Abstract
Background
To evaluate role of AMH as a diagnostic tool for PCOS.
Methods
This was a prospective case–control study on women attending Gynae OPD of Dr RML Hospital, New Delhi, from 1 November 2015 to 31 March 2017. Study comprised of 45 women with PCOS, diagnosed using Rotterdam criteria and 45 women as controls. Clinical history included oligomenorrhea, hirsutism, examination included BMI, Ferriman–Gallwey score, investigations included blood for FSH, LH, estradiol, TSH, prolactin, total testosterone, AMH level and pelvic USG which was done for all women.
Results
Both PCOS cases and control were matched for age and BMI. Median AMH levels of 4.32 ng/ml in PCOS cases was almost twice that of 2.32 ng/ml in controls (p = 0.001). Maximum diagnostic potential of AMH alone for PCOS was at a cut-off of 3.44 ng/ml with sensitivity of 77.78% and specificity of 68.89%. AMH was used as an adjunct to existing Rotterdam criteria as the fourth parameter OA+HA+PCOM+AMH (any three out of four) yielded sensitivity of 80%. However, when PCOM in Rotterdam criteria was replaced by AMH, OA+HA+AMH (any two out of three) or OA/HA+AMH resulted in sensitivity of 86.67 and 71.11%, respectively.
Conclusion
AMH levels were significantly higher in PCOS than in controls. AMH as an independent marker could not effectively diagnose PCOS. However, AMH levels as an adjunct to existing Rotterdam criteria for diagnosis of PCOS had good diagnostic potential.
Keywords: PCOS, AMH, Rotterdam criteria, Hyperandrogenism (HA), Oligomenorrhea (OA), Polycystic ovarian morphology (PCOM)
Introduction
Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder in women of reproductive age group [1]. It is a heterogeneous, multisystem endocrinopathy which presents with wide spectrum of clinical features and delayed sequale like type 2 diabetes mellitus, cardiovascular diseases, metabolic syndrome and endometrial cancer which are preventable [2]. It is caused by imbalance of sex hormones which ultimately leads to menstrual irregularities, infertility, anovulation and other metabolic disturbances [3].
Rotterdam criteria 2003 is the gold standard for diagnosing PCOS, and using it a woman is diagnosed with PCOS if two out of the three following features are present: (1) oligomenorrhea or amenorrhoea (OA), (2) clinical and/or biochemical hyperandrogenism (HA) and (3) polycystic ovarian morphology (PCOM) on ultrasound with a cut-off of more than 12 follicles with a diameter of 2–9 mm or when ovarian volume is more than 10 cucm.
Rotterdam criteria although worldwide accepted has following drawbacks with respect to PCOM criteria: (a) Majority of PCOS are young obese females where TAS is difficult and TVS not possible as most of them are teenagers who are virgin, (b) assessment of AFC is subjective and not standardized with interobserver variability [4], (c) phase of menstrual cycle and oral contraceptive use alter polycystic ovarian morphology and (d) technical advances in imaging have led to an artificial increase in PCOM resulting in confusion over its use as diagnostic criteria [5–7]. Other shortcoming of Rotterdam criteria is that diagnosis of PCOS can be made in the absence of hyperandrogenism which is a basic requisite for NIH and AE-PCOS criteria. Diagnosis of hyperandrogenism is problematic clinically as Ferriman–Gallwey score is subjective and also difficult biochemically because the laboratory tests for androgens are tedious.
Anti-Mullerian hormone (AMH) is a member of transforming growth factor β containing a glycoprotein dimer structure produced by granulosa cells and its levels correlate with number of antral follicles which are 2–6 mm in size. The secretion of AMH from polycystic ovary is 75 times higher than a size-matched granulosa cell of normal ovary indicating that higher AMH in PCOS is not only an indirect measure of AFC but also an indicator for the intrinsic dysregulation in granulosa cells. AMH levels in blood are neither affected by menstrual cycle and nor altered due to usage of oral contraceptive pills and is reproducible from one cycle to another.
The controversy regarding the diagnosis of PCOS still continues due to the complexity of presentation. Feature which should be considered essential for its diagnosis is still a dilemma. Due to the limitations associated with existing Rotterdam criteria, new tool AMH can be used as a potential objective, quantitative and biological diagnostic marker for PCOS [8].
However, a standardized cut-off for AMH in PCOS is still not determined due to conflicting results among various studies because of difference in sample population, sample size and sample selection criteria [9]. AMH can be used alone or as an adjunct to existing Rotterdam criteria to make an effective diagnosis of PCOS. Very few such studies have been reported from India, and therefore, this study was undertaken.
Materials and Methods
The present study was a prospective case–control study which enrolled 90 women in the age group of 18–35 years attending the outpatient Department of Obstetrics and Gynaecology; PGIMER & Dr RML hospital, New Delhi, from 1 November 2015 to 31 March 2017. The study was conducted after approval from the ethical and research review board of the hospital, and written informed consent was taken from all the women. Cases constituted 45 women diagnosed with PCOS according to Rotterdam criteria. Control group consisted of 45 women having regular menstrual cycle, no PCOM on ultrasound and no endocrine abnormalities. Both PCOS cases and control were matched for age and BMI. Women with history of previous ovarian surgery and with intake of COC in past three months were excluded from the study.
Clinical history included complaint of oligomenorrhea, hirsutism and examination included FG score and BMI. Oligomenorrhea was taken as fewer than eight menstrual cycles during the previous 12 months or menstrual interval of more than 35 days. Clinical hyperandrogenism was defined as Ferriman–Gallwey score of > 8 and biochemical hyperandrogenism defined as serum testosterone level of > 2.67 nmol/l. PCOM was diagnosed when either ovary on ultrasound had more than 12 follicles with a diameter of 2–9 mm or when ovarian volume was more than 10 cucm.
A total of 8 ml was withdrawn and put equally in 2 plain vials on day 2–3 of menses or after withdrawal bleeding. Samples were then centrifuged at 3000 rpm in centrifugation machine at the biochemistry laboratory for serum analysis. One vial of centrifuged sample was stored at − 80 degrees in deep freezer for batch analysis of AMH. AMH levels were run on ELISA kit by Immunoconcept bio-detect which consisted of 96 wells with six standards. Analysis was done using a competitive enzyme immunoassay technique utilizing a monoclonal anti-AMH antibody and an AMH-HRP conjugate on an anti-AMH-coated plate. The minimum detection level for the kit was 0.025 ng/ml.
On the other sample, hormonal assays for T3, T4 and TSH, FSH, LH, estradiol, prolactin and total testosterone was performed using chemiluminiscence immunoassay on the ECiQvitros from Johnson’s and Johnson’s. TAS was performed for all the women using 3 MHz Medison model—SONACE X1 to diagnose PCOM.
Results of the above laboratory investigations and imaging studies were recorded along with clinical data of the patient in a proforma. Patients were classified as PCOS cases and controls according to the inclusion and exclusion criteria mentioned above.
Data were entered in MS EXCEL spreadsheet, and statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0. Qualitative variables were correlated using Chi-square test/Fisher’s exact test. Quantitative variables were compared using unpaired t test/Mann–Whitney test between the two groups and ANOVA/Kruskal–Wallis test between more than two groups. A p value of < 0.05 was considered statistically significant. Receiver operating characteristic curve was used to find out the cut-off of AMH for predicting PCOS. Diagnostic test was used to find sensitivity, specificity, NPV and PPV of AMH as adjunct to Rotterdam criteria for diagnosing PCOS.
Results
This prospective case–control study was conducted in the Department of Obstetrics and Gynaecology, PGIMER & RML Hospital, New Delhi. Total 90 women enrolled in the study included 45 PCOS cases and 45 controls based on inclusion and exclusion criteria. Age range for both groups was from 18 to 34 years with majority in the age group of 21–30 years (cases 77.78% and control—82.22%).The mean age of PCOS cases and control was 24.49 and 25.47 years, respectively, and difference was not statistically significant (p = 0.221). Mean BMI of cases was 24.56 kg/m2 and ranged from 18.3 to 29.3 kg/m2. Similarly for controls, mean BMI was 24.13 kg/m2 ranging from 19.54 to 30 kg/m2 and there was no statistical difference between the two groups (p = 0.440). Majority of PCOS cases (48.89%) and controls (43.3%) were obese.
Oligomenorrhea and hirsutism was complained by 62.22 and 20% PCOS females respectively; however, on detailed history and Ferriman–Gallwey score, oligomenorrhea was found in 86.67% and clinical hyperandrogemia in 71.11%.
PCOM was reported in 84.44% of PCOS cases, but none of controls had it as it was an exclusion criteria for controls. Mean FG score for PCOS cases and control was 10.13 and 4.8 respectively, and it was statistically significant (p < 0.0001). Mean testosterone levels in PCOS cases and controls were 1.22 + 0.67 nmol/L and 1.19 + 0.7 nmol/L, respectively, and it was not statistically significant (p = 0.716).
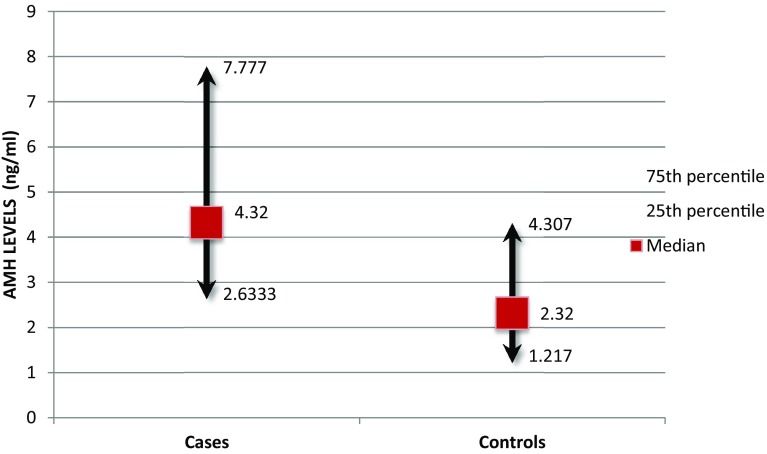
Median (IQR) AMH level in PCOS cases and control was found to be 4.32 (2.633–7.777) ng/ml and 2.32 (1.217–4.307) ng/ml, respectively. Hence, AMH levels in PCOS were statistically higher compared to controls (p = 0.001) (Fig. 1).
Fig. 1.
Median levels of AMH in PCOS cases and controls
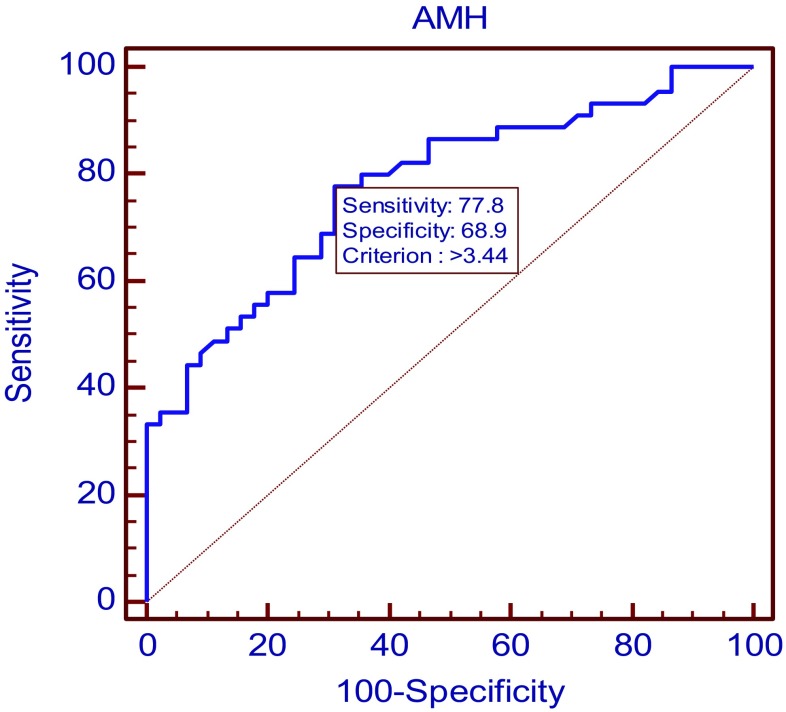
On applying the receiver operating characteristics curve (ROC curve) analysis, area under curve was 0.778 (95% CI 0.678 to 0.859; p value < 0.0001). Maximum diagnostic potency of AMH alone for PCOS was at a cut-off of 3.44 ng/ml with sensitivity of 77.78% and specificity of 68.89% (Fig. 2).
Fig. 2.
ROC curve for AMH as independent diagnostic marker
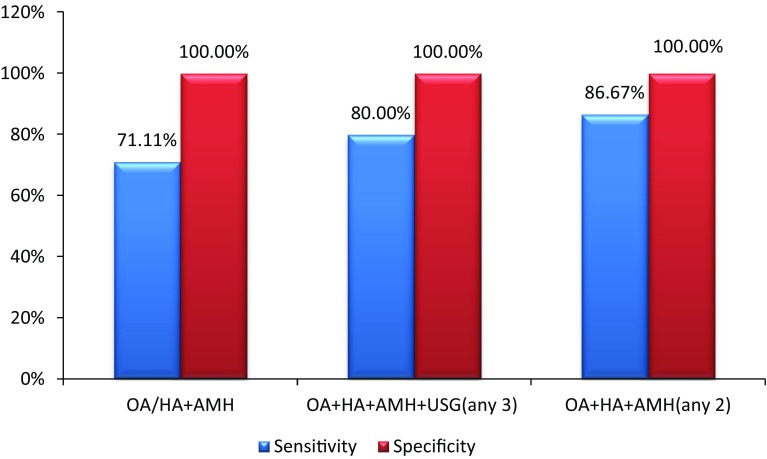
In the present study, AMH was used as an adjunct to existing Rotterdam criteria as the fourth parameter, e.g. OA+HA+PCOM+AMH (any three out of four), yielded sensitivity of 80% (95% CI 0.654 to 0.904) with 100% specificity (95% CI 0.9213 to 0.100). PCOM in Rotterdam criteria was replaced by AMH, OA+HA+AMH (any two out of three), resulted in increased sensitivity of 86.67% (95% CI 0.732 to 0.949) with specificity of 100% (95% CI 0.921 to 0.100) and negative predictive value of 88.24% (95% CI 0.761 to 0.955).
In Rotterdam criteria, the diagnosis of PCOS can be made when either OA or HA was present along with PCOM. It was observed that if only OA or OH was present, then also AMH can be used to replace PCOM (OA/HA+AMH), resulting in sensitivity of 71.11% (95% CI 0.557 to 0.836), specificity of 100% (95% CI 0.921 to 0.100) and negative predictive value of 77.59% (95% CI 0.647 to 0.875) (Fig. 3).
Fig. 3.
Sensitivity and specificity of AMH as an adjunct to Rotterdam criteria for diagnosing PCOS
Discussion
Diagnosis of PCOS requires an objective and quantitative criteria to help clinicians to diagnose and treat patients suffering from this complex endocrine disorder.
In the present study, there was no statistical difference between mean age of PCOS cases and controls and same was observed in previous studies [9–11]. However, there was different age profiles of PCOS cases and controls in a previous study [12]. In the present study, similar BMI was observed in PCOS as well as control and this was in agreement with previous studies [1, 13, 14].
In the present study, AMH level was found to be significantly higher in PCOS as compared to controls, with median AMH levels of 4.32 ng/ml in PCOS cases being almost twice as high of 2.32 ng/ml in controls (p < 0.001). Similar was observed in study by Sahmay et al. where AMH levels were found to be 2–3 times higher in women with PCOS [9]. Higher AMH in PCOS was also found in many previous studies [15, 16]. A study reported the highest AMH in women presenting with all three Rotterdam criteria and with 80% prevalence of PCOS in women with AMH > 11 ng/ml [17]. PCOS was observed in 97% women with AMH higher than 10 ng/ml in a study [18]. A study found that AMH in PCOS was higher whether women were lean or obese [19–21].
In the present study, the best diagnostic potential of AMH was found at cut-off of 3.44 ng/ml with sensitivity and specificity of 77.78% and of 68.89%, respectively. Similar cut-off of AMH of 3.34 ng/ml with a higher sensitivity and specificity of 98% and 93%, respectively, was reported in an Indian study [22]. This was also in congruence with previous studies [13, 23]. Woo et al. and Lin et al. reported sensitivity and specificity similar to our study but at a higher cut-off of 7.82 ng/ml and 7.3 ng/ml, respectively [10, 17]. In contrast Dewailly et al. observed a higher sensitivity and specificity of 92% and 97%, respectively, at a cut-off of 4.9 ng/ml. Hence, they concluded that AMH not only reflects AFC but also the degree of hyperandrogenism making AMH a better marker than follicle numbers per ovary [24]. However, Homburg et al. reported a high specificity of 98.2% but a low sensitivity of 60% of AMH at cut-off of 6.7 ng/ml [14] (Table 1).
Table 1.
AMH as diagnostic tool for PCOS in various studies
| Study | Type of study | PCOS (N) | Age (years) | AMH cut-off (ng/ml) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Hart et al. (2010) | Prospective cohort | 64 | 14.5–17.6 | 4.20 | 53.1 | 69.8 |
| Lin et al. (2011) | Prospective case–control | 126 | 27.7 ± 5.8 | 7.30 | 76.0 | 70 |
| Dewailly et al. (2011) | Prospective case–control | 62 | 20.1–34.0 | 4.90 | 92 | 97 |
| Eilertsen et al. (2012) | Case–control | 56 | 33.3 ± 5.5 | 2.80 | 94.6 | 97.1 |
| Woo et al. (2012) | Prospective cross section | 87 | 22–38 | 7.82 | 75.9 | 86.8 |
| Homburg et al. (2013) | Prospective case–control | 90 | 32.1 | 6.72 | 60 | 98.2 |
| Wiweko et al. (2014) | Case–control | 71 | 29.55 | 4.45 | 76.1 | 74.6 |
| Present study (2017) | Prospective case–control | 45 | 24.49 ± 3.91 | 3.44 | 77.78 | 68.89 |
Li et al. reported a low sensitivity and specificity of AMH of 62% and 65%, respectively, at a cut-off of 3.92 ng/ml with higher AMH in patients having hyperandrogenism [25]. Higher cut-off of 4.7 ng/ml with sensitivity of 79.4% and specificity of 82.8% was reported in a metanalysis [11]. Such difference in cut-off might be because of different types of AMH kits being used. ELISA in these studies was by Diagnostic System Laboratories (DSL) whereas we used kit from Immunoconcept bio-detect.
In the present study when AMH was used alone as a single parameter but not as an adjunct to Rotterdam criteria, it was found to be more sensitive than specific for diagnosing PCOS. Similarly, Cengiz et al. [26] also concluded that AMH when used solely and not as an adjunct to Rotterdam criteria, it was not a reliable predictor for the presence of PCOS in women. In the present study, AMH alone did not prove to be an effective diagnostic tool as a single independent marker and this was in agreement with the previous studies [20, 27].
Sahmay et al. [28] found that when AMH replaced PCOM in Rotterdam criteria and was used as an adjunct when either of OA or HA was present (OA/HA+AMH) resulted in sensitivity of 83%. In congruence to this, in the present study also OA/HA+AMH had sensitivity of 71.11%. In the present study, when AMH was used as an adjunct to Rotterdam criteria as the fourth parameter, i.e. OA+HA+PCOM+AMH (any three out of four), resulted in increased sensitivity of 80% and specificity of 100%. However, in the present study when PCOM in Rotterdam criteria was replaced by AMH levels, i.e. OA+HA+AMH (any two out of three), thereby replacing a subjective criteria with an objective and quantitative criteria, resulted in the highest sensitivity of 86.67% and specificity of 100%. This was in congruence with previous studies which also suggested that AMH could successfully replace PCOM in Rotterdam criteria [28, 29].
Conclusion
PCOS is a complex and common gynaecological condition and PCOM used currently in Rotterdam criteria is highly subjective and poorly reproducible. Though sensitivity and specificity of AMH alone is low and no single cut-off of AMH is diagnostic, still it is a promising diagnostic tool for PCOS as an adjunct to existing Rotterdam criteria especially when it is used to replace PCOM. Additional advantages of AMH as diagnostic tool are that it is biological, objective, quantitative marker not affected by day of menses or OCP intake. So in future, more studies should be undertaken to validate its role as diagnostic tool for PCOS.
Acknowledgement
We thank all the patients who consented to participate in this study.
Dr Upma Saxena
is working as Professor and Senior Gynaecologist in the Department of Obstetrics and Gynaecology, PGIMER & Dr RML Hospital, New Delhi. She is also visiting Gyneaecologist to Parliament House Annexe. She did her MBBS from Maulana Azad Medical college in 1990 and MD from VMMC and Safdarjung hospital in 1994 and has been working as Gynaecologist in CHS for past 22 years. She was conferred FICOG by ICOG in 2008. She has to her credit many publications in national and international journals. She has special interest in high-risk pregnancy, infertility and oncology.
Compliance with Ethical Standards
Conflict of interest
All the authors declare that they have no relevant conflict of interest.
Ethical Approval
The study was approved by Medical Ethical committee of PGIMER & DR RML Hospital, New Delhi.
Informed Consent
Informed consent was taken from all the patients who agreed to participate in the study.
Footnotes
Upma Saxena, Professor and Senior Gynaecologist in the Department of Obstetrics and Gynaecology, PGIMER and Dr RML Hospital, New Delhi; Manisha Ramani, postgraduate student in the Department of Obstetrics and Gynaecology, PGIMER and Dr RML Hospital, New Delhi; Pushpa Singh, Professor and Consultant in the Department of Obstetrics and Gynaecology, PGIMER and Dr RML Hospital, New Delhi.
References
- 1.Begawy A, El-Mazny A, Abou-Salem N, et al. Anti-Müllerian hormone in polycystic ovary syndrome and normo-ovulatory women: correlation with clinical, hormonal and ultrasonographic parameters. Middle East Fertil Soc J. 2010;15(4):253–258. doi: 10.1016/j.mefs.2010.08.005. [DOI] [Google Scholar]
- 2.Mahran A. The relationship between Anti-mullerian hormone and the clinical, biochemical and sonographic parameters in women with polycystic ovarian syndrome. Middle East Fertil Soc J. 2015;21(1):11–15. doi: 10.1016/j.mefs.2015.06.003. [DOI] [Google Scholar]
- 3.Ramanand S, Ghongane B, Ramanand J, et al. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab. 2013;17(1):138–145. doi: 10.4103/2230-8210.107858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broekmans F, de Ziegler D, Howles C, et al. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94(3):1044–1051. doi: 10.1016/j.fertnstert.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 5.Duijkers I, Klipping C. Polycystic ovaries, as defined by the 2003 Rotterdam consensus criteria, are found to be very common in young healthy women. Gynecol Endocrinol. 2010;26(3):152–160. doi: 10.3109/09513590903247824. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone E, Rosen M, Neril R, et al. The polycystic ovary post-rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95(11):4965–4972. doi: 10.1210/jc.2010-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen S, Ramlau-Hansen C, Ernst E, et al. A very large proportion of young Danish women have polycystic ovaries: is a revision of the Rotterdam criteria needed? Hum Reprod. 2010;25(12):3117–3122. doi: 10.1093/humrep/deq273. [DOI] [PubMed] [Google Scholar]
- 8.Streuli I, Fraisse T, Pillet C, et al. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90(2):395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Sahmay S, Atakul N, Aydogan B, et al. Elevated serum levels of anti-Müllerian hormone can be introduced as a new diagnostic marker for polycystic ovary syndrome. Acta Obstetriciaet Gynecologica Scandinavica. 2013;92(12):1369–1374. doi: 10.1111/aogs.12247. [DOI] [PubMed] [Google Scholar]
- 10.Woo H, Kim K, Rhee E, et al. Differences of the association of anti-Mullerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J. 2012;59(9):781–790. doi: 10.1507/endocrj.EJ12-0055. [DOI] [PubMed] [Google Scholar]
- 11.Iliodromiti S, Kelsey T, Anderson R, et al. Can Anti-Müllerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab. 2013;98(8):3332–3340. doi: 10.1210/jc.2013-1393. [DOI] [PubMed] [Google Scholar]
- 12.Köninger A, Koch L, Edimiris P, et al. Anti-Mullerian hormone: an indicator for the severity of polycystic ovarian syndrome. Arch Gynecol Obstet. 2014;290(5):1023–1030. doi: 10.1007/s00404-014-3317-2. [DOI] [PubMed] [Google Scholar]
- 13.Wiweko B, Maidarti M, Priangga M, et al. Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. J Assist Reprod Genet. 2014;31(10):1311–1316. doi: 10.1007/s10815-014-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homburg R, Ray A, Bhide P, et al. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod. 2013;28(4):1077–1083. doi: 10.1093/humrep/det015. [DOI] [PubMed] [Google Scholar]
- 15.Ramezani Tehrani F, Solaymani-Dodaran M, Hedayati M, et al. Is polycystic ovary syndrome an exception for reproductive aging? Hum Reprod. 2010;25(7):1775–1781. doi: 10.1093/humrep/deq088. [DOI] [PubMed] [Google Scholar]
- 16.Villarroel C, Merino P, Lopez P, et al. Polycystic ovarian morphology in adolescents with regular menstrual cycles is associated with elevated anti-Mullerian hormone. Hum Reprod. 2011;26(10):2861–2868. doi: 10.1093/humrep/der223. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Chiu W, Wu C, et al. Antimüllerian hormone and polycystic ovary syndrome. Fertil Steril. 2011;96:230–235. doi: 10.1016/j.fertnstert.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Tal R, Seifer D, Khanimov M, et al. Characterization of women with elevated antimüllerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211(1):59.e1–59.e8. doi: 10.1016/j.ajog.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Cassar S, Teede H, Moran L, et al. Polycystic Ovary Syndrome and Anti-Müllerian hormone: role of insulin resistance, androgens, obesity and gonadotropins. Clin Endocrinol. 2014;81(6):899–906. doi: 10.1111/cen.12557. [DOI] [PubMed] [Google Scholar]
- 20.Sopher A, Grigoriev G, Laura D, et al. Anti-Mullerian hormone may be a useful adjunct in the diagnosis of polycystic ovary syndrome in nonobese adolescents. J Pediatr Endocrinol Metab. 2014;27:1175–1179. doi: 10.1515/jpem-2014-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tayrab E, Ali M, Modawe G, et al. Serum Anti-Müllerian hormone as laboratory predictor in infertile women with and without polycystic ovary syndrome. Am J Res Com. 2014;2(3):61–66. [Google Scholar]
- 22.Saikumar P, KalaiSelvi V, Prabhu K, et al. Anti Mullerian hormone: a potential marker for recruited non growing follicle of ovarian pool in women with polycystic ovarian syndrome. J Clin Diagn Res. 2013;7(9):1866–1869. doi: 10.7860/JCDR/2013/5530.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao K, Ho C, Shyong W, et al. Anti-Mullerian hormone serum level as a predictive marker of ovarian function in Taiwanese women. J Chin Med Assoc. 2012;75(2):70–74. doi: 10.1016/j.jcma.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–3129. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Ma Y, Chen X, et al. Different diagnostic power of anti-Mullerian hormone in evaluating women with polycystic ovaries with and without hyperandrogenism. J Assist Reprod Genet. 2012;29(10):1147–1151. doi: 10.1007/s10815-012-9839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cengiz H, Ekin M, Dagdeviren H, et al. Comparison of serum anti-Müllerian hormone levels in normal weight and overweight–obese adolescent patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2014;180:46–50. doi: 10.1016/j.ejogrb.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Hart R, Doherty D, Norman R, et al. Serum antimullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS) Fertil Steril. 2010;94(3):1118–1121. doi: 10.1016/j.fertnstert.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Sahmay S, Aydin Y, Oncul M, et al. Diagnosis of polycystic ovary syndrome: AMH in combination with clinical symptoms. J Assist Reprod Genet. 2014;31(2):213–220. doi: 10.1007/s10815-013-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eilertsen T, Vanky E, Carlsen S. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: Can morphologic description be replaced? Hum Reprod. 2012;27(8):2494–2502. doi: 10.1093/humrep/des213. [DOI] [PubMed] [Google Scholar]