with and without depression received romantic social feedback during fMRI
The depressed group was emotionally hyperresponsive to rejection and acceptance
Rejection elicited strong activity in the right anterior insula only in depressed women
Happiness after acceptance was mediated by striatal activity only in healthy women
Abnormal responses to social cues may impact the prognosis of depression
Acknowledgments
We are deeply thankful to Ben Sanford for participant recruitment, MRI scanning, and assistance with data processing.
Footnotes
Conflict of Interest
The authors report no financial interests nor potential conflicts of interest.
Contributions
A.A.Y. analyzed data, interpreted results, and wrote manuscript; E.M performed research; B.S. analyzed data; B.J.M. assisted with research design and data interpretation; T.M.L. assisted with research design and data interpretation; S.A.L. assisted with research design and data interpretation; J.K.Z. assisted with research design and data interpretation; D.T.H. designed and performed research, analyzed data, interpreted results, and assisted with manuscript
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayduk O, Downey G, Kim M. Rejection Sensitivity and Depressive Symptoms in Women. Pers Soc Psychol Bull. 2001;27:868–877. [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC. Differential brain responses to social exclusion by one’s own versus opposite-gender peers. Soc Neurosci. 2012;7:331–346. doi: 10.1080/17470919.2011.623181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce P, Hickie I, Parker G, Mitchell P, Wilhelm K, Brodaty H. Interpersonal sensitivity and the one-year outcome of a depressive episode. Aust N Z J Psychiatry. 1992;26:156–161. doi: 10.3109/00048679209072022. [DOI] [PubMed] [Google Scholar]
- Breines JG, Ayduk O. Rejection Sensitivity and Vulnerability to Self-Directed Hostile Cognitions Following Rejection. J Pers. 2015;83:1–13. doi: 10.1111/jopy.12077. [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319. [Google Scholar]
- Chango JM, McElhaney KB, Allen JP, Schad MM, Marston E. Relational stressors and depressive symptoms in late adolescence: rejection sensitivity as a vulnerability. J Abnorm Child Psychol. 2012;40:369–79. doi: 10.1007/s10802-011-9570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Angold A, Costello EJ. Adult psychiatric outcomes of bullying and being bullied by peers in childhood and adolescence. JAMA Psychiatry. 2013;70:419–26. doi: 10.1001/jamapsychiatry.2013.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yücel M. Being liked activates primary reward and midline self-related brain regions. Hum Brain Mapp. 2010;31:660–668. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Ycel M. Increased amygdala response to positive social feedback in young people with major depressive disorder. Biol Psychiatry. 2011;69:734–741. doi: 10.1016/j.biopsych.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Slavich GM, Muscatell KA, Irwin MR, Eisenberger NI. Dorsal anterior cingulate cortex responses to repeated social evaluative feedback in young women with and without a history of depression. Front Behav Neurosci. 2016;10 doi: 10.3389/fnbeh.2016.00064. https://doi.org/10.3389/fnbeh.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Smoski M, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar Depression Does Not Moderate Responses to the Sweet Taste Test. Depress Anxiety. 2010;27:859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- George LK, Blazer DG, Hughes DC, Fowler N. Social support and the outcome of major depression. Br J Psychiatry J Ment Sci. 1989;154:478–485. doi: 10.1192/bjp.154.4.478. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gossen A, Groppe SE, Winkler L, Kohls G, Herrington J, Schultz RT, et al. Neural evidence for an association between social proficiency and sensitivity to social reward. Soc Cogn Affect Neurosci. 2014;9:661–670. doi: 10.1093/scan/nst033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A RATING SCALE FOR DEPRESSION. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EA, Blackwell SE, Burnett Heyes S, Renner F, Raes F. Mental imagery in depression: phenomenology, potential mechanisms, and treatment implications. Annu Rev Clin Psychol. 2016;12:249–280. doi: 10.1146/annurev-clinpsy-021815-092925. https://doi.org/10.1146/annurevclinpsy-021815-092925. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry. 2015;20:193–200. doi: 10.1038/mp.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, et al. Response of the μ-opioid system to social rejection and acceptance. Mol Psychiatry. 2013;18:1211–7. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y, Smith ML, Taylor MJ. Development of ACC-amygdala activations in processing unattended fear. NeuroImage. 2012;60:545–552. doi: 10.1016/j.neuroimage.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Jeffreys SH. The Theory of Probability. Vol. 9 OUP Oxford; 1961. [Google Scholar]
- Joiner T, Coyne JC. The interactional nature of depression: Advances in interpersonal approaches. American Psychological Association (APA); Washington, DC: 1999. [DOI] [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am J Psychiatry. 2014;171:426–435. doi: 10.1176/appi.ajp.2013.13101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Sex differences in the relationship between social support and risk for major depression: a longitudinal study of opposite-sex twin pairs. Am J Psychiatry. 2005;162:250–256. doi: 10.1176/appi.ajp.162.2.250. [DOI] [PubMed] [Google Scholar]
- Koopman J, Howe M, Hollenbeck JR, Sin H-P. Small sample mediation testing: misplaced confidence in bootstrapped confidence intervals. J Appl Psychol. 2015;100:194–202. doi: 10.1037/a0036635. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter GD, Dubois M, Milders M, Reid I, Steele JD. Increased neural response to social rejection in major depression. Depress Anxiety. 2017 doi: 10.1002/da.22665. [DOI] [PubMed] [Google Scholar]
- Leary MR, Baumeister RF. The nature and function of self-esteem: Sociometer theory. Adv Exp Soc Psychol. 2000;32:1–62. [Google Scholar]
- Lee S-Y, Song X-Y. Evaluation of the Bayesian and Maximum Likelihood Approaches in Analyzing Structural Equation Models with Small Sample Sizes. Multivar Behav Res. 2004;39:653–686. doi: 10.1207/s15327906mbr3904_4. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Rohde P, Seeley JR, Lewinsohn PM. Life events and depression in adolescence: Relationship loss as a prospective risk factor for first onset of major depressive disorder. J Abnorm Psychol. 1999;108:606–614. doi: 10.1037//0021-843x.108.4.606. [DOI] [PubMed] [Google Scholar]
- Nuijten MB, Wetzels R, Matzke D, Dolan CV, Wagenmakers E-J. A default Bayesian hypothesis test for mediation. Behav Res Methods. 2014;47:85–97. doi: 10.3758/s13428-014-0470-2. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Turret J, Grunebaum MF, Burke AK, Poh E, Stevenson E, et al. Sex differences in clinical predictors of depression: A prospective study. J Affect Disord. 2013;150:1179–1183. doi: 10.1016/j.jad.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson Ka, Watkins ER, Mullan EG. Rejection sensitivity prospectively predicts increased rumination. Behav Res Ther. 2011;49:597–605. doi: 10.1016/j.brat.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84 doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM. Potential role of childrearing practices in the development of anxiety and depression. Clin Psychol Rev. 1997;17:47–67. doi: 10.1016/s0272-7358(96)00040-2. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton University Press; Princeton, NJ: 1965. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 2):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory Pleasure Predicts Motivation for Reward in Major Depression. J Abnorm Psychol. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cogn Affect Neurosci. 2014;9:1798–1807. doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich H-C, Stippich C, Weisbrod M, et al. Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage. 2010;49:1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Thorton T, Torres LD, Monroe SM, Gotlib IH. Targeted rejection predicts hastened onset of major depression. J Soc Clin Psychol. 2009;28:223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart-Richman L, Leary MR. Reactions to discrimination, stigmatization, ostracism, and other forms of interpersonal rejection: a multimotive model. Psychol Rev. 2009;116:365–383. doi: 10.1037/a0015250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cereb Cortex N Y N 1991. 2010;20:3005–3013. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213. doi: 10.1037/a0028931. [DOI] [PubMed] [Google Scholar]
- Wager T. help:core:brain_masks [Cognitive and Affective Neuroscience Lab - Tor D. Wager, Ph.D.] 2018 at < https://canlabweb.colorado.edu/wiki/doku.php/help/core/brain_masks>.
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci U S A. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Yuan Y, MacKinnon DP. Bayesian Mediation Analysis. Psychol Methods. 2009;14:301–322. doi: 10.1037/a0016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer–Gembeck MJ, Hunter TA, Pronk R. A Model of Behaviors, Peer Relations and Depression: Perceived Social Acceptance as a Mediator and the Divergence of Perceptions. J Soc Clin Psychol. 2007;26:273–302. [Google Scholar]
- The Whole Brain Atlas. at < http://www.med.harvard.edu/aanlib/>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.