Abstract
Hatcheries have the power to spread antimicrobial resistant (AMR) pathogens through the poultry value chain because of their central position in the poultry production chain. Currently, no information is available about the presence of AMR Escherichia coli strains and the antibiotic resistance genes (ARGs) they harbor within hatchezries. Therefore, this study aimed to investigate the possible involvement of hatcheries in harboring hemolytic AMR E. coli. Serotyping of the 65 isolated hemolytic E. coli revealed 15 serotypes with the ability to produce moderate biofilms, and shared susceptibility to cephradine and fosfomycin and resistance to spectinomycin. The most common β-lactam resistance gene was blaTEM, followed by blaOXA-1, blaMOX-like, blaCIT-like, blaSHV and blaFOX. Hierarchical clustering of E. coli isolates based on their phenotypic and genotypic profiles revealed separation of the majority of isolates from hatchlings and the hatchery environments, suggesting that hatchling and environmental isolates may have different origins. The high frequency of β-lactam resistance genes in AMR E. coli from chick hatchlings indicates that hatcheries may be a reservoir of AMR E. coli and can be a major contributor to the increased environmental burden of ARGs posing an eminent threat to poultry and human health.
Introduction
In poultry breeding, the hatchery occupies a central position between breeder farms and poultry production houses. However, because of their intensive production systems and the movement of the produced chicks across long distances, hatcheries can serve as a reservoir and source of pathogenic microorganisms. Some chicks are already infected with Gram-negative bacteria at hatching, which can lead to death from yolk sac infection or bacterial chondronecrosis with osteomyelitis1. The risk of illness through contamination of chickens with pathogenic E. coli should be a concern for all parties from farm to fork, as infection could occur through the environment, equipment, feed and drinking water, insufficient cleaning and disinfection, people and chickens themselves. A pathogen-free hatchery environment therefore plays a crucial role in preventing the spread of pathogens in the poultry value chain2.
Although it has been documented that chickens are the most significant carrier of food-poisoning microorganisms causing illness in humans3, their role in the dispersal of antimicrobial-resistant (AMR) pathogens and antibiotic resistance genes (ARGs) into the food chain has not been given due consideration.
The prevalence of AMR E. coli isolates has increased in low- to moderate-income countries such as Egypt, likely as the result of the very liberal and uncontrolled use of antibiotics, to the extent that it is becoming a threat to medical and veterinary treatment efficacy4. Except for anecdotal information, little information is available about the administration of antibiotics in chicken hatcheries in Egypt. Surveillance data from neglected sources such as hatcheries will be essential to gaining insight into the apparently augmented virulence of E. coli strains. Surveillance data can detect correlation with antibiotic use and resistance leading to better practices to assure human treatment efficacy.
The presence of strains producing extended-spectrum β-lactamases (ESBLs) or AmpC β-lactamases in food products is a cause for particular concern because these phenotypes are usually accompanied by a low susceptibility to other classes of antibiotics5. The distribution of E. coli producing ESBLs worldwide has increased over the past decades and the genes encoding ESBLs have further evolved6. Several studies have characterized producers of ESBLs and AmpC β-lactamases isolated from food-production broiler chickens by testing flocks at the farm level and fecal samples at later production steps as cited by Reich et al.5. However, very little is known about the prevalence and diversity of ESBLs within hatcheries and the effects on hatchling exposure and infection.
We assessed 10 geographically separate hatcheries from Egypt as reservoirs for AMR pathogenic E. coli that have the potential to spread through the food chain and the environment. E. coli isolated from hatchlings and the hatchery environment were examined for hemolytic ability, serotype, ability to form biofilm, and resistance to antibiotics. Furthermore, we assessed the prevalence of ESBL and AmpC β-lactamases genes in the isolates. The results show that AMR E. coli are prevalent in Egyptian hatcheries, and that there is a large variety in the resistance to antibiotics among these isolates. The isolates obtained from the hatchlings did not group with the isolates obtained from various sites within the hatchery, indicating hatchlings may become contaminated with E. coli primarily through contact with other hatchlings.
Results
Prevalence of pathogenic E. coli among Egyptian hatcheries
We obtained samples from 10 different hatcheries located within 40 km of cities in geographically separate regions in Egypt. We collected 45 samples from the meconium of day-old hatchlings from each hatchery, a total of 450 hatchling samples. Environmental samples taken from the hatcheries consisted of 3 samples from 8 different locations (Table 1) making a total of 24 samples per a hatchery or 240 total environmental samples. Each sample was examined for pathogenic E. coli by isolation on selective and differential media followed by confirmatory PCR amplification of the uidA gene. Isolates were considered pathogenic based on presence of hemolytic activity. Sixty-five pathogenic E. coli isolates were recovered – 30 from the day-old hatchling meconium samples and 35 from sites in the hatcheries (Table 1). No E. coli was detected in 3 of the 10 hatcheries analyzed and the remaining hatcheries showed presence of E. coli in both the hatchling meconium and hatchery locations. The positive relationship between presence of E. coli in the environment and the hatchling suggested that the hatchlings may have been exposed through its environment. To identify if the isolates from the environment were the same of similar as found in the hatchling, the isolates were further characterized.
Table 1.
Number of E. coli isolates per matrix and hatchery.
| Sampled Matrices | Hatcheries (H) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | ||
| Day-old hatchling meconium | 4/45 (8.9%) |
4/45 (8.9%) |
4/45 (8.9%) |
5/45 (11.1%) |
3/45 (6.7%) |
5/45 (11.1%) |
5/45 (11.1%) |
0/45 (0.0%) |
0/45 (0.0%) |
0/45 (0.0%) |
30/450 (6.7%) |
| Air tunnels | 0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
3/30 (10.0%) |
| Incubators | 0/3 (0.0%) |
0/3 (0.0%) |
3/3 (100%) |
1/3 (33.3%) |
1/3 (33.3%) |
1/3 (33.3%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
7/30 (23.3%) |
| Hatchery machines | 0/3 (0.0%) |
0/3 (0.0%) |
2/3 (66.6%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
5/30 (16.7%) |
| Infertile eggs | 2/3 (66.6%) |
2/3 (66.6%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
7/30 (23.3%) |
| Water | 0/3 (0.0%) |
2/3 (66.6%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
4/30 (13.3%) |
| Workers’ hands | 1/3 (33.3%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
3/30 (10.0%) |
| Egg refrigerators | 0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
3/30 (10.0%) |
| Floors | 0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
1/3 (33.3%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
3/30 (10.0%) |
| Total | 7/69 (10.1%) |
11/69 (15.9%) |
10/69 (14.5%) |
11/69 (15.9%) |
6/69 (8.7%) |
11/69 (15.9%) |
9/69 (13.0%) |
0/69 (0.0%) |
0/69 (0.0%) |
0/69 (0.0%) |
|
H1–H10 = Hatcheries 1 to 10; Hatchery samples were obtained from two visits. A total of 45 hatchling samples and 3 samples per environmental site were obtained per a hatchery.
Number of E. coli isolates/number of samples (percentage of isolates).
Summary of the Characterization of E. coli isolates
To identify if the isolates from the environment are phenotypically the same or similar to those from the hatchling meconium, the isolates were examined for hemolytic ability, serotype, ability to form biofilm, and resistance to antibiotics. The E. coli isolates were serotyped to determine association with known pathogenic serotypes. Fifteen different E. coli serotypes were identified from different sources in the hatcheries, with O128:K71 the most prevalent serotype (Tables S1 and S2). The O128:K71 was the only serotype detected of isolates from the environment and hatchlings of the same hatchery (H1) suggesting little overlap between the environmental and hatchling isolates. Of the 65 isolates, 28 were identified with serotypes previously associated with isolates from chicken hatcheries or pathogenic strain serotypes O1, O2, O8, O78, O119, and O126.
Biofilm formation was measured to determine the ability of isolates to colonize surfaces for environmental survival and persistence and a virulence factor. The ability to form biofilm as determined by slime production (assessed by Congo red uptake and an adherence assay in glass tubes) revealed a heterogeneity among the isolates, ranging from weak and moderate to strong biofilm formation (Tables S1 and S2). The greater abundance of strong producers from the environmental isolates compared to the hatchling suggest that slim production may be associated with successful survival or colonization of hatchery surfaces (p = 0.009, Table 2).
Table 2.
Phenotypes and genotypes identified as significantly different (p < 0.05) between the source of isolation (hatchlings vs. hatchery environments).
| Phenotypes | Source | Prevalence (%) | p-value |
|---|---|---|---|
| Ciprofloxacin (R) | Hatchery environment | 62.9 | 0.008 |
| Chicken hatchlings | 30 | ||
| blaSHV (+) | Hatchery environment | 14.3 | 0.019 |
| Chicken hatchlings | 40 | ||
| blaOXA (+) | Hatchery environment | 88.6 | 0.008 |
| Chicken hatchlings | 60 | ||
| blaMOX-like (+) | Hatchery environment | 11.4 | <0.001 |
| Chicken hatchlings | 90 | ||
| blaFOX (+) | Hatchery environment | 0 | 0.001 |
| Chicken hatchlings | 26.7 | ||
| blaCIT-like (+) | Hatchery environment | 8.6 | <0.001 |
| Chicken hatchlings | 66.7 | ||
| CR (+) | Hatchery environment | 8.6 | 0.009 |
| Chicken hatchlings | 36.7 | ||
| CR (+++) | Hatchery environment | 31.4 | 0.009 |
| Chicken hatchlings | 10 |
The susceptibility of the 65 E. coli isolates to 23 antibiotics (Table 3) was evaluated (Tables S1 and S2) through disk diffusion method. All of the 65 E. coli isolates were susceptible to cephradine and fosfomycin and resistant to spectinomycin. All hatchling isolates demonstrated resistance to doxycycline and oxytetracycline, while sensitivity to colistin (Table S1). All environmental isolates showed sensitivity to gentamycin (Table S2). Additionally, the presence of ciprofloxacin resistance was significantly greater in the hatchery environmental isolates compared to the hatchling isolates (p = 0.008, Table 2). The different antibiotic resistances suggested that no isolate from the environment and hatchery were clonal, or of the exact same isolate.
Table 3.
List, classification and prioritization of antimicrobials categorized as critically important in human and veterinary medicine.
| Antibiotic | Disc concentration | Antimicrobial class | Medical importance (53) | Prioritization criterion |
|---|---|---|---|---|
| Colistin | 600 µg | Polymyxins | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Cephradine | 10 µg | Cephalosporins | Highly Important Antimicrobials | NA |
| Ceftiofur | 10 µg | Cephalosporins | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Fosfomycin | 5 µg | Phosphonic acid derivatives | High Priority Critically Important Antimicrobials | P1 and P2 |
| Gentamycin | 20 µg | Aminoglycosides | High Priority Critically Important Antimicrobials | P2 and P3 |
| Neomycin | 30 µg | Aminoglycosides | High Priority Critically Important Antimicrobials | P2 and P3 |
| Streptomycin | 5 µg | Aminoglycosides | High Priority Critically Important Antimicrobials | P2 and P3 |
| Chloramphenicol | 15 µg | Amphenicols | Highly Important Antimicrobials | NA |
| Enrofloxacin | 10 µg | Quinolones and fluoroquinolones | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Ciprofloxacin | 10 µg | Quinolones and fluoroquinolones | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Norfloxacin | 10 µg | Quinolones and fluoroquinolones | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Flumequine | 5 µg | Quinolones and fluoroquinolones | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Pefloxacin | 5 µg | Quinolones and fluoroquinolones | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Amoxicillin | 10 µg | Penicillins | Highest Priority Critically Important Antimicrobials | P2 and P3 |
| Ampicillin | 10 µg | Penicillins | Highest Priority Critically Important Antimicrobials | P2 and P3 |
| Sulfamethoxazole/Trimethoprim | 15 µg | Sulfonamides, dihydrofolate reductase inhibitors combination | Highly Important Antimicrobials | NA |
| Spiramycin | 5 µg | Macrolides and ketolides | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Erythromycin | 20 µg | Macrolides and ketolides | Highest Priority Critically Important Antimicrobials | P1, P2 and P3 |
| Spectinomycin | 5 µg | Aminocyclitols | Important Antimicrobials | NA |
| Rifampicin | 5 µg | Ansamycins | Highest Priority Critically Important Antimicrobials | P1 and P2 |
| Oxytetracyclin | 20 µg | Tetracyclines | Highly Important Antimicrobials | NA |
| Doxycycline | 20 µg | Tetracyclines | Highly Important Antimicrobials | NA |
| Clindamycin | 20 µg | Lincosamides | Highly Important Antimicrobials | NA |
Prioritization criterion 1 (P1): High absolute number of people, or high proportion of use in patients with serious infections in health care settings affected by bacterial diseases for which the antimicrobial class is the sole or one of few alternatives for treating serious infections in humans. Prioritization criterion 2 (P2): High frequency of use of the antimicrobial class for any indication in human medicine, or high proportion of use in patients with serious infections in health care settings, because use may favor selection of resistance in both settings. Prioritization criterion 3 (P3): The antimicrobial class is used to treat infections in people for whom there is evidence of transmission of resistant bacteria (e.g., non-typhoidal Salmonella and Campylobacter spp.) or resistance genes (high for E. coli and Enterococcus spp.) from non-human sources. NA: not available.
As the spread of ESBL and β-lactam resistant bacteria are a human health concern, the presence of the ESBL class A β-lactamase genes blaTEM, blaSHV, blaOXA-1 and the ampC type β-lactamase genes blaMOX-like, blaCIT -like and blaFOX were revealed through PCR analysis in 96.9%, 16.9%, 60.0%, 38.4%, 3.0%, and 27.7% of the E. coli isolates, respectively. These genes were chosen based on large variability in detection in E. coli adding a distinguishing variable and their known association with plasmids allowing horizontal gene transfer. The isolates from hatchlings were significantly greater in prevalence of all ampC type genes blaMOX-like, blaCIT -like and blaFOX by 78.6%, 58.1%, and 26.7%, respectively (p < 0.05, Table 2). The blaFOX gene was not detected in isolates from the hatchery environment. The blaSHV gene was also significantly greater in abundance in the hatchlings compared to the environment (p = 0.019). The blaOXA-1 gene was the only gene showing significantly greater abundance in the environment of the hatchery compared to the hatchlings (p = 0.008). This suggests that the genotype of the isolates from the hatchling and their environment related to the harboring of β-lactamases were different.
Isolation, distribution, virulence, phenotypic features and antibiotic resistance traits of the E. coli isolates from hatchlings
Serotyping of the 30 E. coli isolates from the hatchlings revealed that they belonged to 10 different serotypes: O1:K61 (6.7%), O2:K69 (6.7%), O8:K60 (10.0%), O25:K- (6.7%), O78:K80 (10.0%), O86:K61 (20.0%), O119:K69 (10.0%), O128:K71 (13.3%), O158:K- (10.0%) and O164:K- (6.7%) (Table S1).
Hemolysin production was determined to differentiate between the virulent hemolytic isolates and the avirulent non-hemolytic isolates (Table S1). Out of the 30 hatchling isolates, 29 were capable of producing α-hemolysin; one serotype O86:K61 isolate produced β–hemolysin instead of α–hemolysin. Biofilm formation was measured to determine the ability of hatchling isolates to colonize surfaces for environmental survival and persistence and a virulence factor. Testing the ability to form biofilms, as determined by slime production (assessed by Congo red uptake and an adherence assay in glass tubes) revealed a heterogeneity among the hatchling isolates, ranging from weak and moderate to strong biofilm formation (Table S1). When screened for their adherence to polyvinyl chloride (PVC) 96-well microtiter plates, a moderate ability to form biofilms was observed for all hatchling isolates (OD595 < 1).
The results of the antibiotic resistance profiles for the 30 E. coli isolates from hatchling meconium (Table S1) showed β-lactam antibiotics tested were inactive against many of these isolates, with 0.0%, 90.0%, 90.0% and 56.7% of isolates resistant to cephradine, amoxicillin, ampicillin and ceftiofur, respectively. Norfloxacin was the least active (93.3% resistant isolates) of the fluoroquinolone (FQ) antimicrobials, with flumequine, another FQ, yielding similar results (90.0% resistant isolates) and resistance to pefloxacin (80.0% resistant isolates) and enrofloxacin (60.0% resistant isolates) also common. In contrast, ciprofloxacin was quite active, with resistance in only 30.0% of hatchling isolates. No resistance was detected against colistin and fosfomycin, 10.0% were resistant to gentamycin. Most of the isolates were resistant to most of the remaining antibiotics; in descending order, all were resistant to doxycycline, oxytetracycline and spectinomycin, 96.7% were resistant to clindamycin and erythromycin, 93.3% were resistant to streptomycin, 86.7% were resistant to rifampicin and spiramycin, 76.7% were resistant to sulfamethoxazole-trimethoprim drug combination, 73.3% were resistant to neomycin and 53.3% were resistant to chloramphenicol. The high abundance of antibiotic resistant hatchling isolates, some last resort antibiotics for clinical treatment of human infection, suggest a reservoir of AMR E. coli and potential to disseminate.
Investigation of the class A β-lactamase genes encoding ESBLs, blaTEM, blaSHV, and blaOXA-1, revealed that these genes were present in 93.3%, 40.0% and 60.0% hatchling isolates, respectively. The ampC genes blaFOX, blaMOX-like and blaCIT-like were identified in 90.0%, 26.7% and 66.7% of the hatchling isolates, respectively. The distribution of the resistance genes varied between different hatchling isolates (Table S1). Interestingly, only 6.7% isolates from hatchlings harbored none of the 6 resistance genes, 10% of the isolates harbored 5 of the resistance genes (O78:K80, O86:K61, O128:K71) and 16.7% isolates carried all 6 resistance genes tested (all from serotype O86:K61). The prevalence of these β-lactamase genes and their close association with elements of horizontal gene transfer, plasmid, suggest the possibility of further dissemination of β-lactam resistance.
According to the definition of Souli et al.7, none of the 30 hatchling isolates were pan drug resistant (PDR), however, 63.3% isolates were multiple drug resistant (MDR; excluding XDR) and 36.7% were extensively drug resistant (XDR). These XDR E. coli isolates were resistant to 11 of the 13 classes of antibiotics tested and included three serotype O78:K80, two O128:K71, two O119:K58, two O164:K-, and two O2:K69. Nineteen different MDR and XDR patterns were observed. Interestingly, all serotype O158:K- hatchling isolates had the same resistance profile, showing resistance to 14 antibiotics, whereas 50% serotype O86:K61 isolates were resistant to 17 antibiotics. In addition, 50% serotype O128:K71 isolates shared a common resistance profile, showing resistance to the following 12 antibiotics while 66.7% serotype O8:K60 isolates were resistant to 15 antibiotics. The abundance of MDR and XDR within the hatchling isolates demonstrated a high presence of AMR E. coli that may eventually enter the poultry production chain.
Isolation, distribution, virulence, phenotypic features and antibiotic resistance traits of the E. coli isolated from hatchery environments
Serotyping of the 35 E. coli isolated from hatchery environments revealed seven different serotypes (Table S2).
Investigation of the production of hemolysin by the 35 E. coli isolated from the hatchery environment revealed that 91.4% could produce α-hemolysin, whereas 8.6% isolates, from serotype O126:K71 (isolated from an incubator), O128:K71 (isolated from an infertile egg) and O114:K90 (isolated from water), produced β–hemolysin instead. Analysis of biofilm formation using Congo red uptake and glass test tube assays identified 17.1% hatchery isolates as weak biofilm formers, 54.3% as moderate biofilm formers and 28.6% as strong biofilm formers (Table S2), while all hatchery isolates had a moderate ability to form biofilms on polyvinyl chloride (PVC) (OD595 < 1), as indicated by adherence assays conducted in 96-well PVC microtiter plates.
Antibiotic resistance profiling of the 35 E. coli isolates from hatcheries showed that the β-lactam antibiotics tested were inactive against most of the isolates; all hatchery isolates were sensitive to cephradine, and resistance against amoxicillin and ampicillin was detected in 91.4% hatchery isolates for each antibiotic. Ceftiofur was the most active β-lactam antibiotic with 71.4% of hatchery isolates showing resistance. Among the FQ, pefloxacin was the least active, with 94.3% hatchery isolates displaying resistance. Ciprofloxacin was the most active FQ, with only 62.9% of the hatchery isolates showing resistance. The resistance for chloramphenicol was 42.9% of hatchery isolates. All hatchery isolates were sensitive to fosfomycin and gentamycin. All hatchery isolates were resistant to spectinomycin. Resistance for doxycycline, oxytetracycline, clindamycin and erythromycin was detected in 97.1% of isolates for each antibiotic. For streptomycin, spiramycin, and sulfamethoxazole-trimethoprim, 91.4% resistant isolates were detected, whereas 85.7% isolates were resistant to rifampicin and 80.0% to neomycin. The high abundance of antibiotic resistant isolates from the hatchery environment suggest a reservoir of AMR E. coli and potential infection or dissemination to hatchlings.
The occurrence of class A β-lactamase genes and ampC genes assessed by PCR (Table S2) indicated that their distribution varied in the hatchery isolates. The blaTEM, blaSHV, and blaOXA-1 genes were identified in 97.1%, 14.3% and 88.6% of the hatchery isolates, respectively, whereas the ampC genes blaFOX, blaMOX-like, and blaCIT-like were identified in 0%, 11.4% and 8.6% of the hatchery isolates, respectively. Three isolates contained only one of the six resistance genes, 22 isolates contained two resistance genes and 10 isolates contained three resistance genes. We identified 7 isolates that produced an ESBL that also carried an ampC gene. The high abundance of ESBL related genes blaTEM and blaOXA-1 indicate that the hatchery environment is a reservoir of these genes and may potentially disseminate through the poultry production change through hatchlings.
Multiple resistance patterns and distribution among E. coli serotypes
Of the 35 E. coli hatchery isolates, none were PDR, 22.9% were XDR, and 77.1% of isolates were MDR (excluding XDR; Table S2). Of the XDR isolates, one serotype O78:K80 and one serotype O119:K69 isolate were resistant to 12 of the 13 antibiotic classes tested. The other XDR isolates were one O119:K69, one O128:K71, one O119:K69, two serotype O128:K71, one O126:K71 serotype isolate were to 12 classes of antibiotics tested. There was 32 different MDR and XDR patterns observed, and interestingly 30 of them were unique to a single E. coli hatchery isolate. The diversity of resistance patterns suggest a potentially diverse pool of resistance genes or associations of genes such a plasmid within the hatchery environment.
Associations between serotype, isolation source and phenotypic and genotypic traits
The relationship of the strength of biofilm formation and serotype was performed to determine possible associations in the isolates. Serotype may have a relationship with biofilm forming ability in PVC microtiter plates, with serotype O8:K60 isolates being stronger biofilm formers and serotype O25:K- isolates forming the weakest biofilms (Fig. 1), however, more isolates are needed to demonstrate this relationship with any statistical significance. There were no significant differences between serotypes in the formation of biofilm as determined by the CTM and MTP methods (p = 0.30 and 0.31 respectively). While statistical analysis suggested a possible significant difference in serotypes impact on biofilm formation determined by CR (p = 0.013) there was no significant difference upon pairwise comparisons (p > 0.171) there was also no significant correlation between CTM and CR results (p > 0.05, Fig. 2).
Figure 1.
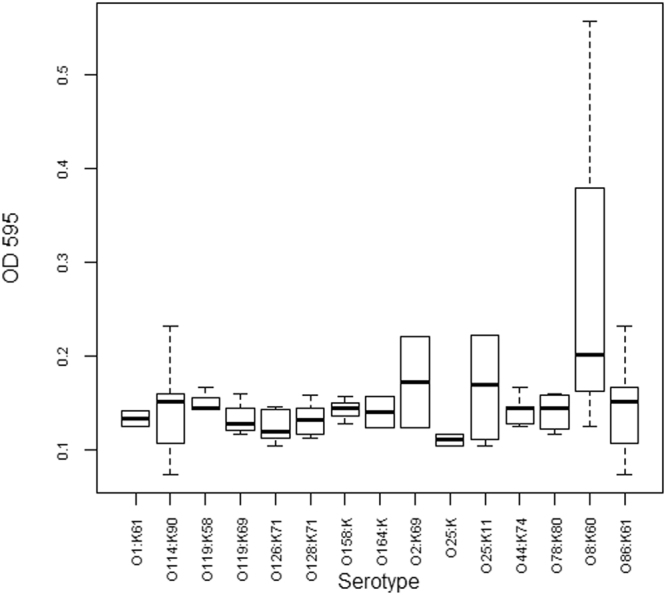
Biofilm formation in the microtiter plate assay by serotype.
Figure 2.
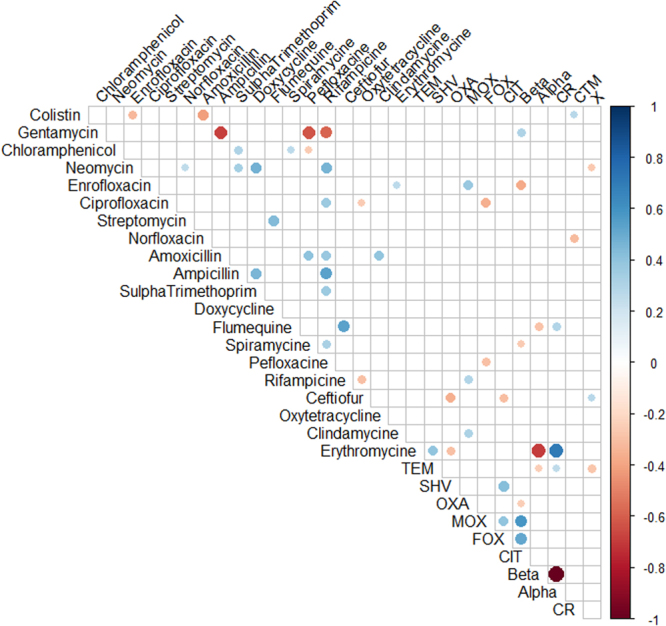
Correlation matrix of phenotypic (antibiotic resistance, hemolytic activity and biofilm formation ability) and genotypic (antibiotic resistance genes) features showing significant (p < 0.05) correlations. White spaces are not significantly correlated. Blue circles indicated significant positive correlation and red show significant negative correlation. The size and strength of color represent the numerical value of the Phi correlation coefficient.
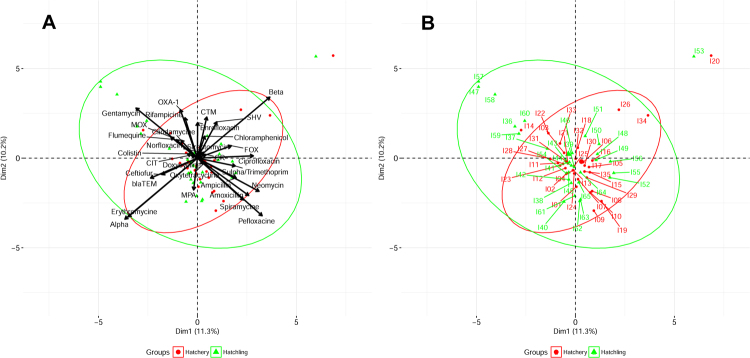
Correlation matrix analysis (Fig. 2), hierarchical clustering (with heatmap) (Fig. 3), principle component analysis (Fig. 4) was used to determine associations between the phenotypic and genotypic traits and source of the isolates. Correlation analysis showed very few positive relationships with the presence of β-lactamase genes and resistance to β-lactams except for resistance to ceftiofur correlating with the presence of the blaSHV gene (Fig. 3, p < 0.05). Significant positive correlations of antibiotic resistances indicated co-occurrence of resistance may be prevalent (p < 0.05, Fig. 3) and confirmed the presence of MDR and XDR strains (above). For example, resistance to the β-lactam amoxicillin was positively correlated with resistance to spiramycine, pefloxacine, and oxytetracycline (p < 0.05). Similarly, resistance to the β-lactam ampicillin was positively correlated with resistance to combination drug sulfamethoxazole-trimethoprim, spiramycine, chloramphenicol, and neomycin (p < 0.05). The Principle Component Analysis (PCA) showed similar relations between these correlations with β-lactam resistance (Fig. 4). However, resistance to the β-lactam ceftiofur did not show any positive correlations with any other antibiotic resistances tested. The presence of ampC related β-lactamase genes of blaMOX-like and blaCIT-like were positively correlated with quinolone resistance to enrofloxacin, ciprofloxacin, or perofloxacine (p < 0.05) also visualized in the PCA.
Figure 3.
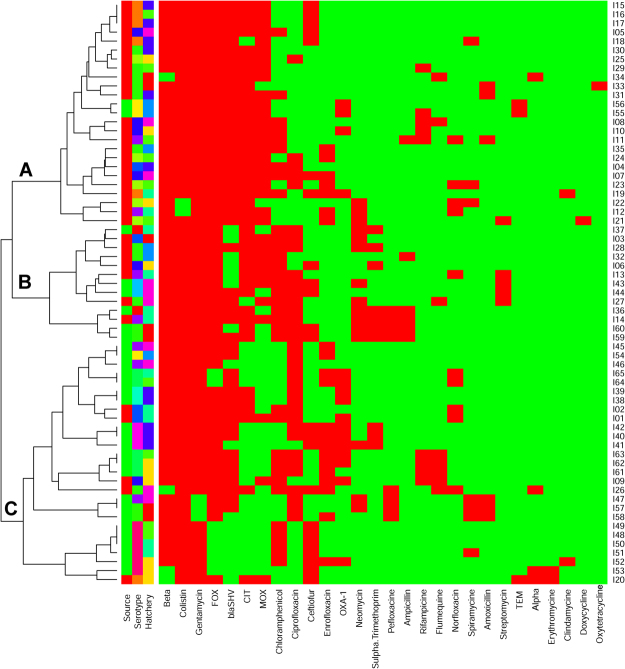
Heatmap and hierarchical clustering of E. coli isolates according to their phenotypic (antibiotic resistance) and genotypic (antibiotic resistance genes) profile of variables showing differences between isolates. Red represent presence and green represented absence of resistance or gene. Left of the heatmap is color representation of the different sources (hatchling in green and hatchery in red), the different serotypes, and the different hatcheries. Hierarchical clustering was perform using Wald’s method and a binary distance matrix. Letters designate the 3 main clusters described in the text.
Figure 4.
Principle component analysis performed on variables showing differences. (A) Visualization of the isolates encompassed in 95% confidence intervals grouping based on source of the isolate (hatchery or hatchling) and (B) labeling of the individual isolates from the same analysis.
Hierarchical clustering analysis allowed the segregation of the 65 E. coli isolates according to their phenotypic (antibiotic resistance profiles, biofilm formation ability, hemolysis) and genotypic (β-lactam resistance gene profiles) traits (Fig. 3). Interestingly, a separation of E. coli isolated from chick hatchlings (Fig. 3, Cluster A) and E. coli isolated from hatchery environmental samples (Fig. 3, Cluster C) was observed. Three main clusters were identified. Cluster A included 23 E. coli isolates from chick hatchlings (from serotypes O128:K71, O78:K80, O158:K-, O86:K61, O164:K-, O8:K60, O119:K58 and O2:K69) and 2 from environmental isolates from water of the same hatchery (serotype O114:K90 and O44:K74), Another large cluster (Cluster C) contained 25 E. coli isolates from the hatchery environment (serotypes: O119:K69, O78:80, O114:K90, O25:K11, O44:K74, O128:K71, and O126:K71) and 2 isolates from hatchlings from the same hatchery (serotype: O119:K58). The third cluster (Cluster B) consisted of 7 hatchling isolates and 6 environmental isolates. Of interest in the third cluster, a hatchling isolate (serotype O78:K80, Isolate 27) and environmental isolate from egg refrigerator (serotype 01:K61, Isolate 44) from the same hatchery were clustered together. While being of different serotypes, these two isolates demonstrated the same phenotype and genotype. The hatchling isolates 36, 59, and 60 clustered together with the hatchery isolate 14 in PCA (Fig. 4) and hierarchical clustering (Fig. 3) most likely driven by the sensitivity to ampicillin, sulfamethoxazole-trimethoprim, and pefloxacine among other shared similarities. Similarly, the clustering of hatchling isolates 47, 57, and 58 were visible in both the PCA and hierarchical clustering and was driven by the presence of gentamycin resistance, as other isolates lacked this property. PCA showed that the hatchery isolates 20 and hatchling isolate 53 clustered far from the majority of isolated and shared the same antibiotic resistances to including sensitivity to erythromycin, which all other isolates showed resistance. There is no evidence that any two isolates had the same phenotype therefore are non-clonal indicating that there was no demonstration of direct transfer between hatchery environment and hatchling, or vis-versa. The differences in associations of traits as demonstrated by clustering, PCA, and correlation indicate the potential for hatchlings and hatchery environment to select for different traits within E. coli.
Discussion
Not only is there a limited amount of information in the literature regarding the prevalence of E. coli and antibiotic resistance in hatcheries, but there is also a considerable variation in those reports8. Several factors may have contributed to these very different estimates of E. coli prevalence. The methods used for obtaining the samples from the hatcheries were not consistent across the different studies. The potential effect of the differences in methodology between studies, such as in the media used for enrichment, selective enrichment, and isolation, can also affect estimates of prevalence9.
The serotyping of the 65 isolates in our study revealed several interesting aspects of the isolates. Among the isolates in Egyptian hatcheries, we identified pathogenic E. coli serogroups, such as O1, O2, and O78, that are usually implicated in field infections10. Furthermore, three of the 65 E. coli isolates belonged to the O8 serogroup, which has been associated with hatchery losses and early chick mortality in India11, while E. coli serotypes O119 and O126, which were also serologically typed from hatchery isolates in Saudi Arabia, were also isolated in the present investigation12. Our sampled hatcheries were geographically separate and situated in a region with a high density of food animal production, which could indicate a common source or selective pressure, while the fertile eggs were supplied by different companies. Fortunately, the six non-O157 Shiga toxin-producing E. coli (STEC) serogroups that increasingly have been associated with serious outbreaks of human infection and often referred to as “the big six”13, O26, O45, O103, O111, O121, and O145, were not identified in our survey.
An important microbial characteristic associated with virulent avian E. coli is a hemolytic reaction on blood agar plates14,15 – hemolytic strains are more virulent than non-hemolytic strains16,17. α-Hemolysin, also known as cytotoxic necrotizing factor, is produced by invasive strains of E. coli, which sets the pace for the pathogenesis of renal disease18 and enhances virulence in a number of clinical infections19. The high prevalence of α-hemolysin in our isolates indicates that it is a common exotoxin produced by avian pathogenic E. coli strains. The ciprofloxacin- and FQ-resistant isolates show a lower expression of α-hemolysin and instead produced more β-hemolysin20.
The biofilm-producing abilities of the E. coli from the different other sources in the hatchery were monitored in our study using the CR assay, which is an easy and reproducible standard laboratory method. It has been used to obtain experimental evidence of amyloid formation and for the presence of a biofilm matrix21 and has previously been used to identify potentially pathogenic E. coli strains in hatcheries and brooders22. Several studies have indicated that only pathogenic E. coli can bind this dye and that Congo red-positive E. coli strains will cause disease when inoculated into chicks23. Little has previously been reported on potential differences in biofilm production between serotypes or serogroups of E. coli24, although differences between strains have been reported by several reports25–27. In our study, minor differences in the ability to form biofilm were observed among E. coli serotypes, although strains of serotype O8:K60 formed stronger biofilms on PVC. Nevertheless, strains of environmental origin showed a greater ability to form biofilms than E. coli strains isolated from chick hatchlings, which suggests that biofilm formation is important for environmental survival and persistence. E. coli biofilms can cause serious problems in hatcheries by increasing the resistance of cells to environmental stresses and protecting them from cleaning and sanitation procedures used to decontaminate processing environments28. To the best of our knowledge, no published study has compared biofilm production by poultry-related E. coli isolates with their zoonotic potential. It is conceivable that biofilms could allow bacteria to persist and survive in the hatchery on poultry processing equipment that is mostly made of glass and/or PVC material29,30 and thus can be colonized by pathogenic E. coli strains.
Antibiotic use plays a major role in the emerging public health crisis of antibiotic resistance, which has become a looming problem31. The majority of antibiotic use occurs in agricultural settings31, and agricultural antibiotics are associated with clinical antibiotic resistance31. Indeed, recently it was estimated that annually more than 1500 deaths in the European Union are directly related to antibiotic use in poultry32 and that 56% of the resistance genes in human pathogens were identical to genes derived from E. coli strains isolated from poultry sources32. Globally, billions of chickens receive third-generation cephalosporins, in ovo or as day-old chicks, to treat E. coli infection, a practice that has resulted in large reservoirs of resistant bacteria32. In our study, we show that resistance to antimicrobial classes commonly employed in industrial farming, including macrolides (erythromycin), tetracyclines (tetracycline) and penicillins (penicillin and ampicillin), is more prevalent than resistance to antibiotics that are less frequently used. The exception was lincosamide (clindamycin) resistance, which was relatively, and unexpectedly, low compared with resistance against other antimicrobial classes commonly used in animal rearing. An interesting finding of our study is the low resistance level observed for “the older antibiotics”, i.e., colistin, fosfomycin and cephradine, which are listed on the World Health Organization’s List of Essential Medicines and are considered among the most important drugs needed in a basic health system (Table 3)33. Colistin remains one of the antibiotics of last resort against MDR Enterobacteriaceae that produce the New Delhi metallo-β-lactamase (NDM-1)34 and it remains the only available treatment for XDR infections. None of the isolates were resistant to fosfomycin, a phosphonic acid derivative that has been used for the prophylaxis and treatment of UTI and has activity against ESBL-producing E. coli. Fosfomycin has therefore retained medical importance, either alone or in combination with other antimicrobials, including β-lactams, aminoglycosides, and fluoroquinolones, possibly owing to synergistic effects. In contrast, resistance to 3rd-generation cephalosporins such as ceftiofur was frequent in the hatcheries. Furthermore, we found that resistance to chloramphenicol and tetracycline often co-occurred. Resistance to these antibiotics is often linked to isolates carrying blaSHV alone or in combination with resistance to nalidixic acid5. The co-resistance and the rate of reduced susceptibility found in our study are comparable to findings reported by Dierikx et al.35 and may be caused by treatment regimens similar to those used in conventionally reared broilers in Germany and the Netherlands.
Several E. coli isolates displayed MDR and XDR phenotypes. Correlation analyses showed that co-occurrence of resistance to various antibiotics, as has also been previously described36,37, represents an important concern for human and animal medicine alike. On the other hand, resistance to certain antibiotics was associated with susceptibility to others. For example, resistance to gentamycin appeared to be associated with susceptibility to amoxicillin, pefloxacin and spiramycin. This result is remarkable because it can facilitate the selection of alternative antibiotics when addressing MDR or XDR E. coli strains.
The detection of resistance genes in our investigation highlights the fact that E. coli found in poultry may serve as reservoirs for anti-microbial resistance genes that could potentially be transferred to pathogenic microorganisms infecting humans38. In the current study, the presence of three ESBL-encoding genes (blaTEM, blaSHV, and blaOXA-1) and three ampC-related β–lactamase genes (blaMOX-like, blaCIT-like and blaFOX) in the E. coli isolates was determined. TEM-52, SHV-12, and CTX-M-1 are the most frequently reported ESBL types from the food animal reservoir5,39,40. We found that several antibiotic-resistant isolates lacked the ampC-related genes, indicating that these isolates might encode a different mechanism of resistance, potentially overexpression of chromosomal ampC, which usually results from mutations in the promoter/attenuator region of ampC5,41.
Interestingly, although no clear associations could be observed between serotype and antibiotic resistance phenotype or gene profile, isolates from chick hatchlings were more likely to contain the blaSHV, blaMOX-like, blaCIT-like and blaFOX resistance genes than isolates of environmental origin, which suggests that antibiotic use in poultry production is imposing a selective pressure, leading to the spread of these AMR genes in poultry hosts. However, no significant differences in resistance to the tested antibiotics were observed between isolates of animal and environmental origin, with the exception of ciprofloxacin, as ciprofloxacin resistance was higher in environmental isolates than in isolates of animal origin. Hierarchical clustering of E. coli isolates based on their phenotypic and genotypic profiles allowed for a good separation of isolates from hatchlings (which had a higher prevalence of β-lactamase encoding genes) and those from hatchery environments (which were more resistant to ciprofloxacin and had better abilities to form biofilms), which suggests that isolates from hatchlings and environmental isolates have different origins. The main differences among isolates of animal and environmental origin potentially reflect the different lifestyle of the pathogen in both niches, in that isolates of animal origin are more frequent carriers of ampC genes, likely due to the increased selective pressure imposed by the use of antibiotics in farms. Moreover, isolates of environmental origin were stronger biofilm formers, likely because the biofilm lifestyle helps them persist in the hatchery environment.
The lack of uniform surveillance protocols for pathogenic E. coli and antimicrobial resistance genes at hatcheries constitutes an important gap in the farm-to-fork spectrum of food safety protection and a risk to public health and food safety that needs to be addressed. The findings described in this study are significant to public health because they indicate that the potential benefit of post-Hazard Analysis and Critical Control Point reduction in pathogenic E. coli prevalence at processing plants can be undermined by contamination occurring at the hatchery. Moreover, we have found that resistance to β-lactams and other clinically relevant antibiotics is widespread in hatcheries, including the presence of several MDR and XDR isolates.
Materials and Methods
Study area
Ten hatcheries located in the outskirts of major populated cities at Egypt (at distances not >40 km from the town centres) were monitored for the presence of pathogenic E. coli. These hatcheries were in the Greater Cairo Zone consisting of Cairo Governorate, Giza, Shubra El-Kheima, Helwan, 15th May City, 6th of October City, Shorouk City, Madinaty and Obour City. The total population of this region is estimated at 20,500,000 (as of 2012). The area, density and altitude are 1,709 km2, 10,400 people/km2 and 250–350 metres (820 and 1,150 ft) above sea level, respectively.
Ethics approval and consent to participate.The meconium samples derived from the birds were all taken from hatchlings in routinely process at the hatchery (H1 thru H10 hatcheries). The hatcheries were numbered in this way, to respect their privacy. No animal experiment was conducted. Meconium samples were collected as fresh droppings on fresh broiler paper at hatcheries. Hand wash samples taken from the workers hands, after their informed and written consent, were part of standard screening by the hatchery officials and these samples became available for the research described in this manuscript. In spite of all these conditions, approval by the Animal Care and Use Committee of the Animal work was approved by the Internal Animal Ethics Committee at Cairo University. Swabs from the environment in the hatcheries and the environment of the broiler farms were taken by the researcher. Sampling was carried out following the procedures of the Animal and Plant Health Agency42, formerly Animal Health and Veterinary Laboratories Agency-GOV.UK. Methods and experimental protocols were carried out in accordance with the Helsinki Declaration.
Recovery and identification of pathogenic E. coli from environmental and meconium samples
Wet sampling and handling
During 2015, the following samples were taken from inside ten commercial hatcheries: 10 meconium drops were pooled into one sample to make a total of 45 meconium samples per hatchery (a total of 450 sampled hatchlings); 24 environmental samples per hatchery from the following sources: water, broken eggshells from each tray, infertile eggs, swabs from the workers hands, air tunnels, floors, incubators, hatchery machines and egg refrigerators. The collection of environmental hatchery samples was made using the following protocol: sterile cotton wool moistened swabs (moistened with sterile buffered peptone water, BPW) were used to swab each of the environments from the ten hatcheries. Collected samples were put into sterile plastic bags, and these into boxes, carefully labelled, packed, cooled in an icebox and immediately transported to the laboratory and stored at 4 °C. All samples were immediately processed for microbiological analyses. In order to maximize sample quality, hatchery visits were planned in such a way that they coincided with the day at which fertile eggs were received by the hatchery.
Isolation and identification of pathogenic E. coli
The isolation procedure adopted for E. coli identification was outlined in the Food and Drug Administration Bacteriological Analytical Manual (FDA-BAM)43. In brief, faeces and liquid samples were inoculated in 225 ml brain heart infusion (BHI) broth at 35 °C for 3 h to facilitate resuscitation of injured cells. These pre-enrichments were then transferred to 225 ml of tryptone phosphate (TP) broth and incubated at 44 °C for 20 h. A volume of enriched broth was then plated onto Levine’s eosin-methylene blue (L-EMB; E. coli colonies produce a green metallic sheen) and MacConkey (MAC) agar plates and the plates were incubated for 18–24 h at 35 °C. Colonies revealing the characteristic metallic sheen colonies on EMB agar (3–5 colonies from each sample) were subjected to biochemical tests for the identification of E. coli. Presumptive E. coli colonies were injected into triple sugar iron and urea agar slants and subjected to the indole test. Colonies exhibiting indole, methyl-red, and catalase tests as positive, Voges–Proskauer and citrate tests as negative, and fermenting glucose and lactose sugars, were confirmed as E. coli isolates44. Colonies showing positive results were further identified by using the API 20E test (bioMérieux Clinical Diagnostics, Marcy l’Étoile, France). Enterobacter cloacae American Type Culture Collection (ATCC) 1307 and E. coli ATCC 11775 were included as positive controls and Salmonella Berta ATCC 8392 was included as a negative control.
Detection of O-serogroups
Isolates biochemically confirmed as E. coli in MAC agar were submitted to slide agglutination tests using polyvalent and monovalent sera. Commercial antisera available at the Central Laboratories of Ministry of Public Health, Egypt, were used.
Phenotypic virulence factors
Biofilm formation
Multiple methods (below) of determining biofilm formation, both qualitatively and quantitively, were used to differentiate the propensity of isolate to attach to surfaces and produce biofilms. The Congo red dye assay allows qualitative analysis for biofilm production, the glass test tube assay allows for qualitative analysis of attachment to glass surfaces and subsequence biofilm formation during disturbance and fluid flow, and the microtiter plate method for quantitative analysis of attachment and biofilm formation on plastic surfaces during static conditions.
Congo red (CR) dye uptake
The ability to take up Congo red dye was determined on agar plates supplemented with 50 mg/mL of Congo red dye. Five microliters of each bacterial suspension were streaked onto the plates, and the plates were incubated at 37 °C for 24 h. The strong biofilm producing isolates were visualized as black colored colonies with dry crystalline consistency, while the red colonies are interpreted as non-biofilm producer isolates45 and moderate biofilm producers were variation between.
Glass test tube assay (CTM)
Quantification of biofilms on glass was based on the test tube assay, described by Christensen et al.46. A loopful of each E. coli isolate grown overnight in culture plates was inoculated in Brain Heart Infusion (BHI) broth (10 ml), and incubated for 24 h at 37 °C without shaking. The tubes were decanted, washed with PBS (pH 7.3) and air-dried. Dried tubes were stained with crystal violet (0.1%). Excess stain was removed and tubes were washed with deionized water. Tubes were than dried in inverted position and observed for slime layer formation. Biofilm formation was considered positive when a visible film lined the wall and bottom of the tube. Ring formation at the liquid interface was not indicative of biofilm formation. All the isolates were tested in triplicate. Tubes were examined and the amount of biofilm formation was scored as 0-absent, 1-weak, 2-moderate or 3-strong.
Microtiter plate assay (MPA)
E. coli isolates were recovered from −80 °C glycerol stocks onto tryptic soy agar and were stored at 4 °C. For tests in the static biofilm model, and according to the procedure of Wakimoto et al.47, 200 μL of Dulbecco’s modified Eagle’s medium containing 0.45% glucose in 96-well flat-bottom microtiter polystyrene plates (Becton Dickinson, Franklin Lakes, NJ) were mixed with 5 μl of an E. coli overnight culture grown at 37 °C in Luria broth. 100 µl from this dilution, were inoculated into eight separate wells of a presterilized polyvinyl chloride (PVC) microtiter plate. Eight wells of Modified Welshimer’s broth were included as a negative control. The plates were incubated for 18 h at 37 °C. After 40 h the liquid was removed from the wells, and unattached cells were removed by rinsing three times with 150 μl of sterile water. Biofilms were stained by adding 50 μl of a 0.5% crystal violet solution to each well and incubating for 45 min at room temperature. Unbound dye was removed by rinsing three times with 150 μl of sterile water. The crystal violet was solubilized by adding 200 μl of 95% ethanol and incubating at 4 °C for 30 min. The contents of each well (100 μl) were then transferred to a sterile polystyrene microtiter plate, and the optical density at 595 nm (OD595) of each well was measured in a microplate reader (model 680, Bio rad, USA).
Production of hemolysins
The E. coli hemolytic activity was evaluated by streaking Columbia agar plates (Oxoid) supplemented with 5% sheep blood with each of the isolated E. coli. After 24 h incubation at 37 °C plates were examined for signs of β-hemolysis (clearing zones around colonies), α-hemolysis (a green-hued zone around colonies) or γ-hemolysis (no halo around colonies)48.
Antibiotic susceptibility testing of the E. coli isolates
The antimicrobial susceptibility of each E. coli isolate was tested against a panel of 23 antibiotics (Oxoid) (Table 3), chosen according to their medical importance49, by inoculating a calibrated bacterial suspension (0.5 McFarland) on Mueller-Hinton agar. The antibiotic susceptibilities of the isolates were determined using Kirby Bauer’s disk diffusion method as described in the guidelines of the Clinical and Laboratory Standards Institute (CLSI) and the results were interpreted according to CLSI guidelines50. Escherichia coli ATCC 25922 was used as the quality control strain for the antibiotic susceptibility tests and the results were interpreted as per CLSI criteria.
Molecular typing methods
Detection of uidA, ampC and class A β-lactamase genes
E. coli isolates were grown overnight in 3 ml BHI broth at 37 °C for 18–24 h, after which 200 μl aliquots were transferred to 1.5-ml Eppendorf centrifuge tubes and centrifuged at 13,000 × g for 2 min. The bacterial pellets were resuspended in 200 μl of sterile water by vortexing. The suspension was boiled for 10 min and centrifuged for 5 min, after which 150 μl of the supernatant containing DNA was used as the DNA template.
Species identification was confirmed using an E. coli specific PCR amplification protocol for the uidA gene (Table 4)51. The optimized protocol was carried out with a PCR mix of 25 μL that contained 2.5 mM MgCl2, 20 mM Tris-HCl (pH 9.0 at 25 °C), 50 mM KCl, and 0.1% Triton X-100), 1 mM dNTP mixture, 1 μM of each of the primers, 1 U of Taq polymerase and 1 μL of the DNA template. For the presence of the following resistance genes in the E. coli isolates: the plasmid-mediated ampC genes was investigated using a multiplex PCR assay targeting blaMOX-like, blaCIT-like and blaFOX, as described by Pérez-Pérez and Hanson52 and class A β-lactamase genes (blaTEM, blaSHV and blaOXA-1) as reported by Colom et al.53 and indicated in Table 4. The PCR reaction was performed with a final volume of 50 µl in 0.5-ml thin-walled tubes. Each reaction contained 20 mM Tris-HCl (pH 8.4); 50 mM KCl; 0.8 mM dNTP mixture; 1.5 mM MgCl2; 0.6 µM primers blaMOX-like and blaCIT-like; 0.4 µM primers blaFOX; and 1.25 U of Taq DNA polymerase. Template DNA (2 µl) was added to 48 µl of the master mixture. All PCR reactions were overlaid with oil and PCR was performed in a Veriti 96-well thermal cycler. PCR amplicons were then analyzed on a 1.5% agarose gel, stained with ethidium bromide and visualized by Gel Documentation System. A 100 bp DNA ladder (Promega, USA) was used as a size marker.
Table 4.
PCR-specific oligonucleotide primers, amplicon size and conditions for uidA, ampC and class A β-lactamase genes.
| Name | Target Enzyme(s) | Amplicon Size (bp) | Cycle Number | Annealing Temperature (°C) | Primer Sequence | Conferred Resistance (or Purpose) | Reference |
|---|---|---|---|---|---|---|---|
| uidA | β-glucuronidase specific for E. coli | 486 | 35 | 60 | P1: 5′-ATCACCGTGGTGACGCATGTCGC | Confirmation of E. coli | 51 |
| P2: 5′-CACCACGATGCCATGTTCATCTGC | |||||||
| AmpC-like β-lactamase (plasmid associated) | |||||||
| blaMOX-like | MOX-1, MOX-2, CMY-1, CMY-8 to CMY-11 | 520 | 25 | 64 | MOXMF: 5′-GCTGCTCAAGGAGCACAGGAT | Enzymes are known to confer resistance to penicillins, oxyimino group cephalosporins and 7-α-methoxy group. Possible resistance to monobactam aztreonam. Unchanged sensitivity for cefepime and carbapenems62. | 52 |
| MOXMR: 5′-CACATTGACATAGGTGTGGTGC | |||||||
| blaCIT-like | LAT-1 to LAT-4, CMY-2 to CMY-7, BIL-1 | 462 | 25 | 64 | CITMF: 5′-TGGCCAGAACTGACAGGCAAA | ||
| CITMR: 5′-TTTCTCCTGAACGTGGCTGGC | |||||||
| bla FOX | FOX-1 to FOX-5b | 190 | 25 | 64 | FOXMF: 5′-AACATGGGGTATCAGGGAGATG | ||
| FOXMF: 5′-CAAAGCGCGTAACCGGATTGG | |||||||
| Extended-spectrum β-lactamase (plasmid associated) | |||||||
| bla OXA-1 | OXA-1 | 609 | 54 | 32 | OXA-G: 5′-TCAACTTTCAAGATCGCA | ESBL enzymes retain ability to confer resistance to penicillins and confer resistance to expanded-spectrum cephalosporins. Unchanged sensitivity for cephamycins, cefoxitin, and cefotetan63. | 53 |
| OXA-H: 5′-GTGTGTTTAGAATGGTGA | |||||||
| bla SHV | SHV | 392 | 54 | 32 | SHV-F: 5′-AGGATTGACTGCCTTTTTG | ||
| SHV-R: 5′-ATTTGCTGATTTCGCTCG | |||||||
| bla TEM | TEM | 516 | 54 | 32 | TEM-C: 5′-ATCAGCAATAAACCAGC | ||
| TEM-H: 5′-CCCCGAAGAACGTTTTC | |||||||
Statistical analysis
Antibiotic resistance phenotypic profiles and gene presence were converted into binary or numerical coding for statistical analysis. Sensitivity to an antibiotic was represented as 0 and resistance was represented as 1. Presence or absence of a specific gene (eg. blaTEM) was represented as 1 and 0, respectively. Because of the qualitative nature of CTM and Congo red (CR) analysis, results were converted to presence or absence similar to antibiotic resistance profiles. Statistical analysis was performed using the open statistical program R54. Correlations for binary variables were calculated using the ‘cor’ function and using ‘cor.test’ function to determine significance by invoking the Pearson method to yield the binary equivalent of the Pearson correlation coefficient, Phi coefficient. Significant correlations were visualized utilizing the ‘corrplot’ function from the ‘corrplot’ R package55. A heatmap and hierarchical clustering was performed heatmap3 R package56. Distance matrices were calculated for hierarchical clustering analysis by the ‘dist’ function using the ‘binary’ method and clustered by ‘hclust’ using Ward’s method57. The R packages ‘FactoMineR’58 and ‘factoextra’59 were used to calculate and visualize Principal component analysis (PCA). Comparison of serotype impact on CTM and CR was performed using ‘glm’ function with guassian model and followed by post-hoc analysis with ‘glht’ from the ‘MultiComp’ package60,61. The impact of serotype on MTP results was statistically determined by use of ‘aov’ function and the post-hoc test ‘TukeyHSD’, if needed. Comparisons between frequencies of occurrence of each phenotypic or genotypic feature in E. coli isolates from hatchlings or hatchery environments were made by contingency table χ2 tests (at p < 0.05).
Electronic supplementary material
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No.: RGP-162.
Author Contributions
K.O. and M.E.H. conceived, designed the experiments and wrote the paper; W.A.G. revised the manuscript; F.E.M. was responsible for the medical human patients during the process of diagnosis, sampling and consequent analysis with A.O. and H.M.Y.Y., who collected the veterinary samples, A.M., T.D., H.A.H., I.M. and A.H. contributed reagents/materials/analysis tools and performed the experiments. A.D.K. helped in statistical analysis and preparation of the manuscript. All authors read, discussed the results and implications and commented on the manuscript at all stages and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23962-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wideman RF. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult Sci. 2016;95:325–44. doi: 10.3382/ps/pev320. [DOI] [PubMed] [Google Scholar]
- 2.de Lange, G. Preventing cross-contamination in the hatchery Pas Reform The future of hatchery technologies at your finger tips. Pas Reform Hatchery Technologies, Bovendorpsstraat 117038 CH P.O. Box 27038 ZG Zeddam The Netherlands (Last accessed 17/9/2016).
- 3.FDA. The U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition. Foodborne Illness-Causing Organisms in the U.S.: What You Need to Know (Page Last Updated: 01/07/2016).
- 4.AMR Control. Published in Official Association with the World. Alliance against Antibiotic Resistance (WAAAR), Global Health Dynamics 20 Quayside, Woodbridge, Suffolk IP12 1BH, UK, www.globalhealthdynamics.co.uk (2015).
- 5.Reich F, Atanassova V, Klein G. Extended-Spectrum ß-Lactamase– and AmpC-Producing Enterobacteria in Healthy Broiler Chickens, Germany. Emerg Infect Dis. 2013;19:1253–9. doi: 10.3201/eid1908.120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souli, M., Galani, I. & Giamarellou, H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill 13(47), pii=19045, Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19045 (2008). [PubMed]
- 8.Boulianne M, et al. Drug use and antimicrobial resistance among Escherichia coli and Enterococcus spp. isolates from chicken and turkey flocks slaughtered in Quebec, Canada. Can J Vet Res. 2016;80:49–59. [PMC free article] [PubMed] [Google Scholar]
- 9.Myint, M. S. Epidemiology of Salmonella contamination of poultry meat products: knowledge gaps in the farm to store products. Dissertation submitted to the Faculty of the Graduate School of the University of Maryland, College Park, in partial fulfillment of the requirements for the degree of Doctor of Philosophy, USA (2004).
- 10.ECDPC. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: The Centre (2011).
- 11.Burkhanova KHK. Properties of E. coli strains isolated from diseased fowl. Vet Moscow. 1980;10:66–68. [Google Scholar]
- 12.Al-khalaf AN, et al. Bacterial Contamination of Hatcheries. J Agric Vet Sci Qassim Univ. 2009;2:67–76. [Google Scholar]
- 13.Nesse LL, et al. Potentially Pathogenic Escherichia coli Can Form a Biofilm under Conditions Relevant to the Food Production Chain. Appl Environ Microbiol. 2014;80:2042–2049. doi: 10.1128/AEM.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Siek, K. E., Giddings, C. W., Doetkott, C., Johnson, T. J., Fakhr, M. K. & Nolan, L. K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology151, 2097–2110 (2005). [DOI] [PubMed]
- 15.Vidotto MC, et al. Virulence factors of avian Escherichia coli. Avian Dis. 1990;34:531–538. doi: 10.2307/1591241. [DOI] [PubMed] [Google Scholar]
- 16.Jaryal SC, Bareja R, Grover PS. Significance of haemolytic Escherichia coli among clinical isolates. Int J Rec Sci Res. 2016;7:9093–9096. [Google Scholar]
- 17.Vaish R, Pradeep MSS, Setty CR, Kandi V. Evaluation of Virulence Factors and Antibiotic Sensitivity Pattern of Escherichia coli Isolated from Extraintestinal Infections. Cureus. 2016;8:e604. doi: 10.7759/cureus.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia TA, Ventura CL, Smith MA, Merrell DS, O’Brien AD. Cytotoxic Necrotizing Factor 1 and Hemolysin from Uropathogenic Escherichia coli Elicit Different Host Responses in the Murine Bladder. Infect Immun. 2013;81:99–109. doi: 10.1128/IAI.00605-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herlax V, Henning M, Bernasconi A, Goni F, Bakas L. The lytic mechanism of Escherichia coli α-hemolysin associated to outer membrane vesicles. Health. 2010;2:484–492. doi: 10.4236/health.2010.25072. [DOI] [Google Scholar]
- 20.Drews SJ, et al. Decreased prevalence of virulence factors among ciprofloxacin-resistant uropathogenic Escherichia coli isolates. J Clin Microbiol. 2005;43:4218–4220. doi: 10.1128/JCM.43.8.4218-4220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shewmaker F, et al. The functional curli amyloid is not based on in-register parallel beta-sheet structure. J Biol Chem. 2009;284:25065–25076. doi: 10.1074/jbc.M109.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Styles, D. & Flammer, K. Preliminary Investigation…Congo Red Binding in Escherichia coli. AFA Watchbird 17, No 4 (1990).
- 23.Osman KM, Mustafa AM, Elhariri M, AbdElhamed GS. Identification of serotypes and virulence markers of Escherichia coli isolated from human stool and urine samples in Egypt. Indian J Med Microbiol. 2012;30:308–313. doi: 10.4103/0255-0857.99492. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Medina M, et al. Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC) BMC Microbiol. 2009;9:202. doi: 10.1186/1471-2180-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salo J, et al. Biofilm formation by Escherichia coli isolated from patients with urinary tract infections. Clin Nephrol. 2009;71:501–507. doi: 10.5414/CNP71501. [DOI] [PubMed] [Google Scholar]
- 26.Cremet L, et al. Orthopaedic-implant infections by Escherichia coli: molecular and phenotypic analysis of the causative strains. J Infect. 2012;64:169–175. doi: 10.1016/j.jinf.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Bono JL, Kalchayanand N, Shackelford S, Harhay DM. Biofilm formation by Shiga toxin-producing Escherichia coli O157:H7 and non-O157 strains and their tolerance to sanitizers commonly used in the food processing environment. J Food Prot. 2012;75:1418–1428. doi: 10.4315/0362-028X.JFP-11-427. [DOI] [PubMed] [Google Scholar]
- 28.Ryu JH, Beuchat LR. Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and curli production on its resistance to chlorine. Appl Environ Microbiol. 2005;71:247–254. doi: 10.1128/AEM.71.1.247-254.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simões M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT - Food Sci Technol. 2010;43:573–583. doi: 10.1016/j.lwt.2009.12.008. [DOI] [Google Scholar]
- 30.Wang, X., Demirci, A. & Puri, V. M. Biofilms in Dairy Products and Dairy Processing Equipment and Control Strategies, in Biofilms in the Food Environment. Second Edition (eds Pometto, A. L. & Demirci, A.) John Wiley & Sons, Ltd, Chichester, UK, 10.1002/9781118864036.ch8 (2015).
- 31.Landers TF, Cohen B, Wittum TE, Larson EL. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012;127:4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collignon P, Aarestrup FM, Irwin R, McEwen S. Human Deaths and Third-Generation Cephalosporin use in Poultry, Europe. Emerg Infect Dis. 2013;19:1339–1340. doi: 10.3201/eid1908.120681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. 19th WHO Model List of EssentialMedicines (April 2015) (PDF). WHO. April 2015. Retrieved May 10, (2015).
- 34.Garonzik SM, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically-ill patients from a multi-center study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dierikx C, van Essen-Zandbergen A, Veldman K, Smith H, Mevius D. Increased detection of extended spectrum beta-lactamase producing Salmonella enterica and Escherichia coli isolates from poultry. Vet Microbiol. 2010;145:273–278. doi: 10.1016/j.vetmic.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Ciccozzi M, et al. Phylogenetic analysis of multidrug-resistant Escherichia coli clones isolated from humans and poultry. New Microbiol. 2013;36:385–394. [PubMed] [Google Scholar]
- 37.Szmolka A, Nagy B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front Microbiol. 2013;4:258. doi: 10.3389/fmicb.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell NM, Johnson JR, Johnston B, Curtiss R, III, Mellata M. Zoonotic Potential of Escherichia coli Isolates from Retail Chicken Meat Products and Eggs. Appl Environ Microbiol. 2015;81:1177–87. doi: 10.1128/AEM.03524-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantón R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9:466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Coque, T. M., Baquero, F. & Canton, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 20, 13(47), pii: 19044 (2008). [PubMed]
- 41.Peter-Getzlaff S, et al. Detection of AmpC beta-lactamase in Escherichia coli: comparison of three phenotypic confirmation assays and genetic analysis. J Clin Microbiol. 2011;49:2924–2932. doi: 10.1128/JCM.00091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Animal and Plant Health Agency (formerly Animal Health and Veterinary), Woodham Lane, New Haw, Addlestone, Surrey, KT15 3NB (2013).
- 43.Feng, P., Weagant, S. D., Grant, M. A. & Burkhardt, W. Bacteriological Analytical Manual Chapter 4 Enumeration of Escherichia coli and the Coliform Bacteria, U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) (2015).
- 44.Marinho CM, Santos T, Gonçalves A, Poeta P, Igrejas G. A Decade-Long Commitment to Antimicrobial Resistance Surveillance in Portugal. Front Microbiol. 2016;7:1650. doi: 10.3389/fmicb.2016.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niveditha S, Pramodhini S, Umadevi S, Kumar S, Stephen S. The Isolation and the Biofilm Formation of Uropathogens in the Patients with Catheter Associated Urinary Tract Infections (UTIs) J Clin Diagc Res: JCDR. 2012;6:1478–1482. doi: 10.7860/JCDR/2012/4367.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakimoto N, et al. Quantitative biofilm assay using a microtiter plate to screen for enteroaggregative Escherichia coli. Am J Trop Med Hyg. 2004;71:687–690. [PubMed] [Google Scholar]
- 48.Maragkoudakis PA, et al. Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J. 2009;16:189–199. doi: 10.1016/j.idairyj.2005.02.009. [DOI] [Google Scholar]
- 49.WHO. World Health Organization. Critically important antimicrobials for human medicine – 5th rev. Geneva: World Health Organization; Licence: CC BY-NC-SA3.0 IGO (2017).
- 50.CLSI. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement CLSI document M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute, (2014).
- 51.Heininger A, et al. PCR and blood culture for detection of Escherichia coli bacteremia in rats. J Clin Microbiol. 1999;37:2479–2482. doi: 10.1128/jcm.37.8.2479-2482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colom K, et al. Simple and reliable multiplex PCR assay for detection of blaTEM, blaSHV and blaOXA-1 genes in Enterobacteriaceae. FEMS Microbiol Lett. 2003;223:147–151. doi: 10.1016/S0378-1097(03)00306-9. [DOI] [PubMed] [Google Scholar]
- 54.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://doi.org/http://www.R-project.org/ (2017).
- 55.Wei, T., & Simko, V. The corrplot package. CRAN Repository. Retrieved from http://www.sthda.com/french/wiki/matrice-de-correlation-la-fonction-r-qui-fait-tout (2016).
- 56.Zhao S, Guo Y, Sheng Q, Shyr Y. Heatmap3: an improved heatmap package with more powerful and convenient features. BMC Bioinformatics. 2014;15(Suppl 10):P16. doi: 10.1186/1471-2105-15-S10-P16. [DOI] [Google Scholar]
- 57.Murtagh F, Legendre P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? Journal of Classification. 2014;31(3):274–295. doi: 10.1007/s00357-014-9161-z. [DOI] [Google Scholar]
- 58.Lê S, Josse J, Husson F. FactoMineR: An R Package for Multivariate Analysis. J. of Statistical Software. 2008;25(1):1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 59.Kassambara, A. & Mundt, F. Factoextra: extract and visualize the results of multivariate data analyses. R Package Version, 1(3) (2016).
- 60.Hothorn T, Bretz F, Westfall P. “Simultaneous Inference in General Parametric Models”. Biometrical Journal. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 61.Bretz, F., Hothorn, T. & Westfall, P. Multiple Comparisons Using R. Boca Raton, Florida, USA: Chapman \& Hall/CRC Press, ISBN: 978–1–58488–574–0 (2010).
- 62.Philippon A, Arlet G, George AJacoby. Plasmid-determined AmpC-type β-lactamases. Antimicrobial agents and chemotherapy. 2002;46(1):1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradford PatriciaA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clinical microbiology reviews. 2001;14(4):933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.