Inflammation is considered a key driver of cardio-renal dysfunction in heart failure (HF). Among pro-inflammatory cytokines, interleukin 6 (IL-6) is of particular interest as it occupies a central pathophysiologic role in several chronic inflammatory conditions and has emerged as a promising therapeutic target.(1, 2) IL-6 has theoretical clinical relevance to cardio-renal syndrome (CRS) as it has been shown, in murine models, to stimulate the renal epithelial sodium (ENaC) channel and worsen neurohormonal activation.(3) Our goal was to understand the relationship between IL-6 and parameters of renal dysfunction in human HF.
We enrolled 98 consecutive patients receiving high-dose loop diuretics in an ambulatory HF unit at the Yale University School of Medicine. IL-6 levels in plasma were used to query systemic inflammation and IL-6 in a pre-diuretic spot urine samples were used to quantify inflammation at the level of renal tissue. Cumulative urine collection during the treatment period was performed to determine total sodium output and efficacy of the administered diuretic (Supplementary Methods).
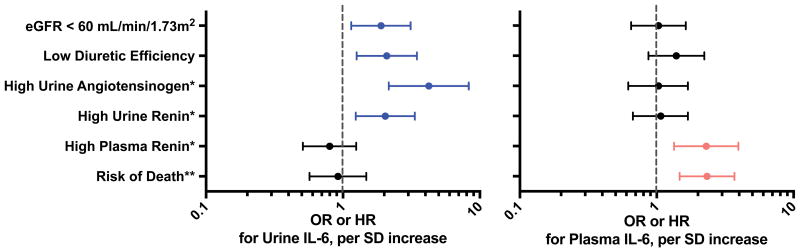
Plasma and urine IL-6 were modestly correlated (r=0.40, P<0.001). Higher levels of either plasma or urine IL-6 were associated with parameters consistent with greater disease severity including higher NT pro-BNP and lower eGFR (Supplementary Table 1). As shown in Figure 1, plasma, but not urine, IL-6 was associated with systemic neurohormonal activation (OR for high plasma renin=1.9, 95% CI 1.2-3.0, P=0.008) and higher risk of mortality (adjusted HR=2.3, 95% CI=1.5-3.7, P<0.001). However, levels of urine IL-6 were closely and independently associated with measures of renal dysfunction such as diuretic resistance (OR=2.3, 95% CI 1.4-3.8, P=0.001), lower eGFR (OR=1.9, 95% CI=1.2-3.1, P=0.01) and increased renal tissue-level neurohormonal activation (OR for high urine renin=2.1, 95% CI 1.3-3.4, P=0.002; OR for high urine angiotensinogen=4.2, 95% CI 2.2-7.9, P<0.001). Plasma IL-6 was not independently associated with diuretic efficiency or tissue-level neurohormonal activation.
Figure 1.
Association between Urine and Plasma IL-6 and Parameters of Cardiorenal Syndrome.
Whiskers represent 95% CI. Analyses of urine IL-6 were adjusted for plasma IL-6; analyses of plasma IL-6 were adjusted for urine IL-6. IL=interleukin. SD=standard deviation. eGFR=estimated glomerular filtration rate. *adjusted for use of ACEI/ARB. **adjusted for baseline characteristics including age, race, NT-proBNP, use of ACE-I/ARB, home loop diuretic dose, and eGFR. Urine IL-6 levels indexed to urinary creatinine. Due to the skewed distribution of urine and plasma IL-6 variables, a log transform was applied before performing logistic and Cox regressions.
In this contemporary HF cohort, IL-6 elevations in both plasma and urine were associated with features of CRS including decreased GFR, decreased diuretic responsiveness, and increased neurohormonal activation. Plasma IL-6 was associated primarily with systemic neurohormonal activation and risk of death. This is consistent with prior studies that have shown plasma IL-6 levels to be strongly associated with adverse outcomes in HF patients, presumably via directly induced myocardial dysfunction.(4) Conversely, urine IL-6 was linked to indicators of local renal processes but not risk of mortality, suggesting a primarily tissue-level importance for this molecule. Though the role of IL-6 in renal disease progression is less well understood, animal model data indicates that IL-6 can exacerbate acute kidney injury, mediate the damaging effects of angiotensin II, and activate renal ENaC channels, promoting sodium retention.(3, 5, 6) To our knowledge, this is the first examination of urine IL-6 and cardio-renal parameters in HF patients. Overall, our results suggest that plasma and urine IL-6 reflect distinct aspects of cardio-renal pathophysiology.
The following limitations of our study should be considered. It is a single-center, cross-sectional nature and small sample size. There is potential for selection bias given that the defining clinical phenotype of our cohort was requirement for focused outpatient diuresis. The study focused on IL-6 as a marker of inflammation. Therefore, additional studies will be necessary to evaluate multiple markers of inflammation in CRS and validate our results in clinically distinct cohorts. Nonetheless, these intriguing results indicate that further research is required to determine whether a therapeutically modifiable relationship exists between IL-6 and features of CRS in HF.
Supplementary Material
Acknowledgments
This publication was made possible, in part, by CTSA Grant Number UL1TR001863 and by the George M O'Brien Kidney Center at Yale, NIH grant P30DK079310.
Funding: NIH Grants, K23HL114868, L30HL115790, and R01HL128973 (JMT); NIH Grant K23DK097201 to FPW; Research Grant from Corvidia Therapeutics. The funding sources had no role in study design, data collection, analysis or interpretation.
Footnotes
Conflict of Interest Disclosures: Research support from Corvidia therapeutics and RK is an employee of Corvidia therapeutics.
References
- 1.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998 Feb;31(2):391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 2.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011 Sep;121(9):3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, Dong Y. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Physiol Regul Integr Comp Physiol. 2010 Aug;299(2):R590–595. doi: 10.1152/ajpregu.00207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plenz G, Song ZF, Tjan TD, Koenig C, Baba HA, Erren M, Flesch M, Wichter T, Scheld HH, Deng MC. Activation of the cardiac interleukin-6 system in advanced heart failure. Eur J Heart Fail. 2001 Aug;3(4):415–421. doi: 10.1016/s1388-9842(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 5.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005 Nov;16(11):3315–3325. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, Tao L, Sun H, Kellems RE, Blackburn MR, Xia Y. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension. 2012 Jan;59(1):136–144. doi: 10.1161/HYPERTENSIONAHA.111.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.