Abstract
The MADS-box genes encode transcriptional regulators with various functions especially during floral development. A total of 54 putative Morus notabilis MADS-box genes (MnMADSs) were identified and phylogenetically classified as either type I (17 genes) or type II (37 genes). The detected genes included three FLOWERING LOCUS C-like (MnFLC-like) genes, MnMADS33, MnMADS50, and MnMADS7. MnFLC-like proteins could directly or indirectly repress promoter activity of the mulberry FLOWERING LOCUS T-like (MnFT) gene. Transgenic Arabidopsis thaliana overexpressing MnFLC-like genes exhibited delayed flowering and down-regulated expression of FT and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1). The gene expression analyses in floral bud indicated that MnMADS33 expression increased, while MnFT expression decreased during the induction of dormancy in response to cold conditions. Dormancy release resulted in the down-regulation of MnMADS33 expression and the up-regulation of MnFT expression. Furthermore, abscisic acid promoted the transcription of MnMADS33 and MnFT, although the expression level of MnFT gradually decreased. These results are consistent with the hypothesis that MnMADS33 negatively regulated the expression of MnFT to repress dormancy release and flowering in mulberry. This study may be relevant for future investigations regarding the effects of MnMADS genes on mulberry flowering development.
Introduction
The MADS-box gene family is not specific to plants as it is also commonly found in animals and fungi. The MADS-box genes, which encode transcription factors, are conserved among eukaryotes1,2. The DNA-binding domain at the N-terminus of these transcription factors consists of a conserved region (approximately 58 amino acids) called the MADS-box domain, which is also involved in dimerization reactions and the binding of accessory factors3. The CArG-box [C(A/T)8G, C(C/T)(A/T)G(A/T)4(A/G), or C(C/T)(A/T)6(A/G)G] is a consensus MADS-box transcription factor-binding site4–6. In the model plant Arabidopsis thaliana, there are two types of MADS-box genes that differ regarding their conserved sequences and structures. Type I MADS-box genes encode only the MADS-box domain, and are further divided into the Mα, Mβ, and Mγ subclasses. In addition to the MADS-box domain, type II genes encode the intervening (I), keratin-like (K), and C-terminal (C) domains that ultimately form the so-called MIKC-type domain structure7,8. The I and K domains affect protein-protein interactions, resulting in the formation of homodimeric or heterodimeric complexes, while the highly variable C domain influences transcriptional regulation and contributes to the formation of protein complexes2,9. Type II genes are classified into the MIKCc and MIKC* subfamilies7. Based on their sequence characteristics and functions, the MIKCc genes have been further divided into 13 subcategories, which were named after their first identified members1,10,11.
There is a limited amount of information regarding the A. thaliana type I MADS-box genes. In addition, the type II genes include the floral homeotic genes, whose functions are related to the determination of floral organ identities, which can be explained by the well-studied ABCDE model12–14. All ABCDE model genes belong to the MIKCc subfamily, with the exception of AP215. The APETALA1/FRUITFULL (AP1/FUL), APETALA3/PISTILLATA (AP3/PI), AGAMOUS (AG), SHATTERPROOF/SEEDSTICK (SHP/STK), and SEPALLATA (SEP) subfamily genes encode proteins that interact with one another to form a complex regulatory network, that controls the development of the floral organ (e.g., sepals, petals, stamens, carpels, and ovules)2. Moreover, accumulating evidence suggests that the type II genes play crucial regulatory roles in various developmental processes throughout the A. thaliana life cycle, including embryogenesis16, flowering time17, pollen maturation and tube growth18, and seed dormancy19. The functions of the MADS-box family genes have been summarized in detail20. Although the diverse functions of MADS-box genes in annuals have been described, their roles in perennials remain relatively unknown.
Perennials survive periods of seasonally-induced stress as dormant buds21. However, the molecular mechanism underlying this dormancy has not been fully characterized22. SHORT VEGETATIVE PHASE (SVP) is a MADS-box gene that represses flowering in A. thaliana23. Homologs of SVP are known as dormancy-associated MADS-box (DAM) genes and have been identified in peach (Prunus persica)24, leafy spurge (Euphorbia esula)25, Japanese apricot (Prunus mume)26, and Japanese pear (Pyrus pyrifolia)27. For example, the P. persica DAM5 and DAM6 expression patterns correspond with dormancy induction and release. Specifically, DAM5 and DAM6 expression levels are up-regulated in lateral floral buds throughout autumn and are down-regulated during the dormancy release stage of the endodormancy cycle28. Meanwhile, the FLOWERING LOCUS T (FT), which affects flower development29, influences the development of dormancy30,31. Overexpression of poplar FT1 in the plum (Prunus domestica) leads to continuous flowering as well as impaired initiation of dormancy32. Chromatin immunoprecipitation assays in the leafy spurge using endodormant crown buds indicated that a DAM-like protein likely binds to the CArG-boxes in the FT promoter regions to suppress FT expression as part of dormancy regulation33. A previous study revealed that the overexpression of mulberry FT induces early flowering in A. thaliana34, but the involvement of MnFT in dormancy remains unexplored.
Dormancy release requires a period of accumulative chilling, which is analogous to the chilling requirement of vernalization for flowering in winter annuals35. In A. thaliana, the FLOWERING LOCUS C (FLC) MADS-box gene has been extensively studied. This gene encodes a flowering repressor involved in the vernalization response36. The FLC protein binds to the CArG-box element in the first intron of FT to suppress the expression of FT37. Additionally, FLC can form a dimer with SVP to repress the floral transition in A. thaliana38. The MADS AFFECTING FLOWERING 1 (MAF1) –MAF5 genes are closely related to FLC, with MAF1–MAF4 encoding floral repressors and MAF5 encoding a floral activator in A. thaliana39. A recent meta-analysis of microarray data suggested that the MAF3-like gene expression in the underground adventitious buds of leafy spurge might be useful as an endodormancy marker40. An analysis of the transcription profile of apple (Malusx domestica) revealed that the expression of two MdFLC-like genes, MdMADS135 and MdMADS136, increased during the winter dormancy period and decreased considerably at dormancy release41. Similarly, PtFLC expression levels in trifoliate orange (Poncirus trifoliata) increased after September, peaked in November, and then decreased during spring42. Several reports have suggested that dormancy in woody plants and vernalization in annual herbaceous plants are regulated by overlapping pathways43–46. Consequently, considering the similar expression profiles of DAM and FLC-like genes during the dormancy period, FLC-like genes are likely important for dormancy. However, there is a lack of published research regarding the roles of FLC-like genes related to dormancy regulation in perennials.
Mulberry (order: Rosales; family: Moraceae) is a perennial woody flowering plant that is widely cultivated in Asia, Europe, Africa, and North and South America47. Although mulberry is mainly used for raising silkworms, its fruit is converted into wine, juice, jam, and canned food and is also used in medicinal products48. Mulberry belongs to the “indirect flowering” group, in which the differentiation of flower buds occurs before dormancy, while blooming and fruit development take place after dormancy release49. In a previous study, 36 members of the Morus alba MADS-box genes were detected in the floral buds50. However, a comprehensive analysis of mulberry MADS-box genes based on genome sequences have not yet been reported.
The importance of MADS-box proteins for plant development prompted us to investigate their functions in mulberry plants. In the present study, we conducted a comprehensive analysis of the MADS-box gene family in the M. notabilis genome51. We identified 54 MnMADS genes, including three FLC-like genes, MnMADS33, MnMADS50, and MnMADS7. We also examined MnMADS phylogenetic relationships, gene structures, and encoded conserved protein motifs. The expression of type I genes was nearly undetectable, while type II genes were expressed at high levels in flower-related organs. Of these genes, MnMADS1, MnMADS19, MnMADS33, MnMADS45, and MnMADS46 were abundantly expressed in the buds of differentiating inflorescence primordia, dormant buds, and catkins. Moreover, subcellular localization of MnFLC-like proteins was investigated by ectopic expression in tobacco cells. MnFLC-like-GFP proteins were mainly localized to the nucleus, while MnMADS33-GFP was also detected in the epicyte and epidermal cell organelles of tobacco. The ectopic and constitutive expression of MnFLC-like genes in A. thaliana plants resulted in late-bolting phenotypes with down-regulated expression of FT and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1). Analyses of mulberry floral buds indicated that MnMADS33 and MnFT expression levels increased and decreased, respectively, in cold-treated mulberry floral buds under field and artificially controlled conditions. Dormancy release was associated with the down- and up-regulated expression of MnMADS33 and MnFT, respectively. MnMADS33 could directly or indirectly suppress the promoter activity of MnFT to repress the expression of downstream report gene in vivo. These results along with the conserved function of FLC down-regulating FT in other systems37,52, suggesting that MnMADS33 might down-regulate the expression of MnFT to repress the dormancy release and flowering in mulberry. The mulberry genes described herein warrant further study to characterize their regulatory effects on flowering.
Results
Identification of MADS-box genes from the mulberry genome
We detected 63 M. notabilis genes based on HMM and BLASTP analyses. Nine sequences (i.e., Morus001839, Morus002012, Morus002795, Morus016120, Morus016684, Morus018169, Morus020771, Morus023144, and Morus025559) did not contain a complete MADS domain-coding sequence and were excluded from further analyses. The remaining 54 genes were considered putative MnMADS genes (i.e., MnMADS1–MnMADS54). The full-length cDNA sequences of the truncated genes (MnMADS7, MnMADS16, MnMADS21, MnMADS50, MnMADS51, and MnMADS52) were verified manually based on previously reported de novo transcription data50. The MnMADS7, MnMADS16, MnMADS21, MnMADS50, MnMADS51, and MnMADS52 genes corresponded to c79593_g1, c68277_g1, c70333_g2, c78979_g1, c79142_g3, and c74310_g1, respectively. Details regarding the putative MnMADS proteins, including length (i.e., 79–509 amino acids), molecular weight, and isoelectric point, are provided in Table S1. The 54 MnMADS genes were detected on 42 scaffolds. Scaffolds 1731 and 336 contained the most MnMADS genes (three genes), followed by scaffolds 1100, 261, 262, 271, 553, 651, 73, and 948 (two genes). The other 31 scaffolds consisted of only one MnMADS gene. Additionally, MnMADS genes on the same scaffold were located relatively close to each other and apparently formed clusters.
We identified potential paralogous pairs among the 54 MnMADS genes using MCScanX. The MnMADS gene types are presented in Table S1. Eight paralogous pairs were detected, including three whole genome/segmental duplications (i.e., MnMADS16/MnMADS46, MnMADS45/MnMADS53, and MnMADS17/MnMADS45) and five tandem duplications (i.e., MnMADS42/MnMADS43, MnMADS27/MnMADS28, MnMADS9/MnMADS10, MnMADS26/MnMADS27, and MnMADS2/MnMADS3) (Table S2). The Ka/Ks ratio was calculated for the coding sequences of eight paralogous pairs to determine the type of Darwinian selection underlying the gene divergences after duplication events. The Ka/Ks ratios of whole genome/segmental duplications were <0.1, while the Ka/Ks ratios for tandem duplications were 0.03–1.57 (average: 0.58). There were two pairs of MnMADS genes (i.e., MnMADS26/MnMADS27 and MnMADS2/MnMADS3) for which the Ka/Ks ratio was >1.
Phylogenetic analyses of the Arabidopsis thaliana and mulberry MADS-box genes
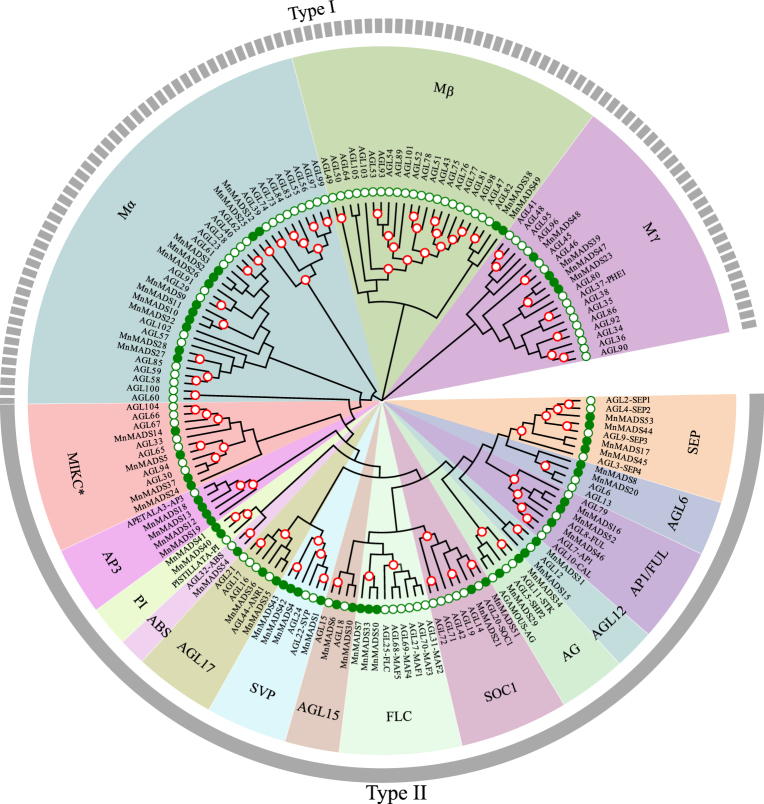
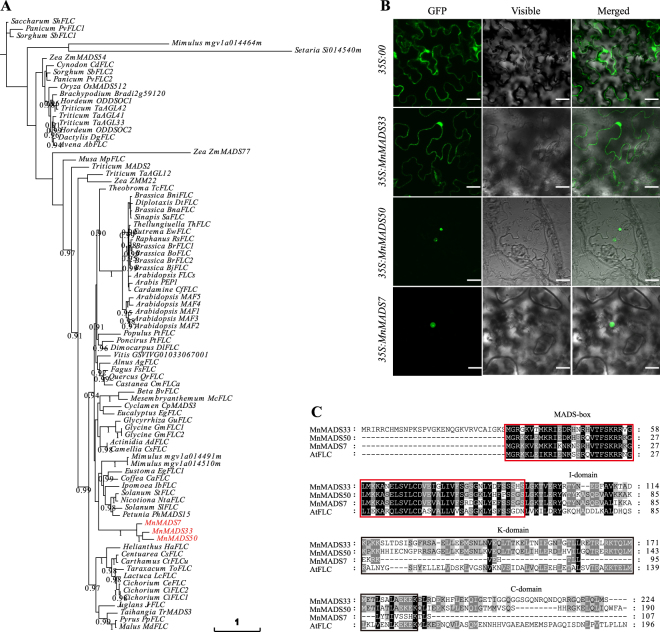
To analyze the phylogenetic relationships among MnMADS genes, an unrooted neighbor-joining (NJ) tree was constructed based on the alignment of the full-length amino acid sequences of 54 putative MnMADS proteins and 103 A. thaliana MADS-box transcription factors. A phylogenetic analysis revealed that 17 type I and 37 type II MADS-box genes encoded the 54 MnMADS proteins (Fig. 1). According to the A. thaliana MADS-box subgroup classifications, the type II proteins were further divided into the following 14 groups: SEP, AGL6, AP1/FUL, AGL12, AG, SOC1, FLC, AGL15, SVP, AGL17, ARABIDOPSIS BSISTER (ABS), PI, AP3, and MIKC*. Most of these subgroups were named according to extensively investigated MADS-box genes. To further validate the evolutionary relationships among MnMADSs, an unrooted NJ tree was constructed using the MADS-box protein sequences of A. thaliana, grape, and mulberry (Supplementary Fig. S1). Moreover, the evolutionary relationships among the 54 MnMADS proteins were investigated using a maximum likelihood (ML) method (Fig. 2A). It is noteworthy that the FLC subfamily was adjacent to AP1, SEP, and AGL6 in the ML tree. Furthermore, the evolutionary relationships of the MnMADS proteins in Fig. 2A were consistent with the relationships in the NJ phylogenetic tree (Fig. 1).
Figure 1.
Unrooted neighbor-joining tree constructed using all full-length MADS-box proteins from mulberry (green dot) and Arabidopsis thaliana (green circle). Bootstrap values over 90% are labeled with red circles. The MADS-box genes are divided into two groups (i.e., type I and type II). Fourteen groups in type II are highlighted in different colors.
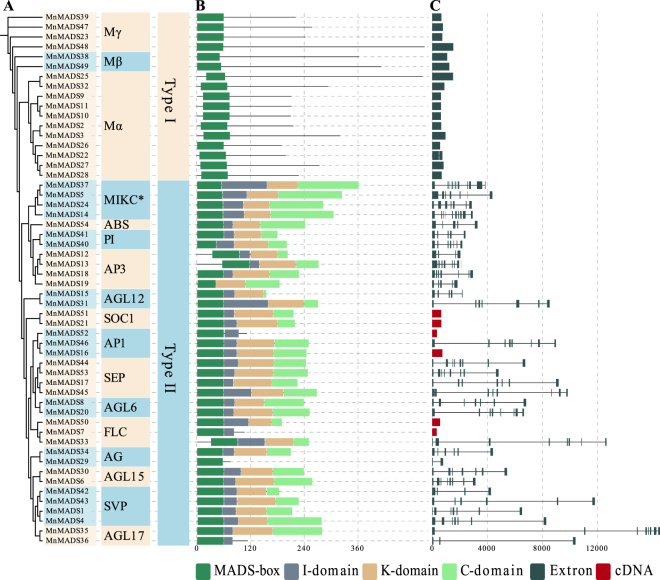
Figure 2.
Phylogenetic relationships and structural analyses of mulberry MADS-box genes. (A) The unrooted maximum likelihood tree of 54 MnMADS proteins was reconstructed using a JTT + I + G model. Groups are shown in different colors. (B) Domains of MADS-box, intervening (I), keratin-like (K), and C-terminal (C) are indicated by different colored broad lines. The length corresponds to motif length. (C) The broad grey lines and thin lines indicate exons and introns, respectively. Broad red lines indicate the full-length cDNA of MnMADS genes, which are manually modified according to the de novo transcriptome assembly data reported by Shang et al.50.
Mulberry MADS-box protein domains, gene structures, and classes
All MnMADS proteins consisted of a MADS-box domain. As shown in Fig. 2B, except for MnMADS7, MnMADS29, MnMADS36, and MnMADS52, all the type II MnMADS proteins contained a K-domain. Additionally, the MnMADS gene structures were investigated using comparative alignments of the corresponding genomic and coding sequences. The number of introns varied from 0 to 10, with genes in the same family containing a similar number of introns. Most of the type I genes had no introns, while the majority of type II genes had six. The genes indicated by broad red lines in Fig. 2C are manually corrected cDNA sequences. For most MADS-box gene families and subfamilies, there were more A. thaliana genes than mulberry genes (or an equal number). For example, we identified 17 type I mulberry genes (31% of the total number of MnMADS genes), while there were 58 type I A. thaliana genes (56% of the total number of A. thaliana MADS-box genes) (Supplementary Fig. S2). In contrast, the AP3 and PI subfamilies contained more mulberry genes than A. thaliana genes. We observed that A. thaliana carried only one AP3 and one PI gene, while mulberry contained four AP3 and two PI genes. Additionally, mulberry also had four SVP/AGL24 genes, while A. thaliana consisted of only two.
Expression profiles of mulberry MADS-box genes in various organs and flower-related tissues
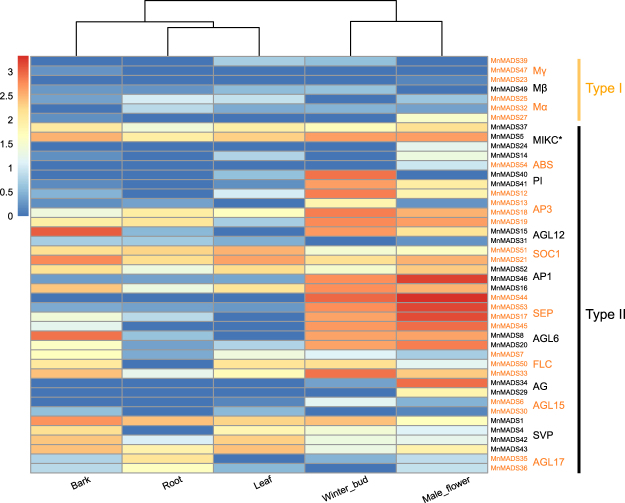
Analyses of the MnMADS expression profiles in the roots, leaves, bark, winter buds, and male flowers resulted in the identification of 44 MnMADS genes that were expressed in at least one of these organs, including all 37 type II genes. The expression level of seven type I genes was generally low, while the expression of the remaining 10 type I genes was undetectable in all examined organs. Overall, the MnMADS expression levels were lowest in the roots, followed by the leaves. Most genes with more than 50 RPKM were associated with the ABCDE flowering model and were preferentially expressed in winter buds and male flowers. Several genes exhibited high expression levels in various tissues. The most highly expressed gene was MnMADS44, with an expression level of 535 RPKM in male flowers. It was also highly expressed in winter buds. MnMADS46 was the next most highly expressed gene, with an expression level of 304 RPKM in male flowers. The most highly expressed gene in bark was MnMADS15 (147 RPKM). Additionally, the expression level of MnMADS33 (i.e., FLC homolog) was biased in winter buds (>80 RPKM), while the expression level of the SVP subfamily MnMADS genes was relatively low (Fig. 3, Table S3).
Figure 3.
Expression profiles of MnMADS genes in different organs. Mulberry roots, bark, winter buds, male flowers, and leaves were used. The homologs of the corresponding mulberry genes were separately presented with black and orange colors. The names of subfamily were marked on the right side. The color scale bar at the bottom left of the figure represents log2 (RPKM+1) values.
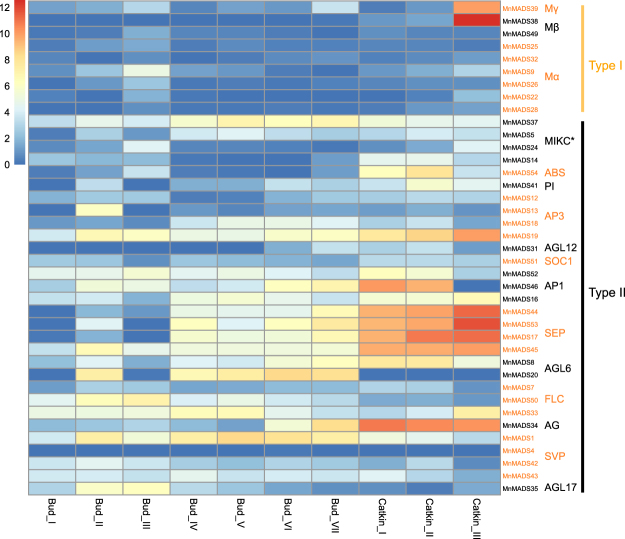
To further associate MnMADS functions with specific developmental processes, a quantitative real-time polymerase chain reaction (qRT-PCR) was used to analyze expression profiles in various flower-related mulberry tissues. Of the 54 MnMADS genes, 39 were expressed in at least one tissue of the mulberry species ‘Jinqiang 63’ (JQ63) (Table S4). Gene expression patterns for the buds of differentiating inflorescence primordia (buds I-III), during endodormancy and dormancy release (buds IV-VII), as well as in the different catkin development stages (catkins I-III) are shown in Fig. 4. Prominent among the nine genes in type I, both of MnMADS38 and MnMADS39 were highly expressed in catkin III. Of the type II genes, five (i.e., MnMADS1, 19, 33, 45, and 46) were abundantly expressed in all three stages. For example, MnMADS33 was most highly expressed in catkin III but also expressed in buds I–III and buds IV-V. Meanwhile, MnMADS1 (i.e., SVP homolog) was abundantly expressed in bud II and III, as well as in catkin I. The mulberry genome includes four MnMADS homologs of SVP/AGL24 (i.e., MnMADS1, 4, 42, and 43). We observed that MnMADS1 was expressed at a continuously high level during the endodormancy and dormancy release periods. The remaining genes in the SVP/GAL24 family were expressed at very low expression levels during these periods.
Figure 4.
The expression of MnMADS genes in the flower-related tissues during inflorescence development. Bud I, II, and III represent initial, mid-term, and later stage of differentiating inflorescence primordial, respectively. Paraffin sections were prepared to ensure the correct stages (Supplementary Fig. S7). Bud IV and V are dormant buds. Bud VI and VII were dormancy release buds. Catkin I, catkin II, and catkin III were before, mid-term, and later stage of pollination, respectively. The data presented here represent two biological replicate experiments. The qRT-PCR is repeated three times for each biological replicate. RPL15 is used as the internal control. This heat map is created by method mentioned in Fig. 3.
FLOWERING LOCUS C homologs in mulberry
To provide additional evidence for the relationship between FLC and MnMADS33/MnMADS50/MnMADS7, we reanalyzed the previously reported phylogenetic relationships among the FLC-like proteins from mulberry and other species53. MnMADS33, MnMADS50, and MnMADS7 were clustered in the same clade and were closely related to the Mimulus guttatus FLC homologs (Fig. 5A). An investigation of the subcellular localization in N. benthamiana leaves indicated that MnMADS50 and MnMADS7 were mainly located in the nucleus. Meanwhile, MnMADS33 localized to the epicyte and organelles including guard cells, but primarily the nucleus (Fig. 5B). Analyses of protein structures revealed that MnMADS33 and MnMADS50 contained the classic MIKC motifs. Additionally, MnMADS7 comprised the MADS-box and I- domains, but the K- and C- domains were incomplete (Figs 2 and 5C). An amino acid sequence alignment confirmed that there are 31 amino acids before the MnMADS33 MADS-box domain. Moreover, we observed a 51.08% amino acid identity between MnMADS33 and MnMADS50. In contrast, A. thaliana FLC shared only 29.00% and 37.00% amino acid sequence identity with MnMADS33 and MnMADS50, respectively (Fig. 5C). Dual luciferase assays indicated that MnFLC-like proteins (i.e., MnMADS33, 50, and 7) could directly or indirectly suppress the promoter activity of MnFT and repressed the expression of the luciferase gene (Fig. 6A). According to the yeast two-hybrid assays, the interaction between MnMADS50 and MnMADS1 is strong. In addition, a very weak interaction between MnMADS33 and MnMADS1, and no interaction between MnMADS7 and MnMADS1 were detected (Fig. 6B). β-galactosidase activity assays were further conducted to confirm the true interactions between MnMADS33 and MnMADS50 with MnMADS1. The β-galactosidase activity for the interaction combinations pGBKT7-MnMADS1/pGADT7-MnMADS33, pGBKT7-MnMADS1/pGADT7-MnMADS50, pGADT7-MnMADS1/pGBKT7-MnMADS33, pGADT7-MnMADS1/pGBKT7-MnMADS50, pGADT7-T7/pGBKT7-p53, and pGADT7-T7/pGBKT7-lam were 17.51, 15.30, 15.06, 16.27, 15.68, and 1.00 units, respectively (Supplementary Fig. S3).
Figure 5.
Mulberry FLOWERING LOCUS C-like genes. (A) Phylogenetic relationship of FLC homologs from multi-species. Phylogenetic tree of FLC homologs is generated using PhyML (version 3.1) by maximum likelihood method with GTR + I + G model. The mulberry genes are marked in red. (B) Analyses of subcellular localization of MnMADS33, MnMADS50, and MnMADS7 in Nicotiana benthamiana leaves. Agrobacterium-infiltrated tobacco leaves, expressing the MnMADS33-GFP, MnMADS50-GFP, and MnMADS7-GFP fusion proteins driven by the CaMV 35S promoter, were used for obtaining images under green fluorescence, merged light, and visible light. 35S:00 represent the empty expression vector. Scale bar = 25 μm. (C) Comparison of A. thaliana FLC, MnMADS33, MnMADS50, and MnMADS7 amino acid sequences. The MADS-box, I, K, and C-terminal domains are labelled.
Figure 6.
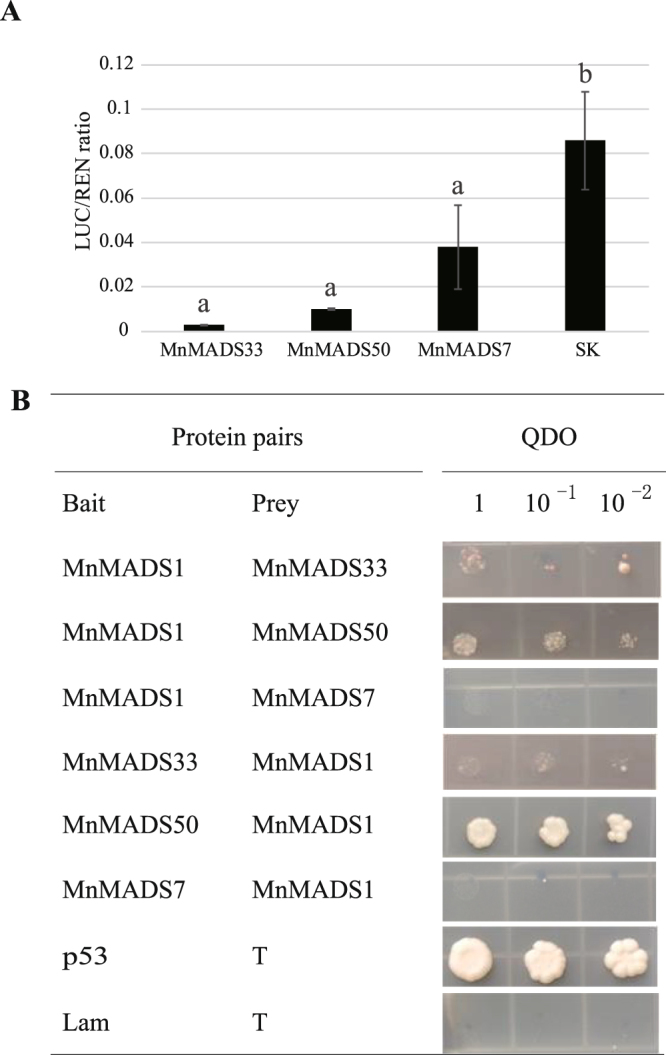
In vivo interaction of MnFLC-like proteins with MnFT promoter and MnMADS1 protein. (A) In vivo associations of MnFLC-like proteins and MnFT promoter obtained from transient assays in tobacco leaves using dual luciferase assay. SK represents empty vector. Error bars indicate SE from three biological replicates (P < 0.05). (B) Yeast co-transformation interaction assessment between MnFLC-like and SVP-like (MnMADS1). Yeasts were plated on quadruple dropout (QDO) medium after diluting 1, 10, and 100 times.
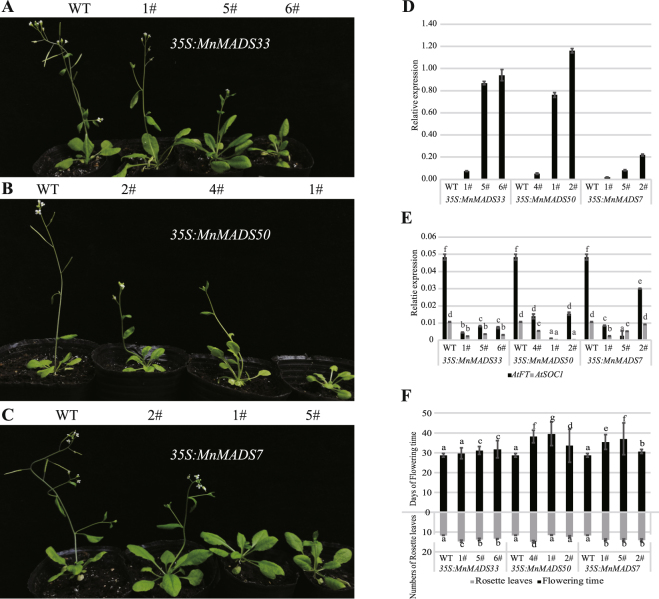
Ectopic expression of mulberry FLOWERING LOCUS C-like genes in Arabidopsis thaliana resulted in a late-bolting phenotype
To investigate the potential functions of MnMADS33, MnMADS50, and MnMADS7 genes regarding the flowering transition, transgenic A. thaliana plants expressing these genes under the control of the CaMV 35S promoter were examined. We selected three transgenic lines for each gene and analyzed their flowering phenotypes and gene expression levels (Fig. 7A–C). Gene-specific primers were used to conduct qRT-PCR analyses of WT and transgenic A. thaliana to validate the phenotypic assessment of the transgenic lines and the expression of flowering-related genes (Fig. 7D,E). The FT and SOC1 expression levels were lower in all transgenic lines than in the WT (Fig. 7E). Meanwhile, the transgenic lines exhibited varying degrees of delayed flowering and produced more rosette leaves than those of the the WT plants (Fig. 7F).
Figure 7.
Phenotypes of the wild-type and transgenic Arabidopsis thaliana plants overexpressing MnMADS33, MnMADS50, and MnMADS7. (A–C) Five-week-old wild and transgenic seedlings under long-day conditions (16/8 h of light/dark). (D) Expression levels of MnMADS33, MnMADS50, and MnMADS7 determined by qRT-PCR in wild-type and transgenic A. thaliana. (E) qRT-PCR analyses of AtSOC1 and AtFT in wild-type and transgenic A. thaliana. (F) The leaf numbers of rosette in wild and transgenic A. thaliana plants. For qRT-PCR analyses in (D,E), A. thaliana Actin7 is used as the internal control. Error bars represent the standard deviations of three independent experiments. Significant differences are indicated by different lower-case letters resulting from one-way Duncan’s test (P < 0.05).
Relationships between endodormancy and the FLOWERING LOCUS C- and FT-like genes in mulberry
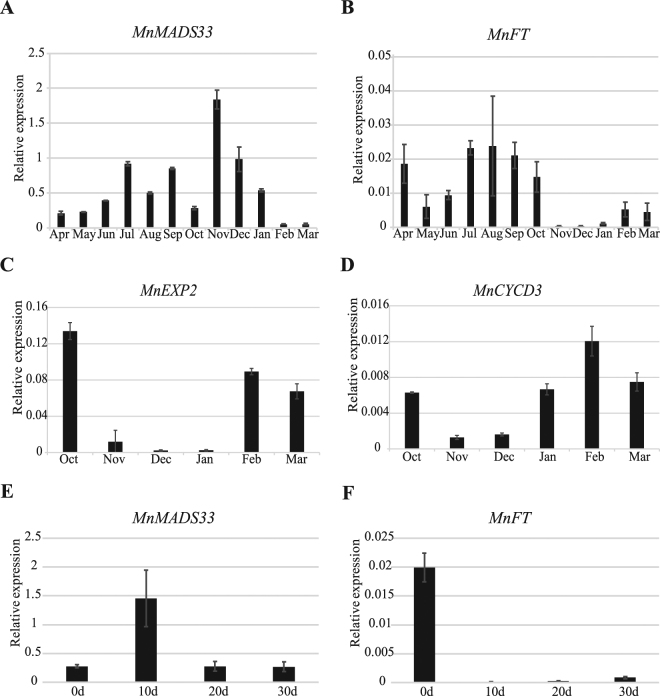
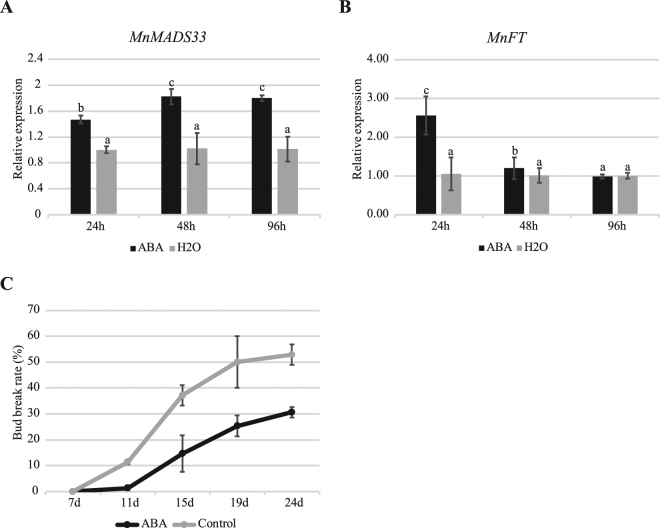
A qRT-PCR was conducted to clarify the relationship between the seasonal periodicity of floral bud development and the expression of MnFLC-like genes and MnFT-like gene (Fig. 8A,B, Supplementary Fig. S4). An expansion gene (MnEXP2; Morus011237) and a cyclin D-type gene (MnCYCD3; Morus027452) were used to provide evidence of the endodormancy cycle activities54. The MnEXP2 and MnCYCD3 expression levels were rapidly down-regulated in November and recovered during dormancy release (Fig. 8C,D). Meanwhile, the MnMADS33 and MnFT expression levels fluctuated before endodormancy, after which MnMADS33 expression was sharply up-regulated, peaking in November, and then down-regulated until dormancy release in the spring. The MnFT gene exhibited a contrasting expression pattern during the same period. The expression of MnMADS50 and MnMADS7 were relatively low during endodormancy (Supplementary Fig. S4). An analysis of floral buds of plants grown under artificially controlled cold conditions revealed that MnMADS33 expression was rapidly up-regulated after 10 days of chilling, while MnFT exhibited the opposite expression pattern (Fig. 8E,F). We also investigated the expression levels of genes related to ABA biosynthesis and catabolism [e.g., ABA 8-hydroxylase gene (MnCYP707A4; Morus027528), RING-H2-type zinc-finger gene (MnXERICO; Morus006867), and 9-cis-epoxycarotenoid dioxygenase gene (MnNCED1; Morus012507)] in mulberry floral buds exposed to low temperatures under field and artificially controlled conditions. The genes exhibited similar expression profiles under the two experimental conditions (Supplementary Fig. S5). The MnCYP707A4 and MnNCED1 expression levels were up-regulated after endodormancy, and then down-regulated before dormancy release. The expression of MnNCED1 coincided with that of MnMADS33. Furthermore, MnXERICO expression was down-regulated during endodormancy and up-regulated before dormancy release. Mulberry floral buds were treated with ABA to confirm the relationships between ABA and the expression of MnMADS33 and MnFT (Fig. 9). The ABA treatment up-regulated the expression of MnMADS33 and MnFT; however, while the increased MnMADS33 expression level was stable, the ABA-induced MnFT expression level gradually decreased (Fig. 9A,B). The expression of MnMADS50 and MnMADS7 was up-regulated at 24 h and then down-regulated at 48 h and 96 h after ABA treatment (Supplementary Fig. S6). Furthermore, bud break was repressed by ABA treatment (Fig. 9C).
Figure 8.
Expression of MnMADS33 and MnFT genes in floral buds. (A,B) Expression of MnMADS33 and MnFT in adult mulberry buds over one year. (C,D) Expression analyses of MnEXP2 and MnCYCD3 in the mulberry floral buds during endodormancy. (E,F) Expression of MnMADS33 and MnFT in mulberry floral buds under controlled cold conditions in 30 days. Gene expression is measured by qRT-PCR using the RPL15 as a reference (n = 3, mean ± measurement range).
Figure 9.
Abscisic acid treatment influences the expression of MnMADS33 and MnFT. (A,B) Expression profiles of MnMADS33 and MnFT after treatment for 24, 48, and 96 hours. (C) The break rate of dormant buds after treatment for 7, 11, 15, 19, and 24 days. Gene expression is measured by method mentioned in Fig. 8.
Discussion
Woody plants are characterized by a long growth cycle. Mulberry has a moderately long juvenile phase, and starts to flower 2–3 years after seeds are sown55. The extensive use of mulberry fruit in food and medicinal products compelled us to focus on mulberry flower development, which is partly influenced by the MADS-box gene family. Advances in whole-genome sequencing technology have facilitated the comprehensive analysis of this particular gene family. In the present study, we used systematic bioinformatics analyses and molecular methods to investigate the role of the mulberry MADS-box gene family in terms of floral transition and flower bud development.
Mulberry MADS-box family genes are conserved in structures and functions during evolution
We identified and characterized 54 MnMADS genes in mulberry (Table S1). The MnMADS gene structures and encoded protein domains were highly similar to those of the corresponding genes from other species2,3,56–61. The ABCDE model for annual plants is the most well-known floral development model. We detected AP1, AP3, PI, AG, STK, and SEP homologs among the identified 54 MnMADS genes (Figs 1 and 2, Supplementary Fig. S2). In most cases, homologs of the ABCDE model genes produced similar expression profiles (Figs 3 and 4). For example, four SEP-like genes in the E class were expressed in a floral-related-organ-biased manner. This result was consistent with the SEP function in A. thaliana floral organ development10,13. The A. thaliana MADS-box gene family is regulated by a complex network and is crucial for floral transitions. We detected the A-, B-, and E- class genes in the differentiating inflorescence primordia, suggesting a functional conservation among floral signal integration pathways.
Conserved evolution of mulberry type I and type II MADS-box genes
Despite the lack of an assembled genome, 10 clusters containing 22 genes were detected based on their locations on the scaffolds (Table S1). A previous study involving synteny network analyses indicated that AGL6-SOC1 and SEP-AP1 tandems are conserved across all angiosperms62. In the present study, we observed one AGL6-SOC1 and three SEP-AP1 tandems. Moreover, we detected three tandem duplication clusters, including eight Mα genes, while no duplication events were observed in the Mβ and Mγ subfamilies. This may explain why there are more Mα genes than Mβ and Mγ genes in mulberry. An analysis of the evolution of A. thaliana and Oryza sativa MADS-box genes suggested that type I genes underwent a faster birth-and-death evolution than type II genes, in part because of a higher frequency of segmental gene duplication and weaker purifying selection in type I than in type II genes63. Generally, Ka/Ks = 1 indicates a neutral selection, Ka/Ks <1 corresponds to a purifying selection, and Ka/Ks >1 suggests an accelerated evolution with positive selection64. In our study, the Ka/Ks ratios of three whole genome/segmental duplication paralogs from type II were <0.1, consistent with a strong purifying selection pressure. The paralogous pairs of the type I Mα subfamily genes had a higher Ka/Ks ratio, including two pairs (MnMADS26/MnMADS27 and MnMADS2/MnMADS3) with Ka/Ks ratios >1, suggesting the Mα genes evolved under a positive selection pressure. There were three pairs of MIKCc genes distributed in a tandem duplication pattern. According to the duplication types of the paralogous pairs (Table S2), the expansion of the type I gene group was due to tandem duplications, whereas segmental duplications played a leading role in the expansion of the type II gene group. The expansion patterns of the mulberry type I and type II MADS-box genes are similar to those reported for A. thaliana3, O. sativa65, and P. mume58.
Identification of mulberry FLOWERING LOCUS C-like genes
A previous study concluded that FLC functions as a flowering repressor, which delays flowering by inhibiting the expression of the activators of flowering such as FT and SOC137. A recent transcriptome study in mulberry determined that MnMADS33, MnMADS50, and MnMADS7 (i.e., c75631_g1, c78979_g1, and c79593_g1, respectively) are AGL6 homologs based on gene annotations50. In the present study, MnMADS33, MnMADS50, and MnMADS7 were identified as FLC-like genes according to genome-wide identification and re-analysis of the phylogenetic relationships among MnMADS genes (Table S1). Our data was further verified by completing a multi-species phylogenetic analysis (Fig. 5). The mulberry FT promoter has been cloned66. Four CArG-box like elements were identified in the MnFT promoter (Supplementary File 1). MnFLC-like proteins can directly or indirectly suppress the MnFT promoter activity and suppressed the expression of the luciferase gene in the dual luciferase assay (Fig. 6A). Moreover, the interactions of MnMADS33/MnMADS1 and MnMADS50/MnMADS1 were detected. All these results demonstrated that MnMADS33, MnMADS50, and MnMADS7 might be FLC-like genes. Although, ectopically expressed MnMADS33, MnMADS50, or MnMADS7 in A. thaliana slightly delayed flowering, the heterologous system results in a weaker phenotype than expected potentially because the binding affinity to the interacting partner or target DNA might be weaker than the original one.
MnMADS33 may down-regulate the expression of mulberry FLOWERING LOCUS T in dormant buds to inhibit dormancy release
In A. thaliana, FT is expressed in leaves and the resulting protein migrates to the shoot apex where it induces the formation of floral primordial67. In P. persica, DAM5 and DAM6 expression levels are up-regulated during dormancy development and down-regulated during the winter chilling period68. A recent study suggested that DAM5 and DAM6 are key regulators of dormancy35. Similarly, we observed that MnMADS33 expression in mulberry floral buds is dramatically up-regulated after endodormancy and then down-regulated, while MnFT exhibits the opposite expression pattern (Fig. 8), suggesting that MnMADS33 affects dormancy and negatively regulates the expression of MnFT during the dormancy period.
For plants in the indirect flowering group, such as mulberry, the formation and development of the inflorescence primordia occur in the buds in summer and then cease growth during endodormancy until next bud burst in spring. Additionally, dormant mulberry buds contain floral and leaf primordia50. For the relatively high MnMADS33 and MnMADS50 expression levels in differentiating inflorescence primordial, we propose that the encoded proteins help to maintain the inflorescence meristems in buds and inhibit bud burst and flowering by repressing the localized expression of MnFT. Before endodormancy, the MnFT and MnMADS33 expression levels fluctuate in floral buds, and the balance between these levels likely prevents the bursting of the floral buds. MnMADS50, MnMADS7, and MnMADS33 are three FLC-like genes. However, the low expression level in floral buds during endodormancy gathered with the down-regulation by ABA indicated that MnMADS50 and MnMADS7 had little relationship to endodormancy.
A recent study suggested that P. pyrifolia PpDAM1 up-regulates PpNCED3 expression and forms a feedback regulatory loop with ABA metabolism and signaling pathways to control endodormancy69. In A. thaliana, ABSCISIC ACID-INSENSITIVE 4 (ABI4), which is a key component of the ABA signaling pathway, directly promotes FLC transcription and negatively regulates the floral transition70. In our study, MnNCED1 expression was up-regulated which is consistent with the hypothesis that high MnMADS33 expression level during the induction of endodormancy up-regulates ABA signaling and promotes endodormancy induction (Supplementary Fig. S4). Moreover, an ABA treatment up-regulated MnMADS33 and MnFT expression, although the MnFT expression level was subsequently down-regulated at 48 and 96 h after treatment (Fig. 9). Our results support the hypothesis that dormancy and flowering share overlapping pathways46. We also provide evidence supporting the hypothesis that FLC-like genes affect dormancy by directly or indirectly down-regulating the expression of FT-like genes. However, whether MnMADS33 and MnFT function in the same regulatory pathway or act independently during dormancy remains to be determined.
Conclusions
In summary, 54 putative MADS-box family members were identified in the mulberry genome. We investigated the MnMADS phylogenetic relationships, gene structures, and encoded protein domains. A comparison of the expression patterns of paralogous pairs provided insights into the functional conservation of MADS-box proteins from mulberry and other species. Mulberry FLC-like proteins (i.e., MnMADS33, MnMADS50, and MnMADS7) were mainly localized to the nucleus, although MnMADS33 was also detected in the epicyte and organelles. MnFLC-like proteins directly or indirectly suppressed the promoter activity of the mulberry MnFT gene in vivo. Phenotypic analyses of WT and MnFLC-like-overexpressing A. thaliana plants indicated that the transgenic plants exhibited delayed flowering and down-regulated FT and SOC1 expression levels. Additionally, we propose that MnMADS33 might interact with MnFT to regulate dormancy and flowering. The data presented herein enhance our understanding of the roles of MnMADS genes related to the regulation of flowering.
Methods
Plant material
Mulberry JQ63 was used for gene expression analysis and vector construction. Mulberry materials were obtained from the Southwestern University mulberry germplasm for field sampling. Floral buds were collected before, during, and after the differentiation of inflorescence primordia (termed bud I, bud II, and bud III) in April 2016. Paraffin sections were prepared to ensure correct stages (Supplementary Fig. S7)71. Floral buds during endodormancy (November and December 2015) (as bud IV and bud V) and dormancy releasing stages (January and February 2016) (termed bud VI and bud VII) were gathered. Mulberry catkins were collected in three development stages (before, during, and after pollinated stages; termed catkin I, catkin II, and catkin III) in May 2016. Mulberry cuttings for chilling and abscisic acid (ABA) treatment were obtained on September 28, 2016 and November 4, 2016, respectively. For chilling treatment, cuttings were exposed in 4 °C and were sampled once per 10 days for total RNA extraction. For ABA treatment, cuttings were immersed in vases with 10 μM ABA (Sangon Biotech, Shanghai, China) solution in a growth chamber at 24 °C under a 14/10 h light/dark regime. After incubation for 96 h, cuttings were transferred to tap water. The control was treated with water all the time.
Identification of mulberry MADS-box genes
We searched the M. notabilis genome database (http://morus.swu.edu.cn/morusdb/) to identify MADS-box genes. Information and sequences of A. thaliana MADS-box proteins were downloaded from The Arabidopsis Information Resource at website (https://www.arabidopsis.org/). To identify the maximum number of MADS-box domain-containing sequences, the mulberry peptide database was searched using two methods. First, all 107 A. thaliana MADS-box proteins were used in a BLASTP search (e-value of 1e−10)72. Second, the hidden Markov model (HMM) profile of the MADS-box domain (accession no. PF00319) was downloaded from the Pfam database (http://pfam.xfam.org/)73. The HMMER (version v3.1b2)74 was used to search MADS-box genes in the mulberry database using the HMM profile. All sequences obtained using above two methods were used as queries to BLAST the de novo transcriptome assembly data50. The truncated sequences were manually corrected based on the de novo transcriptome assembly data. We used the GENSCAN web server (http://genes.mit.edu/GENSCAN.html)75 to analyze the nucleotide sequences. The gene sequences were uploaded to the website to predict the coding regions, with A. thaliana as the reference organism. Furthermore, the protein sequences were analyzed on the SMART website (http://smart.embl-heidelberg.de/)76 for domain prediction. Proteins with MADS domains were considered candidate MnMADS proteins.
Analyses of gene and protein properties
Schematic diagrams representing MADS-box gene structures (e.g., introns and exons) were generated using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn)77. We calculated coding sequence lengths and determined the number of amino acids, molecular weights, and isoelectric points of the encoded proteins using the Compute pI/Mw tool on the ExPASy website (http://web.expasy.org/compute_pi/)78. The MCScanX program79 was used to classify duplication types of MnMADS genes based on genome sequences encoding proteins and alignments with default parameters. The ratio of the number of nonsynonymous substitutions per non-synonymous site to the number of synonymous substitutions per synonymous site (Ka/Ks ratio) of each duplicated gene pair was calculated using DnaSP (version 5.10.01)80 on the full-length coding sequences. The STRING program (version 10.0)81 was used to construct possible protein–protein interaction networks based on experimental evidence with the highest confidence values (i.e., >0.900) in A. thaliana MADS-box networks. Furthermore, homologous genes encoding these interacting proteins were identified according to phylogenetic analyses. Their expression patterns in the differentiating inflorescence primordia stage were investigated by qRT-PCR.
Phylogenetic reconstruction
Full-length protein sequences of all A. thaliana, grape, and mulberry MADS-box proteins were used to analyze their phylogenetic relationships. The amino acid sequences were aligned with ClustalW and an unrooted neighbor-joining (NJ) phylogenetic tree was constructed using a bootstrapping method (1,000 replicates) in MEGA 7.082 with p-distance and complete deletion parameters. All MnMADS protein sequences were used to build maximum likelihood (ML) tree to ensure the reliability of the constructed tree. The unalignable regions at N and C terminals were manually trimmed to decrease phylogenetic noise. ProtTest 383 was used to determine the best-fit model of amino acid sequence evolution, which took the JTT + I + G model under the Akaike information criterion. We used PhyML (version 3.1)84 to constructed ML tree with 100 bootstrap replicates. The sequence alignment analysis of A. thaliana FLC, MnMADS33, MnMADS50, and MnMADS7 was performed using the pairwise alignment program within the GeneDoc software85. Phylogenetic relationship of FLC homologs (Table S5) was generated by ML method using GTR + I + G model. The gene structures, protein structures, and all the phylogenetic trees were visualized using EvolView software on the website (http://www.evolgenius.info/evolview/)86.
Gene expression analyses
We used reads per kilobase of exon per million mapped reads (RPKM) to compare the gene expression levels among samples (Table S3). Values for the roots, bark, winter floral buds, male flowers, and leaves of MnMADSs were obtained from RNA sequencing data (http://morus.swu.edu.cn/morusdb/), which were from single samples. A heatmap was generated based on the log2-transformed (RPKM+1) values using R project software. The expression patterns of MnMADSs during flowering were evaluated using qRT-PCR. Total RNA was extracted from the collected samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Contaminating genomic DNA in the total RNA was removed using RQ1 RNase-free DNase (Promega, Madison, WI, USA). RNA (1 μg) was used for cDNA synthesis with Moloney Murine Leukemia Virus (M-MLV) Reverse Transcriptase (Promega, Madison, WI, USA). Fourfold-diluted cDNA was used for qRT-PCR. The primers for qRT-PCR were designed with Primer Premier 5 (Table S6) and then checked by melting curves. The qRT-PCR was conducted using SYBR Premix Ex Taq II (Takara, Dalian, PR China) and the StepOnePlus Real Time PCR system (Applied Biosystems, Foster City, CA, USA), initiated by 30s at 95 °C and followed by 40 cycles of 95 °C for 5s, 60 °C for 30s, and completed with a melting-curve analysis program. The PCR mixture (20 μl total volume) comprised 10 μl of 2 × SYBR® Premix Ex Taq, 0.4 μl of each primer (10 μM), 0.4 μl of 50 × ROX Reference Dye II, 2 μl of diluted cDNA and 6.8 μl PCR-grade H2O. The mulberry RPL15 gene (Morus024083) was used as an internal control gene. The relative expression of genes for each sample was calculated using the formula 2− [Ct (target gene) − Ct (control gene)]. The log2-transformed (the relative fold changes+1) values of the 39 MnMADSs were visualized in heat maps prepared with R project software. The expression data of 39 MnMADSs were from duplicates of two biological replicates. Three biological replicates were performed for gene expression analyses in anniversary monthly floral buds, chilling-treated floral buds, and ABA-treated floral buds. The qRT-PCR analyses were performed three times for each biological replicate.
Cellular localization
The open reading frames (ORFs) of MnMADS33, MnMADS50, and MnMADS7 without stop codons were obtained via PCR amplification using gene-specific primers (Table S7). The purified PCR products were ligated into the pLGNL 35S-GFP vector after digestion with Kpn I and BamH I enzymes. Linker sequences comprising four glycine and one serine residue with three repetitions (GGAGGAGGAGGATCAGGAGGAGGAGGATCAGGAGGAGGAGGATCA) were added between target genes and GFP. The fusion vectors were introduced into Agrobacterium tumefaciens strain GV3101 (pMP90)87. The A. tumefaciens cells, containing the pLGNL 35S-GFP, pLGNL 35S-MnMADS33-GFP, pLGNL 35S-MnMADS50-GFP, or pLGNL 35S-MnMADS7-GFP vectors, were grown to OD600 = 1.0 in LB liquid medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) containing 35 μg/mL rifampicin, 50 μg/mL kanamycin, and 30 ng/mL acetosyringone. Cells were collected by centrifugation at 5,000 g at room temperature for 10 min and placed for 3–5 hours at 25 °C after resuspending to OD600 = 0.4 using infiltration medium (2 g/L 2-morpholinoethanesulfonic acid, 1 g/L Magnesium chloride, and 30 ng/mL acetosyringone, pH 5.8). Infiltration was carried out by injecting N. benthamiana epidermal cells. The N. benthamiana samples were kept in darkness for 12 hours and then grown in a chamber at 24 °C under a 16/8 h light/dark regime for 2 days. The subcellular localization results were scanned by FV1200 laser scanning confocal microscope (Olympus, Tokyo, Japan).
Dual luciferase assay
The promoter of the MnFT gene was amplified with the primers described in Table S7. Full-length MnMADS33/50/7 sequences were inserted into the pGreenII 62-SK vector (SK). The promoter of MnFT was inserted into the pGreenII 0800-LUC vector88. N. benthamiana leaves were used in dual luciferase assays. The Agrobacterium-mediated method was performed as mentioned in cellular localization. The firefly luciferase (LUC) and Renilla luciferase (REN) were assayed using dual luciferase assay reagents (Promega, Madison, WI, USA) after infiltration for 3 days. Three biological replicates were performed (three technical replicates in each independent experiments).
Yeast two-hybrid and β-galactosidase activity assays
pGBKT7 (bait) and pGADT7 (prey) vectors were used for a yeast two-hybrid assay. Primers used for vector construction are listed in Table S7. The autoactivation and toxicity of both the bait and prey were tested. Positive controls were pGADT7-T and pGBKT7-53, and negative controls were pGBKT7-Lam and pGADT7-T. For each co-transformation pair, 200 ng of bait recombinant plasmid and 100 ng of prey recombinant plasmid were transformed into Saccharomyces cerevisiae Y2HGold, and then the cells were grown at 30 °C on synthetic medium lacking both leucine (Leu) and tryptophan (Trp) (Double Dropout, DDO). The positive clones were grown on synthetic medium without Trp, Leu, histidine (His), and adenine (Ade) (Quadruple Dropout, QDO) after diluting 1, 10, and 100 times. Three replications were conducted for each pair of proteins. The yeast strain AH109 was used for β-galactosidase activity analysis using Yeast β-galactosidase Assay Kit (Thermo Fisher Scientific, Hudson, NH, USA) following the manufacturer’s protocol. Yeast transformation and culture methods were the same as mentioned above. Three biological replicates were performed for each combination.
Generation of transgenic Arabidopsis thaliana plants and expression analyses of downstream target genes
The full-length cDNA of MnMADS33, MnMADS50, and MnMADS7 were amplified by PCR and were inserted into the Kpn I/BamH I cloning sites of a binary vector driven by the cauliflower mosaic virus (CaMV) 35S promoter and followed at the 3′ end by the nopaline synthase gene (NOS) terminator (pLGNL 35s-NOS). The primers used for plasmid construction are listed in Table S7. Overexpression of MnMADS33, MnMADS50, and MnMADS7 in A. thaliana was carried out in wild-type (WT) ecotype Columbia (Col-0) by A. tumefaciens-mediated plant transformation, which was performed by the floral dipping method89. Transformed seedlings were obtained from selective medium containing 50 mg/L kanamycin, and then transferred to soil. Homozygous lines of transgenic A. thaliana plants from T3 generation were used for further analyses. Plants were grown in chamber at 24 °C under a 16/8 h light/dark regime. Flowering time and the number of rosette leaves were recorded when the primary inflorescence was 0.5 cm long90. To understand the function of mulberry FLC-like genes in transgenic A. thaliana, the expressions of the flowering time genes, FT and SOC1, were investigated using qRT-PCR analyses. A. thaliana Actin7 (At5g09810) was used as the internal control to normalize the expression levels. Conditions for total RNA isolation, reverse transcription, and qRT-PCR are the same as mentioned above. Primers used for expression analyses of downstream target genes in transgenic plants were listed in Table S6.
Statistical analysis
Statistical analyses were performed by the SPSS (version 17.0). Three independent results were presented as mean values ± SD. Differences between samples were analyzed using one-way analysis of variance (ANOVA). Comparisons among means were made by Duncan’s test calculated at P < 0.05.
Electronic supplementary material
Acknowledgements
This project was funded by the research grants from the National Hi-Tech Research and Development Program of China (No. 2013AA100605-3), Natural Science Foundation of China (No. 31572323), China Postdoctoral Science Foundation funded projects (No. 2013M540694, No. 2014T70845 and No. 2016M592622), and the “111” Project (B12006).
Author Contributions
Y.L., Z.X. and N.H. conceived and designed the experiments. Y.L. and H.L. performed the experiments. Y.L. and N.H. analyzed data. Y.L. and N.H. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23985-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Becker A, Theissen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 2003;29:464–489. doi: 10.1016/S1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann K, Melzer R, Theissen G. MIKC-type MADS-domain proteins: structural modularity, protein interactions and network evolution in land plants. Gene. 2005;347:183–198. doi: 10.1016/j.gene.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Parenicova L, et al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell. 2003;15:1538–1551. doi: 10.1105/tpc.011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riechmann JL, Wang M, Meyerowitz EM. DNA-binding properties of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA and AGAMOUS. Nucleic Acids Res. 1996;24:3134–3141. doi: 10.1093/nar/24.16.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jetha K, Theissen G, Melzer R. Arabidopsis SEPALLATA proteins differ in cooperative DNA-binding during the formation of floral quartet-like complexes. Nucleic Acids Res. 2014;42:10927–10942. doi: 10.1093/nar/gku755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, et al. A MADS-box gene NtSVP regulates pedicel elongation by directly suppressing a KNAT1-like KNOX gene NtBPL in tobacco (Nicotiana tabacum L.) J. Exp. Bot. 2015;66:6233–6244. doi: 10.1093/jxb/erv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henschel K, et al. Two ancient classes of MIKC-type MADS-box genes are present in the moss Physcomitrella patens. Mol. Biol. Evol. 2002;19:801–814. doi: 10.1093/oxfordjournals.molbev.a004137. [DOI] [PubMed] [Google Scholar]
- 8.Pnueli L, et al. The MADS box gene family in tomato: temporal expression during floral development, conserved secondary structures and homology with homeotic genes from Antirrhinum and Arabidopsis. Plant J. 1991;1:255–266. doi: 10.1111/j.1365-313X.1991.00255.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Fanning L, Jack T. The K domain mediates heterodimerization of the Arabidopsis floral organ identity proteins, APETALA3 and PISTILLATA. Plant J. 2003;33:47–59. doi: 10.1046/j.0960-7412.2003.01473.x. [DOI] [PubMed] [Google Scholar]
- 10.Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MFB. and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- 11.Theissen G, Kim JT, Saedler H. Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J. Mol. Evol. 1996;43:484–516. doi: 10.1007/BF02337521. [DOI] [PubMed] [Google Scholar]
- 12.Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 13.Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr. Biol. 2004;14:1935–1940. doi: 10.1016/j.cub.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, et al. Expression of floral MADS-box genes in basal angiosperms: implications for the evolution of floral regulators. Plant J. 2005;43:724–744. doi: 10.1111/j.1365-313X.2005.02487.x. [DOI] [PubMed] [Google Scholar]
- 15.Rijpkema AS, Vandenbussche M, Koes R, Heijmans K, Gerats T. Variations on a theme: changes in the floral ABCs in angiosperms. Semin. Cell Dev. Biol. 2010;21:100–107. doi: 10.1016/j.semcdb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Heck GR, Perry SE, Nichols KW, Fernandez DE. AGL15, a MADS domain protein expressed in developing embryos. Plant Cell. 1995;7:1271–1282. doi: 10.1105/tpc.7.8.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo E, et al. Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator. FLC. Plant Cell. 2009;21:3185–3197. doi: 10.1105/tpc.108.063883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamczyk BJ, Fernandez DE. MIKC* MADS domain heterodimers are required for pollen maturation and tube growth in. Arabidopsis. Plant Physiol. 2009;149:1713–1723. doi: 10.1104/pp.109.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang GC, Barua D, Kramer EM, Amasino RM, Donohue K. Major flowering time gene, flowering locus C, regulates seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2009;106:11661–11666. doi: 10.1073/pnas.0901367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smaczniak C, Immink RG, Angenent GC, Kaufmann K. Developmental and evolutionary diversity of plant MADS-domain factors: insights from recent studies. Development. 2012;139:3081–3098. doi: 10.1242/dev.074674. [DOI] [PubMed] [Google Scholar]
- 21.Cooke JEK, Eriksson ME, Junttila O. The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant Cell Environ. 2012;35:1707–1728. doi: 10.1111/j.1365-3040.2012.02552.x. [DOI] [PubMed] [Google Scholar]
- 22.Fadón, E., Herrero, M. & Rodrigo, J. In Advances in Plant Dormancy (ed. Anderson, J. V.) 123–136 (Springer, 2015).
- 23.Hartmann U, et al. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J. 2000;21:351–360. doi: 10.1046/j.1365-313x.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- 24.Bielenberg DG, et al. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet. Genome. 2008;4:495–507. doi: 10.1007/s11295-007-0126-9. [DOI] [Google Scholar]
- 25.Horvath DP, Chao WS, Suttle JC, Thimmapuram J, Anderson JV. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.) BMC genomics. 2008;9:536. doi: 10.1186/1471-2164-9-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki R, et al. Functional and expressional analyses of PmDAM genes associated with endodormancy in Japanese apricot. Plant Physiol. 2011;157:485–497. doi: 10.1104/pp.111.181982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito T, et al. Expression and genomic structure of the dormancy-associated MADS box genes MADS13 in Japanese pears (Pyrus pyrifolia Nakai) that differ in their chilling requirement for endodormancy release. Tree Physiol. 2013;33:654–667. doi: 10.1093/treephys/tpt037. [DOI] [PubMed] [Google Scholar]
- 28.Yamane H, et al. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. J. Exp. Bot. 2011;62:3481–3488. doi: 10.1093/jxb/err028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kardailsky I, et al. Activation tagging of the floral inducer FT. Science. 1962;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed R, et al. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 2010;62:674–688. doi: 10.1111/j.1365-313X.2010.04185.x. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Yuceer C. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc. Natl. Acad. Sci. USA. 2011;108:10756. doi: 10.1073/pnas.1104713108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan C, Dardick C, Callahan A, Scorza R. Plum (Prunus domestica) trees transformed with poplar FT1 result in altered architecture, dormancy requirement, and continuous flowering. PLoS ONE. 2012;7:e40715. doi: 10.1371/journal.pone.0040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao X, Chao W, Yang Y, Horvath D. Coordinated expression of FLOWERING LOCUS T and DORMANCY ASSOCIATED MADS-BOX-like genes in leafy spurge. PLoS ONE. 2015;10:e0126030. doi: 10.1371/journal.pone.0126030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su-Li, W. U. et al. Expression vector construction of mulberry FLOWERING LOCUS T gene and early flowering in transgenic Arabidopsis thaliana. Science of Sericulture (2014).
- 35.Liu, Z., Zhu, H. & Abbott, A. In Advances in Plant Dormancy (ed. Anderson, J. V.) 75–105 (Springer, 2015).
- 36.Bastow R, et al. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- 37.Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006;46:183–192. doi: 10.1111/j.1365-313X.2006.02686.x. [DOI] [PubMed] [Google Scholar]
- 38.Li D, et al. A repressor complex governs the integration of flowering signals in. Arabidopsis. Dev. Cell. 2008;15:110–120. doi: 10.1016/j.devcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Ratcliffe OJ, Kumimoto RW, Wong BJ, Riechmann JL. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell. 2003;15:1159–1169. doi: 10.1105/tpc.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doğramacı, M., Horvath, D. P. & Anderson, J. V. In Advances in Plant Dormancy (ed. Anderson, J. V.) 197–219 (Springer, 2015).
- 41.Kumar G, et al. Comparative phylogenetic analysis and transcriptional profiling of MADS-box gene family identified DAM and FLC-like genes in apple (Malusx domestica) Sci. Rep. 2016;6:20695. doi: 10.1038/srep20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang JZ, Li ZM, Mei L, Yao JL, Hu CG. PtFLC homolog from trifoliate orange (Poncirus trifoliata) is regulated by alternative splicing and experiences seasonal fluctuation in expression level. Planta. 2009;229:847–859. doi: 10.1007/s00425-008-0885-z. [DOI] [PubMed] [Google Scholar]
- 43.Chouard P. Vernalization and its relations to dormancy. Annu. Rev. Plant Physiol. 1960;11:191–238. doi: 10.1146/annurev.pp.11.060160.001203. [DOI] [Google Scholar]
- 44.Horvath DP, Anderson JV, Chao WS, Foley ME. Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci. 2003;8:534–540. doi: 10.1016/j.tplants.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Horvath D. Common mechanisms regulate flowering and dormancy. Plant Sci. 2009;177:523–531. doi: 10.1016/j.plantsci.2009.09.002. [DOI] [Google Scholar]
- 46.Brunner AM, Evans LM, Hsu CY, Sheng X. Vernalization and the chilling requirement to exit bud dormancy: shared or separate regulation? Front. Plant Sci. 2014;5:732. doi: 10.3389/fpls.2014.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang HP, Ou TT, Wang CJ. Mulberry (sang shèn zǐ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement. Med. 2013;3:7–15. doi: 10.4103/2225-4110.106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim HG, et al. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson’s disease models. Brit. J. Nutr. 2010;104:8–16. doi: 10.1017/S0007114510000218. [DOI] [PubMed] [Google Scholar]
- 49.Grainger J. Studies upon the time of flowering of plants: anatomical, floristic and phenological aspects op the problem. Ann. Appl. Biol. 2010;26:684–704. doi: 10.1111/j.1744-7348.1939.tb06994.x. [DOI] [Google Scholar]
- 50.Shang JZ, Liang JB, Xiang ZH, He NJ. Anatomical and transcriptional dynamics of early floral development of mulberry (Morus alba) Tree Genet. Genome. 2017;13:40. doi: 10.1007/s11295-017-1122-3. [DOI] [Google Scholar]
- 51.He N, et al. Draft genome sequence of the mulberry tree Morus notabilis. Nat. Commun. 2013;4:2445. doi: 10.1038/ncomms3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin SI, et al. Differential regulation of FLOWERING LOCUS C expression by vernalization in cabbage and Arabidopsis. Plant Physiol. 2005;137:1037–1048. doi: 10.1104/pp.104.058974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruelens P, et al. FLOWERING LOCUS C in monocots and the tandem origin of angiosperm-specific MADS-box genes. Nat. Commun. 2013;4:2280. doi: 10.1038/ncomms3280. [DOI] [PubMed] [Google Scholar]
- 54.Saito T, et al. Development of flower buds in the Japanese pear (Pyrus pyrifolia) from late autumn to early spring. Tree physiol. 2015;35:653–662. doi: 10.1093/treephys/tpv043. [DOI] [PubMed] [Google Scholar]
- 55.Venkateswarlu M, et al. A first genetic linkage map of mulberry (Morus spp.) using RAPD, ISSR, and SSR markers and pseudotestcross mapping strategy. Tree Genet. Genome. 2006;3:15–24. doi: 10.1007/s11295-006-0048-y. [DOI] [Google Scholar]
- 56.Hou XJ, Liu SR, Khan MRG, Hu CG, Zhang JZ. Genome-wide identification, classification, expression profiling, and SSR marker development of the MADS-box gene family in citrus. Plant Mol. Biol. Rep. 2014;32:28–41. doi: 10.1007/s11105-013-0597-9. [DOI] [Google Scholar]
- 57.Wei B, et al. Genome-wide analysis of the MADS-box gene family in Brachypodium distachyon. PLoS ONE. 2014;9:e84781. doi: 10.1371/journal.pone.0084781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Z, et al. Genome-wide identification, characterisation and expression analysis of the MADS-box gene family in Prunus mume. Mol. Genet. Genomics. 2014;289:903–920. doi: 10.1007/s00438-014-0863-z. [DOI] [PubMed] [Google Scholar]
- 59.Tian Y, et al. Genome-wide identification and analysis of the MADS-box gene family in apple. Gene. 2015;555:277–290. doi: 10.1016/j.gene.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Wei X, et al. Genome-wide identification and analysis of the MADS-box gene family in sesame. Gene. 2015;569:66–76. doi: 10.1016/j.gene.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Ma J, et al. Genome-wide identification and analysis of the MADS-box gene family in bread wheat (Triticum aestivum L.) PLoS ONE. 2017;12:e0181443. doi: 10.1371/journal.pone.0181443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao T, et al. Phylogenomic synteny network analysis of MADS-box transcription factor genes reveals lineage-specific transpositions, ancient tandem duplications, and deep positional conservation. Plant Cell. 2017;29:1278–1292. doi: 10.1105/tpc.17.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nam J, et al. Type I MADS-box genes have experienced faster birth-and-death evolution than type II MADS-box genes in angiosperms. Proc. Natl. Acad. Sci. USA. 2004;101:1910–1915. doi: 10.1073/pnas.0308430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akhunov ED, et al. Comparative analysis of syntenic genes in grass genomes reveals accelerated rates of gene structure and coding sequence evolution in polyploid wheat. Plant Physiol. 2013;161:252–265. doi: 10.1104/pp.112.205161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arora R, et al. MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC genomics. 2007;8:242. doi: 10.1186/1471-2164-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao, L. et al. Cloning and functional site analysis of the promoter of mulberry flowering locus T gene. Science of Sericulture (2016).
- 67.Corbesier L, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 68.Li Z, Reighard GL, Abbott AG, Bielenberg DG. Dormancy-associated MADS genes from the EVG locus of peach [Prunus persica (L.) Batsch] have distinct seasonal and photoperiodic expression patterns. J. Exp. Bot. 2009;60:3521–3530. doi: 10.1093/jxb/erp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuan, P. A., Bai, S., Saito, T., Ito, A. & Moriguchi, T. Dormancy-associated MADS-box (DAM) and abscisic acid pathway regulate pear endodormancy through a feedback mechanism. Plant Cell Physiol., 10.1093/pcp/pcx074 (2017). [DOI] [PubMed]
- 70.Shu K, et al. ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis FLOWERING LOCUS C transcription. J. Exp. Bot. 2016;67:195–205. doi: 10.1093/jxb/erv459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye M, et al. Study of seed hair growth in Populus tomentosa, an important character of female floral bud development. BMC genomics. 2014;15:475. doi: 10.1186/1471-2164-15-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Finn RD, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eddy SR. Accelerated profile HMM searches. PLoS Comput. Biol. 2011;7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 76.Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:D302–305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo AY, Zhu QH, Chen X, Luo JC. GSDS: a gene structure display server. Yi chuan. 2007;29:1023–1026. doi: 10.1360/yc-007-1023. [DOI] [PubMed] [Google Scholar]
- 78.Gasteiger, E. et al. In The Proteomics Protocols Handbook (ed. Walker, J. D.) 571–607 (Humana Press, 2005).
- 79.Wang Y, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- 81.Szklarczyk D, et al. STRINGv10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 85.Nicholas, K. & Nicholas, H. GeneDoc: a tool for editing and annotating multiple sequence alignments. distributed by the author. 14 (1997).
- 86.He Z, et al. Evolviewv2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016;44:W236–241. doi: 10.1093/nar/gkw370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koncz C, Schell J. The promoter of TL -DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 1986;204:383–396. doi: 10.1007/BF00331014. [DOI] [Google Scholar]
- 88.Hellens RP, et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 90.Voogd C, Wang T, Varkonyi-Gasic E. Functional and expression analyses of kiwifruit SOC1-like genes suggest that they may not have a role in the transition to flowering but may affect the duration of dormancy. J. Exp. Bot. 2015;66:4699–4710. doi: 10.1093/jxb/erv234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.