Abstract
Objective
To explore the feasibility and safety of a single-lead, fully implantable peripheral nerve stimulation system for the treatment of chronic shoulder pain in stroke survivors.
Participants
Participants with moderate to severe shoulder pain not responsive to conservative therapies for six months.
Methods
During the trial phase, which included a blinded sham introductory period, a percutaneous single-lead peripheral nerve stimulation system was implanted to stimulate the axillary nerve of the affected shoulder. After a 3-week successful trial, participants received an implantable pulse generator with an electrode placed to stimulate the axillary nerve of the affected shoulder. Outcomes included pain, pain interference, pain-free external rotation range of motion, quality of life, and safety. Participants were followed for 24 months.
Results
Twenty-eight participants underwent trial stimulation and five participants received an implantable pulse generator. The participants who received the implantable generator experienced an improvement in pain severity (p=0.0002.) All five participants experienced a 50% or greater pain reduction at 6 and 12 months, and four experienced at least a 50% reduction at 24 months. There was an improvement in pain interference (p<0.0001.) There was an improvement in pain-free external ROM (p=0.003). There were no serious adverse events related to the device or to the procedure.
Conclusion
This case series demonstrates the safety and efficacy of a fully implantable axillary PNS system for chronic HSP. Participants experienced reduction in pain, reduction in pain interference, and improved pain-free external rotation ROM. There were no serious adverse events associated with the system or the procedure.
Keywords: Stroke, Peripheral Nerve Stimulation
Background
Hemiplegic shoulder pain (HSP) is a common condition affecting the impaired shoulder of stroke survivors. It is estimated that 29% of all stroke survivors experience shoulder pain within the first 12 months of their stroke.1 HSP is associated with poor rehabilitation outcomes, including interference with activities of daily living (ADLs)2 and poor quality of life (QoL).3 Unfortunately, many who develop HSP do not find relief with current standard of care.1 There is a need for better treatment options for those with HSP.
Percutaneous peripheral nerve stimulation (PNS) has been shown to be efficacious in producing long-term relief in those with chronic HSP.4–6 Treatment with percutaneous PNS involves the temporary placement of a percutaneous electrode into muscles around the painful shoulder to stimulate motor nerves to produce muscle contraction. The most recent randomized controlled trial (RCT) was of PNS delivered through a single-electrode to stimulate the axillary nerve to produce contraction of the middle and posterior deltoid muscles for 6-hours per day for 3 weeks.5 In that trial, 67% of participants who received PNS experienced sustained pain relief, compared to 25% who received physical therapy (PT). Some who receive PNS experience short-term relief of pain but with a return of pain within weeks of ending stimulation.7 These patients may benefit from a permanently placed system for long-term treatment. A fully implantable PNS system was developed and a case study reported successful pain relief for at least 12 months.8
This is a case-series of an axillary PNS system for chronic, refractory HSP involving a percutaneous trial stage followed by a fully implantable stage for those with successful pain relief. A two-stage treatment increases the likelihood that only those who respond to axillary PNS will undergo the procedure to receive the implantable pulse generator (IPG) for long-term treatment. Participants who completed the implant stage were followed for two years to determine the durability of pain relief and to accumulate safety data.
Methods
The study protocol was approved by the local institutional review boards (IRBs) of the four participating institutions and conducted under an Investigational Device Exemption from the United States Food and Drug Administration. A convenience sample of stroke survivors with chronic HSP was recruited. Inclusion criteria included age at least 21 years; shoulder pain with a severity ≥4 on the Brief Pain Inventory Short Form question 3 (BPI-SF3); ≥6 months after the stroke; pain refractory to two or more conservative therapies for a total period of at least 6 months since the onset of post-stroke shoulder pain; hemiplegia of the affected upper limb (shoulder abduction in synergy or if isolated movement is present, shoulder abduction ≤4/5 on Medical Research Council (MRC) scale); cognitive ability to fulfill study requirements. Exclusion criteria included evidence of joint or overlying skin infection of the affected limb; use of any opioid medication for shoulder pain or for any other chronic pain condition; intra-articular or sub-acromial steroid injections or botulinum toxin injections to the affected shoulder in the previous 3 months; history of arrhythmia with hemodynamic instability; valvular heart disease; evidence of non-stroke related shoulder pathology with continuing symptoms; bleeding disorder or international normalized ratio (INR) >3.0 for those on warfarin; unable to stop antiplatelet or anticoagulant medications for at least 7 days prior to IPG implantation; receiving outpatient physical or occupational therapy for shoulder pain; compromised immune system; severely impaired communication skills; uncontrolled seizure disorder; an implanted electronic device; and pregnancy.
Intervention
Trial Stage
The trial stage consisted of a 3-week blinded sham introductory period and a 3-week active stimulation period. Two external stimulators were used during this trial (Rehabilicare ® NT2000, Empi, Inc., St. Paul, MN or SPRINT™ (formerly SMARTPATCH™) PNS SYSTEM, SPR Therapeutics, LLC, Cleveland, Ohio). The procedure for placing the electrode has been described in detail previously.9 In brief, after sterilizing the skin over the affected shoulder, a percutaneous single-lead electrode system was implanted to stimulate the axillary nerve of the affected shoulder. Placement of the electrode was determined by placing monopolar needle electrodes at the locations of the axillary nerve motor points of the middle and posterior deltoids, with confirmation from strong contractions of the muscles. A third monopolar needle electrode was placed at the midpoint between the 2 prior locations to obtain strong contraction of both the middle and the posterior deltoid muscles. The location was marked and the depth of the third needle electrode was recorded. A 20-gauge introducer loaded with a percutaneous lead was then inserted perpendicular to the skin surface to the depth and location indicated by the third needle electrode. The introducer was withdrawn leaving the electrode in place. Stimulation was delivered through the lead to the axillary nerve to ensure stimulation produced comfortable contraction of the middle and posterior deltoid, confirming proper placement.
After placement of the electrode an external stimulator was connected to the lead. Participants completed the 3-week blinded sham period at home with an external stimulator, which appeared to function as normal but delivered no stimulation to the electrode. Participants were informed that different levels of stimulation would be prescribed and that they may not be able to detect stimulation. Participants were instructed on the use of the stimulator and were prescribed 6 hours of stimulation per day. After the 3-week blinded sham introductory period the participant returned for reprogramming of stimulator for the 3-week active stimulation period. The stimulator provided a biphasic waveform at an amplitude of 20mA, frequency of 12 Hz, and a 50% duty cycle. The pulse duration (40–200μs) was adjusted to produce the strongest muscle contraction that was comfortable for the participant. Participants were prescribed 6 hours of stimulation per day for 3 weeks, to be completed at home in single or divided doses, for a total of 126 hours of stimulation. The devices recorded the total time of stimulator usage. At the conclusion of the 3-week active stimulation period, the lead was removed by applying gentle traction on its exposed end.
Successful Trial Stage was defined as at ≥ 2-point reduction in shoulder pain intensity (BPI-SF3) at the end of the Trial Stage relative to end of the blinded sham period or baseline, whichever is lower, or if the participant reported a BPI-SF3 score of zero at the end of the Trial Stage. In the original protocol design, participants who were Trial Stage successes were automatically able to proceed to the Implant Stage. After the participation of the first eight participants, the study design was modified such that participants must have been a Trial Stage success and experience a return of pain to proceed to the implant stage. A return of pain was defined as an increase in BPI-SF3 score of ≥2 points relative to the end of the Trial Stage resulting in a BPI-SF3 score ≥4 within 6 months of the end of the Trial Stage that was sustained for at least two consecutive weeks.
Implant Stage
The IPG was a single-channel stimulator (MICROPULSE; NDI Medical, Cleveland, OH) that provided an identical stimulation waveform to the external stimulator. The IPG contains a lithium-ion battery that is charged via transcutaneous radio frequency magnetic field. The participant is able to charge the IPG at home for 5 hours every 1 to 2 weeks.
The procedure for implanting the IPG has been described previously.8 In brief, the IPG was placed under local anesthesia and conscious sedation or general anesthesia, at the discretion of the surgeon. Placement of the implantable lead10 was determined after placement of monopolar needle electrodes at the location to stimulate the axillary nerve motor points of the middle and posterior deltoids. Proper placement was confirmed by strong contraction of the middle and posterior deltoid muscles. The needle introducer with external sheath was inserted approximately 1 cm deep into the muscle and the needle was removed. The lead was inserted into the introducer sheath and the sheath was withdrawn, leaving the lead anchored in place. The proximal end of the lead was tunneled subcutaneously from posterolateral shoulder to the anterior chest with lead slack coiled for tension relief. The IPG was placed in a subcutaneous pocket in the chest wall. Participants were discharged the same day. After one week, the participant returned for programming of the stimulator via an external programming device. Participants were prescribed 6-hours of stimulation per day, in single or divided doses, controlled by an external remote control device.
Outcomes
Outcomes included pain, pain interference with activities of daily living, pain-free external rotation range of motion (ROM), quality of life, patient report of efficacy, and safety. Outcomes assessments were performed at baseline, at the completion of the 3-week introductory sham period, and at the completion of the 3-week active stimulation period. After placement of the IPG, outcomes assessments were performed at 3-weeks, 6-weeks (BPI-SF3 and 9 only), 12 weeks, 6-months, 9-months, 12-months, and 24 months (BPI-SF3 and 9 only). The stimulator was deactivated at least 1 hour prior to BPI administration and participants were instructed to focus on their shoulder pain during responses.
The primary outcome measure was pain severity measured by question 3 from the BPI-SF3. The BPI-SF3 asks participants to rate their worst shoulder pain within the last week on a 0 to 10 numeric rating scale (NRS), where “0” indicates “No pain” and “10” indicates “Pain as bad as you can imagine.” A reduction of 30% in this measure is clinically relevant.11 In this trial we selected a more conservative 50% pain reduction criterion as clinically successful pain relief.
Pain interference with activities of daily living was measured with the BPI-SF question 9 (BPI-SF9). The BPI-SF9 assesses the degree to which pain interferes with general activity, mood, walking ability, normal work, interpersonal relationships, sleep and enjoyment of life on a 0–10 NRS, where “0” indicates no interference and “10” indicates complete interference. A clinically meaningful difference is defined as a reduction in the pain interference score by 1 or greater.11 Pain-free external rotation ROM was measured by a therapist with participants in a supine position and the hemiplegic shoulder in 0° abduction and full internal rotation, elbow in 90° flexion, and forearm in neutral. The participant’s shoulder was externally rotated passively until pain was reported. A goniometer was used to measure the angle of pain.12 Quality of life (QoL) was measured with the SF-36v2, a population-norm based health-related QoL measure across multiple domains. A higher score is indicative of a higher QoL, and the population average equals a score of 50 with a standard deviation of 10.13 The Patient Global Impression of Change (PGIC) scale14 was used to measure patient-centered report of efficacy since the beginning of the study using a 7-point Likert scale ranging from “very much worse – very much improved.” at 6, 9, 12, and 24 months. Safety was evaluated at all outcomes assessments via questionnaires and open ended inquiry. Adverse events were categorized as serious or non-serious, and related or unrelated to the device or study. For an event to be considered serious, the event outcome must result in death, be life-threatening, result in hospitalization, disability or permanent damage, cause congenital anomaly/birth defect, require intervention to prevent permanent impairment/damage, or other serious (important medical events). All other outcomes result in a non-serious classification of the adverse event. Adverse events were summarized and reported to the IRB and FDA.
Statistical Analysis
Outcomes were analyzed longitudinally using a linear mixed model for repeated measures for each outcome measure. A compound symmetry covariance structure was used, since it is reasonable to assume correlation between assessments. The model assessed whether the outcomes change over time. When a significant time effect was found, pairwise comparisons between discrete time points of the end of the blinded sham introductory period and follow-up time points of 6, 12, and 24 months. To control for family-wise error with multiple comparisons, the p-values for pairwise comparisons were adjusted using the Dunnett procedure.15 We define α = 0.05 for our level of significance in all statistical tests. All statistical tests are two-tailed.
Results
Twenty-eight participants enrolled in the Trial Stage (Table 1) and underwent implantation of percutaneous leads. Sixteen participants (57.1%) had a successful percutaneous trial, defined as having at least a 2-point reduction during active stimulation. Six participants (21.4%) experienced successful trial stage with subsequent return of pain that was required to proceed to the Implant Stage, though one participant opted not to proceed and withdrew from the study. Five participants progressed to the Implant Stage and received the implantable system (Table 1). The flow of participants can be seen in Figure 1.
Table 1.
Demographics
| Trial Stage | Implant Stage | |
|---|---|---|
| n=28 | n=5 | |
| Female (%) | 46.4 | 40.0 |
| Age (years, median (Q1 – Q3)) | 54.0 (49.0 – 60.1) | 62.7 (49.4 – 66.0) |
| Race/Ethnicity (%) | ||
| White | 50.0 | 40.0 |
| Black | 50.0 | 60.0 |
| Hispanic/Latino | 0 | 0 |
| Time Since Stroke (years, median, (Q1 – Q3)) | 2.3 (0.9 – 3.3) | 3.1 (2.3 – 3.8) |
| Right Side Lesion (%) | 67.9 | 100 |
| Hemorrhagic Stroke (%) | 28.6 | 20 |
Abbreviations: Q1= quartile 1; = quartile 3
Figure 1.
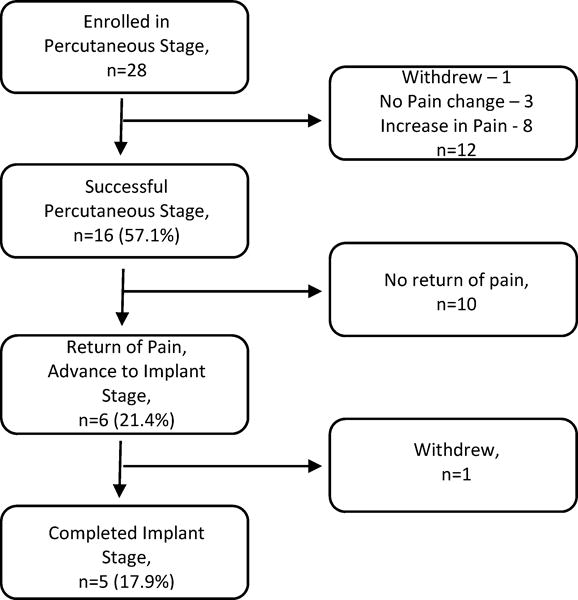
Participant flow.
Data from outcomes are shown in Table 2. There was an improvement in pain severity as measured by the BPI-SF3 (Figure 2, F(8,32)=5.6, p=0.0002.) Compared with the end of the sham introductory period, there was a significant reduction at 6 months of 3.6 (69.2%, 95% CI [1.8 – 5.5], p=0.003), at 12 months of 4.4 (84.6%, 95% CI [2.6 – 6.3], p=0.0002), and at 24 months of 3.6 (69.2%, 95% CI [1.7 – 5.5], p=0.003). All five participants experienced a 50% or greater pain reduction at 6 and 12 months, and four experienced at least a 50% reduction at 24 months.
Table 2.
Outcomes Assessments for Implant Stage Participants, n=5
| Baseline | End of Sham | End of Trial | 6 months | 12 months | 24 months | |
|---|---|---|---|---|---|---|
| Worst pain 7d (+/−SE) | ||||||
| 8.2 | 5.2 (+/− 0.7) | 2.4 (+/− 0.7) | 1.6 (+/− 0.7) | 0.8 (+/− 0.7) | 1.6 (+/− 0.7) | |
| Pain Interference 7d (+/−SE) | ||||||
| 5.8 | 4.2 (+/− 0.4) | 1.4 (+/− 0.4) | 0.3 (+/− 0.4) | 0.1 (+/− 0.4) | 0.4 (+/− 0.4) | |
| External Rotation ROM (degrees) | ||||||
| 69.2 | 96.6 (+/− 9.1) | 134.2 (+/− 9.1) | 141.2 (+/− 9.1) | 151.4 (+/− 9.1) | ||
| SF-36v2 (+/−SE) | ||||||
| Physical Functioning | ||||||
| 28.9 | 30.5 (+/− 6.1) | 33.4 (+/− 6.1) | 31.3 (+/− 6.1) | 31.3 (+/− 6.1) | ||
| Role-Limitations Physical | ||||||
| 29.4 | 35.6 (+/− 4.2) | 38.3 (+/− 4.2) | 37.1 (+/− 4.2) | 30.6 (+/− 4.2) | ||
| Bodily Pain | ||||||
| 30.6 | 34.8 (+/− 3.4) | 42.0 (+/− 3.4) | 45.1 (+/− 3.4) | 50.1 (+/− 3.4) | ||
| General Health | ||||||
| 42.4 | 38.7 (+/− 4.5) | 38.7 (+/− 4.5) | 41.7 (+/− 4.5) | 38.0 (+/− 4.5) | ||
| Vitality | ||||||
| 46.0 | 47.2 (+/− 3.7) | 44.8 (+/− 3.7) | 51.4 (+/− 3.7) | 50.2 (+/− 3.7) | ||
| Social Functioning | ||||||
| 39.2 | 42.4 (+/− 4.2) | 47.8 (+/− 4.2) | 47.8 (+/− 4.2) | 44.6 (+/− 4.2) | ||
| Role-Emotional | ||||||
| 35.2 | 34.5 (+/− 7.0) | 39.0 (+/− 7.0) | 47.4 (+/− 7.0) | 43.6 (+/− 7.0) | ||
| Mental Health | ||||||
| 39.6 | 46.3 (+/− 4.6) | 46.3 (+/− 4.6) | 50.7 (+/− 4.6) | 47.9 (+/− 4.6) | ||
The trial stage consisted of a 3-week blinded sham introductory period and a 3-week active stimulation period. Abbreviations: SE- Standard Error; PNS- Peripheral Nerve Stimulation; VGRS- Visual Graphic Rating Scales
Figure 2.
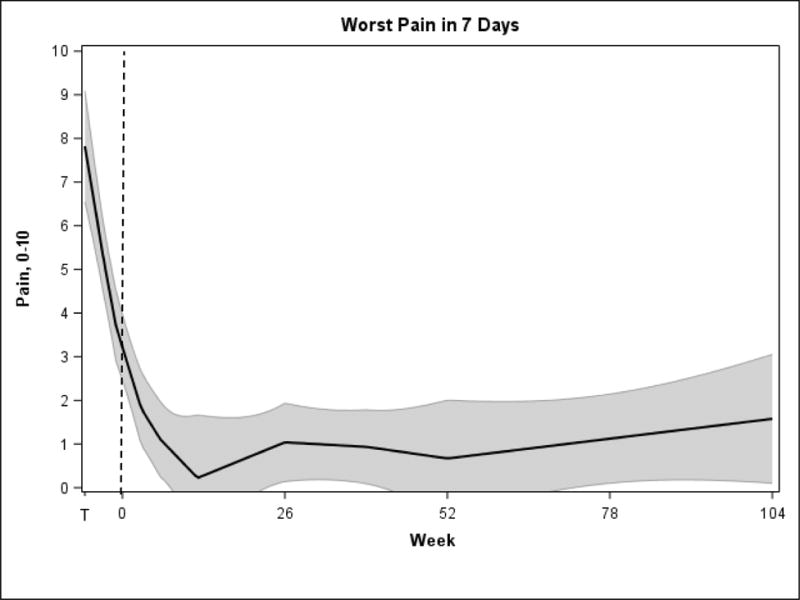
Worst Pain in the Last 7 Days, BPI-SF3. Time T to 0 represents the trial stage, which consisted of a 3-week, blinded sham introductory period and a 3-week active stimulation period. The shaded area represents the 95% confidence intervals.
There was an improvement in pain interference as measured by the BPI-SF9 (F(8,32)=10.1, p<0.0001.). Compared with the end of sham introductory period there was a significant reduction at 6 months of 3.9 (93.5%, 95% CI [2.7 – 5.1], p<0.0001), at 12 months of 4.0 (95.9%, 95% CI [2.9 – 5.2], p<0.0001), and at 24 months of 3.8 (91.1%, 95% CI [2.6 – 5.0], p<0.0001).
There was an improvement in pain-free external ROM (F(6,24)=8.3, p=0.003). Compared with the end of sham introductory period, there were statistically significant increases at 6 months of 44.6 degrees (46.2%, 95% CI [18.3 –70.9], p=0.009) and at 12 months of 54.8 degrees (56.7%, 95% CI [28.5 – 81.1], p=0.001).
There were no significant changes in any domain of the SF36v2. The efficacy, as measured by the PGIC with a question about the improvement in shoulder pain with treatment since enrolling in the study, participants rated themselves as minimally, much, or very much improved at 6, 12, and 24 months (Figure 3).
Figure 3.
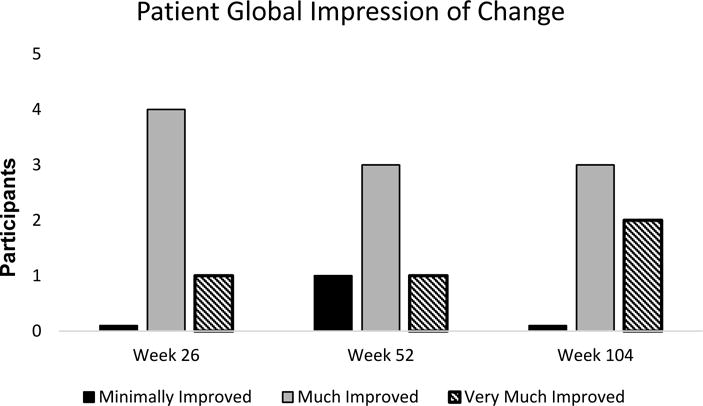
Patient Global Impression of Change.
For all participants of the Trial and Implant stage, 12 serious adverse events occurred during the trial, 11 of which were unrelated to the device or procedure. The causality could not be determined in one serious adverse event in which a participant was hospitalized for chest pain of uncertain origin. There were 54 non-serious adverse events, of which 14 were unrelated to the device or procedure. The 40 non-serious adverse events related to the device or procedure included: 7 related to lead fracture during explant of percutaneous lead without adverse health effects; 8 related to granulomas/erythema/pruritus at the percutaneous lead exit site; 9 related to erythema/pruritus/rashes due to bandages or electrode pads; 5 related to painful stimulation; 3 due to dislodged electrodes; 3 related to erythema/abrasions due to the lead connector; 1 painful keloid at the IPG incision site; 1 non-functioning percutaneous lead requiring second implantation; 1 suboptimal stimulation of percutaneous lead requiring second implantation; 1 distress after being informed of ineligibility; and 1 tenderness after a stimulation session.
Discussion
This trial evaluated the efficacy and safety of a fully implantable, single-lead axillary PNS system for chronic HSP. Participants experienced a clinically important reduction in pain and pain interference at 6, 12, and 24 months after implantation. Participants also experienced an improvement in pain-free external rotation ROM and felt that the treatment had an overall improvement for the two years of follow-up. The device was also safe, with no serious adverse events attributable to the treatment.
A fully implantable system, such as this single-lead axillary PNS system, may represent an important addition to treatment options for those who do not receive long-term relief of pain from other treatments. In spite of available treatments, many with HSP will not find relief. PNS has been shown to hold promise for many with HSP. A multi-center RCT demonstrated the efficacy of a 6-week course of 6-hours daily PNS via 4 percutaneous leads in reducing chronic HSP.4, 6 Additionally, a RCT of a single-lead, 3-week percutaneous axillary nerve PNS system produced a significant and clinically relevant improvement in HSP that was greater than that achieved by physical therapy.16 In both percutaneous PNS trials, more than 30% did not achieve long-term pain relief, possibly related to the duration of time from stroke onset.7 In one post-hoc study, improvement with percutaneous PNS was found to be temporary in those stroke survivors who had HSP and were more than 18 months from their stroke, with return of pain within weeks after undergoing PNS treatment. A fully-implantable PNS treatment, such as that described in this case-series, may provide a solution for those who do not have sustained relief of pain with percutaneous PNS treatment.
The mechanism of pain reduction of HSP with PNS is not known. Unlike many other forms of electrical stimulation, such as TENS or dorsal column stimulation in which afferent sensory nerves are stimulated, the goal of PNS as used in this treatment is to stimulate motor nerves to produce muscle contraction. It is possible that the muscle contraction provides physiologically relevant afferent information to the central nervous system (CNS) from receptors that respond to the contraction, such as golgi tendon organs and muscles spindles. The afferent information may lead to normalization of the pathological function of the CNS that is associated with chronic HSP.17–20 In spite of the improvement in pain-free external ROM in this trial, prior trials provide evidence that the mechanism of pain reduction is not biomechanical improvement of shoulder function.6, 21 Further studies are necessary to determine the mechanism of action of PNS.
There are limitations to keep in mind when reviewing the results of this trial. First, a small sample was studied without a comparison group. Larger studies with a comparison group will be necessary to interpret efficacy. It is also not possible to know from this study whether continuous stimulation is necessary for pain relief. As described in a prior case report8, one participant who had good response to fully implanted PNS for chronic HSP experienced an extended interruption in treatment due to an unrelated serious adverse event. Pain returned within 6 weeks, and pain relief was again experienced when PNS treatment was resumed. This trial was not designed to determine if the other participants would have a similar reaction in response to interrupting treatment. Finally, it is not clear which patients will benefit from a fully implantable PNS system as opposed to a temporary, percutaneous PNS system. Identifying the characteristics of those who will respond to the percutaneous treatment and those who will require continuous stimulation will reduce morbidity and cost associated with multiple procedures.
Conclusion
This case series demonstrates the safety and efficacy of a fully implantable axillary PNS system for chronic HSP. Participants experienced reduction in pain, reduction in pain interference, and improved pain-free external rotation ROM. There were no serious adverse events associated with the system or the procedure.
Acknowledgments
Funding Statement: This research was funded by grants R01HD075542, R21HD068905, R43NS066524, and UL1TR000439 from National Institutes of Health, and by SPR Therapeutics, LLC.
Footnotes
Conflict of Interest: Statement Dr. Wilson is a consultant to SPR Therapeutics. Ms. Bennett is an employee of SPR Therapeutics. Dr. Chae is a consultant and chief medical advisor to SPR Therapeutics and owns equity in SPR Therapeutics. The remaining authors have no potential conflicts of interest to disclose.
Authorship Statement: Drs. Wilson, Nguyen, Bock, O’Dell, Watanabe, Amundson, Hoyen and Chae, and Ms. Bennett designed and conducted the study, including patient recruitment, data collection, and data analysis. Dr. Wilson prepared the manuscript draft with important intellectual input from Drs. Nguyen, Bock, O’Dell, Watanabe, Amundson, Hoyen and Chae, and Ms. Bennett. All authors approved the final manuscript. The National Institutes of Health and SPR Therapeutics, LLC. provided funding for the study. Dr. Wilson and Ms. Bennett had complete access to the study data.
Reprints will not be available from the authors.
Contributor Information
Richard D. Wilson, MetroHealth Rehabilitation Institute of Ohio, MetroHealth Medical Center, Cleveland, OH, United States of America; Case Western Reserve University School of Medicine, Cleveland, OH, United States of America; Cleveland Functional Electrical Stimulation Center, Cleveland, OH, United States of America.
Maria E. Bennett, SPR Therapeutics, LLC., Cleveland, OH, United States of America.
Vu Q.C. Nguyen, Carolinas HealthCare/Charlotte Institute of Rehabilitation, Charlotte, NC, United States of America.
William C. Bock, Carolinas HealthCare/Sanger Heart and Vascular Institute, Charlotte, NC, United States of America.
Michael W. O’Dell, Weill Cornell Medical College, New York, NY, United States of America; Department of Rehabilitation Medicine, New York-Presbyterian Hospital, Weill Cornell Medical Center, New York, NY, United States of America.
Thomas K. Watanabe, Albert Einstein Healthcare Network/Moss Rehabilitation, Elkins Park, PA, United States of America.
Russell H. Amundson, United Healthcare Clinical Services, Philadelphia, PA, United States of America.
Harry Hoyen, Department of Orthopaedic Surgery, MetroHealth Medical Center, Cleveland, OH, United States of America; Case Western Reserve University School of Medicine, Cleveland, OH, United States of America; Cleveland Functional Electrical Stimulation Center, Cleveland, OH, United States of America.
John Chae, MetroHealth Rehabilitation Institute of Ohio, MetroHealth Medical Center, Cleveland, OH, United States of America; Case Western Reserve University School of Medicine, Cleveland, OH, United States of America; Cleveland Functional Electrical Stimulation Center, Cleveland, OH, United States of America.
References
- 1.Adey-Wakeling Z, Arima H, Crotty M, Leyden J, Kleinig T, Anderson CS, et al. Incidence and associations of hemiplegic shoulder pain poststroke: prospective population-based study. Arch Phys Med Rehabil. 2015;96(2):241–7 e1. doi: 10.1016/j.apmr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Chae J, Mascarenhas D, Yu DT, Kirsteins A, Elovic EP, Flanagan SR, et al. Poststroke shoulder pain: its relationship to motor impairment, activity limitation, and quality of life. Arch Phys Med Rehabil. 2007;88(3):298–301. doi: 10.1016/j.apmr.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Adey-Wakeling Z, Liu E, Crotty M, Leyden J, Kleinig T, Anderson CS, et al. Hemiplegic Shoulder Pain Reduces Quality of Life After Acute Stroke: A Prospective Population-Based Study. Am J Phys Med Rehabil. 2016;95(10):758–63. doi: 10.1097/PHM.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 4.Chae J, Yu DT, Walker ME, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2005;84(11):832–42. doi: 10.1097/01.phm.0000184154.01880.72. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J. Peripheral Nerve Stimulation for the Treatment of Chronic Subacromial Impingement Syndrome: A Case Series. Neuromodulation. 2014;17(8):771–6. doi: 10.1111/ner.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu DT, Chae J, Walker ME, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil. 2004;85(5):695–704. doi: 10.1016/j.apmr.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Chae J, Ng A, Yu DT, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular electrical stimulation for shoulder pain in hemiplegia: does time from stroke onset predict treatment success? Neurorehabil Neural Repair. 2007;21(6):561–7. doi: 10.1177/1545968306298412. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VQ, Bock WC, Groves CC, Whitney M, Bennett ME, Lechman TE, et al. Fully implantable peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Am J Phys Med Rehabil. 2015;94(2):146–53. doi: 10.1097/PHM.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil. 2011;92(5):837–40. doi: 10.1016/j.apmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memberg W, Peckham PH, Keith MW. A surgically implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans Rehabil Eng. 1994;2:80–91. [Google Scholar]
- 11.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Andrews AW, Bohannon RW. Decreased shoulder range of motion on paretic side after stroke. Phys Ther. 1989;69(9):768–72. doi: 10.1093/ptj/69.9.768. [DOI] [PubMed] [Google Scholar]
- 13.Keith RA. Functional status and health status. Arch Phys Med Rehabil. 1994;75(4):478–83. doi: 10.1016/0003-9993(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 14.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 15.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. Journal of the American Statistical Association. 1955;50(272):1096–121. [Google Scholar]
- 16.Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93(1):17–28. doi: 10.1097/PHM.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roosink M, Buitenweg JR, Renzenbrink GJ, Geurts AC, Ijzerman MJ. Altered cortical somatosensory processing in chronic stroke: A relationship with post-stroke shoulder pain. NeuroRehabilitation. 2011;28(4):331–44. doi: 10.3233/NRE-2011-0661. [DOI] [PubMed] [Google Scholar]
- 18.Roosink M, Renzenbrink GJ, Buitenweg JR, van Dongen RT, Geurts AC, Ijzerman MJ. Somatosensory symptoms and signs and conditioned pain modulation in chronic post-stroke shoulder pain. J Pain. 2011;12(4):476–85. doi: 10.1016/j.jpain.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Roosink M, Renzenbrink GJ, Geurts AC, Ijzerman MJ. Towards a mechanism-based view on post-stroke shoulder pain: theoretical considerations and clinical implications. NeuroRehabilitation. 2012;30(2):153–65. doi: 10.3233/NRE-2012-0739. [DOI] [PubMed] [Google Scholar]
- 20.Soo Hoo J, Paul T, Chae J, Wilson RD. Central hypersensitivity in chronic hemiplegic shoulder pain. Am J Phys Med Rehabil. 2013;92(1):1–9. doi: 10.1097/PHM.0b013e31827df862. quiz 10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RD, Knutson JS, Bennett ME, Chae J. The Effect of Peripheral Nerve Stimulation on Shoulder Biomechanics: A Randomized Controlled Trial in Comparison to Physical Therapy. Am J Phys Med Rehabil. 2017;96(3):191–8. doi: 10.1097/PHM.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]


