Abstract
Key message
GmDW1 encodes an ent-kaurene synthase (KS) acting at the early step of the biosynthesis pathway for gibberellins (GAs) and regulates the development of plant height in soybean.
Abstract
Plant height is an important component of plant architecture, and significantly affects crop breeding practices and yield. Here, we report the characterization of an EMS-induced dwarf mutant (dw) of the soybean cultivar Zhongpin 661 (ZDD23893). The dw mutant displayed reduced plant height and shortened internodes, both of which were mainly attributed to the longitudinally decreased cell length. The bioactive GA1 (gibberellin A1) and GA4 (gibberellin A4) were not detectable in the stem of dw, and the dwarf phenotype could be rescued by treatment with exogenous GA3. Genetic analysis showed that the dwarf trait of dw was controlled by a recessive nuclear gene. By combining linkage analysis and mapping-by-sequencing, we mapped the GmDW1 gene to an approximately 460-kb region on chromosome (Chr.) 8, containing 36 annotated genes in the reference Willliams 82 genome. Of these genes, we identified two nonsynonymous single nucleotide polymorphisms (SNPs) that are present in the encoding regions of Gmdw1 and Glyma.08G165100 in dw, respectively. However, only the SNP mutation (T>A) at nucleotide 1224 in Gmdw1 cosegregated with the dwarf phenotype. GmDW1 encodes an ent-kaurene synthase, and was expressed in various tissues including root, stem, and leaf. Further phenotypic analysis of the allelic variations in soybean accessions strongly indicated that GmDW1 is responsible for the dwarf phenotype in dw. Our results provide important information for improving our understanding of the genetics of soybean plant height and crop breeding.
Electronic supplementary material
The online version of this article (10.1007/s00122-017-3044-8) contains supplementary material, which is available to authorized users.
Introduction
Ideal plant architecture has recently become a significant breeding objective in many crops (Reinhardt and Kuhlemeier 2002). Height is one of several important components of plant ideotypes, and a relatively shorter stem length contributes to attaining higher yield in crop production (Cooper et al. 1995, 2003). Dwarfism is a desirable characteristic in crop breeding because it confers enhanced resistance to lodging damage from wind and rain and is associated with stable, increased yields by improving the harvest index. For instance, the introduction of semi-dwarf varieties in wheat and rice led to substantial increases in grain yields throughout Asia in the 1960s and 1970s, and prevented many people across the world from starving; this time period is known as the Green Revolution (Peng et al. 1999; Khush 2001; Hedden 2003). In soybean, many semi-dwarf cultivars such as Hobbit87, Charleston, and Apex were also developed. These semi-dwarf cultivars were high yielding and had potential for lodging resistance (Cooper et al. 1995, 2003).
Gibberellins (GAs) regulate diverse biological processes in plant growth and development such as seed germination, stem elongation, leaf expansion, and flowering (Sun and Gubler 2004). Previous studies on dwarf mutants in the model plant species Arabidopsis thaliana (Helliwell et al. 1998; Magome et al. 2004) and in rice (Hong et al. 2003; Ji et al. 2014) have revealed that GAs are one of the most important phytohormones determining plant height. Both gibberellin-deficient and -insensitive mutants showed alterations in plant height. For example, a mutation in the sd1 allele, encoding a gibberellin 20-oxidase gene (GA20oxs), reduced endogenous GA levels and led to the short stature of rice variety IR8 (Sasaki et al. 2002; Spielmeyer et al. 2002). The sd1 seedlings respond to exogenous GAs, which restore height to that of wild-type plants. A similar case happened to another rice semi-dwarf cultivar, Tan-Ginbozu (d35Tan-Ginbozu) with a weak allele of the ent-kaurene oxidase, contributing to the increase in rice yield in Japan in the 1950s (Itoh et al. 2004). The wheat gene Rht (Peng et al. 1999), the maize gene dwarf-8 (d8) (Fujioka et al. 1988), and their orthologue GAI in Arabidopsis (Peng et al. 1997), all encode the DELLA protein, a key component of the molecular GA-GID1-DELLA mechanism controlling plant response to GA (Ueguchi-Tanaka et al. 2005, 2007a, b; Harberd et al. 2009). These mutant alleles always reduce plant height and show reduced responses to GAs. However, the different manner of mutation on the DELLA protein (e.g., SLR1, a rice DELLA protein) can lead to opposite GA response phenotypes: a constitutive GA response slender plant (Ikeda et al. 2001), and a GA-insensitive dwarf (Asano et al. 2009; Hirano et al. 2010).
Mutants play an important role in identifying gene functions in flowering plants. Using spontaneous or artificially induced dwarf mutants, many genes have been cloned and functionally characterized in rice, and many of these are involved in metabolic pathways of plant hormones such as GA, BR (brassinosteroid), and/or strigolactone (SLs) (Itoh et al. 2004; Hirano et al. 2010; Hong et al. 2003; Tong et al. 2009; Lin et al. 2009; Zhou et al. 2013). Within these genes, D35 encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase, and all shortened internodes of d35 plants were restored to the wild-type phenotype by the GA3 treatment (Itoh et al. 2004). However, for another semi-dominant dwarf mutant, Slr1-d4, a mutation in the C-terminal GRAS domain of SLR1 caused its reduced responsiveness to GA3 (Hirano et al. 2010). BR is another important hormone involved in plant height development. The d2 mutants, deficient in the downstream biosynthesis pathway of BR, can respond to exogenous BR treatment, and D2 encoded a cytochrome P450 protein with high similarity to the identified BR synthesis enzymes (Hong et al. 2003). The dwarf and low tillering phenotype of dlt was less sensitive to treatment with BRs, since DLT, a new member of the plant-specific GRAS family, positively regulates the BR signaling pathway in rice (Tong et al. 2009). SLs may also take part in rice stem elongation. D27, a novel iron-containing protein, is involved in biosynthesis of SLs, and the reduced plant height phenotypes of d27 could be rescued to the wildtype by applied strigolactone (Lin et al. 2009). However, d53 is a rice SL-insensitive mutant and the reduced height does not respond to exogenous SL treatment due to a gain-of-function mutation in the D53 protein, acting as a repressor of SL signaling (Zhou et al. 2013). In soybean, increasing numbers of mutants causing dwarfing have been also identified; these mutants occur following ionizing (gamma rays and fast neutrons) radiation (Zhang et al. 2014; Hwang et al. 2015; Cheng et al. 2016) and/or chemical mutagenesis-generated populations (Li et al. 2017). However, except for the peroxidase-encoding gene underlying the del3-15 locus on chromosome 15 (Hwang et al. 2015), few dwarf genes have been cloned and characterized to date.
In this study, we performed a thorough phenotypic characterization of the dw mutant, and reported the molecular identification of the GmDW1 gene by an integrated approach that involved linkage analysis, mapping-by-sequencing, and allelic variation test, which encodes the key enzyme ent-kaurene synthase (KS) that functions in the GA biosynthetic pathway in soybean. Our results indicated that GmDW1 plays a key role in GA-regulated cell elongation in soybean stem internodes.
Materials and methods
Plant materials
The seeds of soybean (Glycine max) cultivars Zhongpin 661(Zp661, ZDD23893), Jidou 12 (JD12, ZDD23040), and Zhonghuang 13 (Zh13, ZDD23876), together with eight other soybean accessions for the allelic variations tests in GmDW1 (Table 8), were obtained from the National Crop Gene Bank, Chinese Academy of Agricultural Sciences. The dw dwarf mutant was isolated from an EMS-mutagenized M3 line in the genetic background of Zp661, which was derived from a cross between the soybean cultivar Williams (PI 548631) and Buffalo (PI 424131) (Li et al. 2017).
Table 8.
Phenotypic analysis and allelic variations in GmDW1 from soybean accessions
| Materialsa | Sequence variation and genotype | SNP effect | Plant height at maturity (cm)c | P valued | Average internode length (cm)c | P valued |
|---|---|---|---|---|---|---|
| Cultivated soybean accessions | ||||||
| ZDD23269 | G-to-A change at nucleotide 5811/Gmdw1-1 | Arg to His at residue 623 | 102.1 ± 4.3 (n = 10) | – | 5.2 ± 0.2 | – |
| ZDD23893 (Zp661) | Wildtype/GmDW1 | Wildtype | 130.9 ± 4.7 (n = 10) | < 0.0001 | 5.8 ± 0.4 | 0.0003 |
| ZDD02315 | Wildtype/GmDW1 | Wildtype | 160.0 ± 11.8 (n = 5) | 0.0111 | 8.8 ± 1.2 | 0.0330 |
| ZDD12910 | Wildtype/GmDW1 | Wildtype | 171 ± 24.1 (n = 5) | 0.0101 | 6.6 ± 0.9 | 0.0455 |
| ZDD03651 | Wildtype/GmDW1 | Wildtype | 180.6 ± 10.4 (n = 5) | < 0.0001 | 8.4 ± 0.2 | < 0.0001 |
| Wild soybean accessions | ||||||
| ZYD02878 | G-to-A change at nucleotide 2837 and 5811/Gmdwb (Gmdw1-1 and Gmdw1-2) | Val to Ile at residue 439 and Arg to His at residue 623 | 7.9 ± 1.7 (n = 6) | – | 1.4 ± 0.3 | – |
| ZYD03687 | Wildtype/GmDW1 | Wildtype | 46.0 ± 15.2 (n = 10) | < 0.0001 | 5.2 ± 1.3 | < 0.0001 |
| ZYD04569 | Wildtype/GmDW1 | Wildtype | 34.5 ± 9.7 (n = 15) | 0.0006 | 3.1 ± 0.8 | 0.0011 |
| ZYD04638 | Wildtype/GmDW1 | Wildtype | 54.2 ± 13.6 (n = 6) | 0.0004 | 4.4 ± 1.2 | 0.0012 |
aCultivated and wild soybean accessions are given a “ZDDxxxxx” or “ZYDxxxxx” number, respectively, and conserved in the National Crop Gene Bank, Chinese Academy of Agricultural Sciences
bThe Gmdw genotype contained both the Gmdw1-1 and the Gmdw1-2 allele
cFor each accession, 5–10 plants (n) were measured for plant height and internode length. All data are given as mean ± SD
dP values for differences between the soybean accession with GmDW1 genotype and the soybean accession with Gmdw1-1 or/and Gmdw1-2 allele were generated by a Student’s t test
Plant hormone treatment and endogenous GA determination
Zp661 and the dw mutant were grown in a growth chamber at 25 °C under conditions of 16-h daylight, 8-h darkness, and 75% humidity. Approximately 14 days after emergence (DAE), 1 g (fresh weight) internode tissue from the mutant or wild-type seedlings was harvested, weighed, immediately frozen in liquid nitrogen, and then stored at − 80 °C. The phytohormone extraction and quantitative profiling of GAs (GA1, GA3, GA4, GA5, GA6, GA7, GA8, GA9, GA12, GA13, GA15, GA19, GA20, GA23, GA24, GA29, GA34, GA44, GA51, and GA53) were performed as described by Chen et al. (2012). These analyses were conducted by the Key Laboratory of Analytical Chemistry for Biology and Medicine of Wuhan University in China.
To assess the response of dw to phytohormones, a range of concentrations of GA3 from 0 to 1.0 mg/L were applied three times for a week to treat the seedlings with fully open true leaves. Uniconazole (Uni) (a GA biosynthesis inhibitor) treatment was carried out at the same time (Itoh et al. 2004). Soybean seeds with no physical damage were soaked in Uni solution (0.6 mg Uni fully diluted in 1 L of double distilled water) for 2 h, and then transferred to vermiculite for normal germination in a growth chamber. Soybean growth condition was set as mentioned above. For each treatment three repeats were prepared, and 1 week later the effect of hormone on stem expansion was evaluated by measuring seedling length.
Scanning electron microscopy
To measure cell size, an internode of 14-DAE dw and Zp661 seedlings was split in half, fixed in 2.5% glutaraldehyde solution (pH 7.4) for 48 h at room temperature, and then processed according to the manual supplied with the scanning electron microscope (Hitachi, S-3000N).
DNA extraction, primer design, and sequencing PCR products
A single young leaf was collected from each plant at the V2 stage (one fully expanded trifoliolate). Genomic DNA was extracted using the modified CTAB method (Saghaimaroof et al. 1984), and diluted to a concentration of 20 ng/μL in ddH2O. Primers were designed online using Primer3 (http://primer3.ut.ee/) based on the Williams 82 reference genome. PCR reactions (25 µL) were composed of 4 μL genomic DNA (20 ng/μL), 2.5 μL PCR buffer (10×), 2.0 μL dNTPs (2 mmol), 2.0 μL MgSO4 (25 mmol), 2.6 μL forward and reverse primers (2 μmol), 0.4 U Kod-Plus-Neo DNA polymerase (TOYOBO, Japan), and sterile water. PCR amplification started with a denaturing step at 94 °C for 3 min, followed by 36 cycles of denaturation at 98 °C for 20 s, annealing at 58–60 °C for 20 s, extension at 68 °C for 50 s, and a final extension at 68 °C for 6–8 min before cooling to 10 °C. PCR products were separated on 2% agarose gels stained with ethidium bromide, visualized in a UV light box, and then sequenced using the Sanger method (Sanger et al. 1977).
Segregation population and genetic mapping
The F1 from a cross between a dw plant and a wild-type (Zp661, JD12, or Zh13) plant was self-crossed to generate an F2 population for genetic analysis and mapping of the dw mutant. The parental lines, ten random F2 recessive individuals, and ten wild-type plants from the F2 population of dw × JD12 were used for bulked segregant analysis. To screen for polymorphic markers, the two parents were genotyped with 567 SSR markers across 20 soybean chromosomes (Song et al. 2010). Together with some newly developed SNP markers, the polymorphic SSR markers were selected for genotyping the two tail bulks and the F2 mutant plants derived from a cross of dw and JD12. The SSR assay was performed using polyacrylamide gel electrophoresis, as described by Wu and Tanksley (1993). Detailed information on the linkage markers for genetic mapping of the dw mutant is shown in Table 1.
Table 1.
Basic information on the SSR markers and developed SNP markers linked to the GmDW1 gene on chromosome 8
| Primer ID | Forward strand sequence (5′–3′) | Reverse strand sequence (5′–3′) | Physical position (Mb) | Product size (bp) |
|---|---|---|---|---|
| GMENOD2Ba (08-0556) | TAGGCAAAAGACTAAAAGAGTA | GCATGTCATTTTGATTGA | 10.19 | 169 |
| BARCSOYSSR_08_0687a (08-0687) | TCTCACCACCACCCTCTTTC | CCTGCAGCAAAACGTCACTA | 12.38 | 226 |
| BARCSOYSSR_08_0692a (08-0692) | TCTGTTAGCAATTCTTATGTAACCG | TCAATTCTTGTTCACAAATCAATAAA | 12.57 | 171 |
| BARCSOYSSR_08_0706a (08-0706) | GGCTAATTTAAGAAAATTTAAAACACG | AATGTTGATAATAAAATCACATGCTTA | 12.89 | 287 |
| BARCSOYSSR_08_0716a (08-0716) | GGGACAATGTGCGAGGTTAG | AAATTGTTGAACCTTTTATTTTTCA | 13.07 | 279 |
| BARCSOYSSR_08_0762a (08-0762) | CACAAGCAATCCCTGACAGA | CAGAAACCGTGGAAACCCTA | 13.95 | 264 |
| BARCSOYSSR_08_0777a (08-0777) | TCGGCCAATGAGTATACGTG | CACGATGGACTTCACGACAT | 14.17 | 258 |
| Sat_129a (08-0818) | GGGGACTCCCTCTCCAGAAGTAAT | GGGAGCAATTGATAAGTGTGAAAATAAT | 14.73 | 239 |
| BARCSOYSSR_08_0935a (08-0935) | TGGATCGATTGTTTTCCAAGA | AAAAATTATCATGGCAGCCG | 16.85 | 231 |
| BARCSOYSSR_08_0941a (08-0941) | AAGGAACAAGTAAAGGAATCATCA | CACCGCACCTTATATTATTACGAA | 16.91 | 285 |
| SNP08-1b | TGCACCAAAACCAGCTCAAT | AGGATCAGAAGGCTTGGGAC | 12.61 | 876 |
| SNP08-2b | CCCGGTGCCAATTTTGAAGT | GATCAAACTTGCTCGTGACCA | 12.69 | 833 |
| SNP08-3b | TCCTCTCGTCAAAAGCTCCA | CCAAGTGTACAGAGCAATCCTTT | 12.85 | 925 |
| SNP08-4b | TGAAAGCCTTGACATTGCGG | GGCAAAAGGAACCCAAGGAT | 12.90 | 703 |
| SNP08-5b | TCTAAAGAGCCTACCGTGGG | AAGCAATGCCCCTCAATGTG | 13.01 | 774 |
| SNP08-6b | CTGGTGTCAAATTCCCCTGC | AAA GGC ACC GAA CAT CTT GC | 13.08 | 848 |
aThe unified or classical name for SSR markers associated with the GmDW1 locus are displayed
bSNP markers were developed based on the SNP mutations indentified in the dw genome, as shown in Table 6
Whole genome resequencing, SNP detection and identification of the candidate interval
A similar strategy to the MutMap+ method (Fekih et al. 2013) was used to isolate the GmDW1 gene. One plant that contained a heterozygous DW locus was selected from an M3 line derived from the EMS-mutagenized population of cv. Zp661. This plant was self-crossed to generate an isogenic M4 segregating population. DNA from 45 mutant or 45 wild-type plants was extracted and equally pooled. According to the manufacturer’s instructions (Illumina Inc.), > 5-μg genomic DNA for each pool was prepared for constructing a sequencing library. Paired-end sequencing libraries with an insert size of approximately 500 bp were sequenced on an Illumina HiSeq 2500 sequencer. Variation calling and annotation was conducted following the protocol of Zhou et al. (2015). Theoretically, for the causal SNP allele, the genotype should be mutated in the raw reads from the mutant pool, while partial reads or no reads containing variant target SNP loci should be present in the wild-type pool. The ED (Euclidean distance) method was used to evaluate differences in allele frequencies between the two phenotype pools for each of the identified SNPs along the 20 chromosomes of soybean. Based on the analysis of the ED values of SNPs, several putative linked regions for the dw dwarf phenotype were detected (Hill et al. 2013; Su et al. 2016).
RNA extraction, reverse transcription PCR, and quantitative real-time PCR
Soybean growth condition was set as mentioned earlier in plant hormone treatment and endogenous GA determination. Fresh tissues from 2-week-old (14 DAE) seedlings were collected, immediately frozen in liquid nitrogen, and stored at − 80 °C for RNA extraction. Total RNA from leaves, stem, and root was extracted using an RNA Prep Pure Plant kit (Tiangen Co., Beijing, China), and treated with DNaseI (Thermo Fisher Scientific Inc., Grand Island, NY). cDNA was synthesized using a SuperScript II kit (TaKaRa). Real-time PCR was performed using a SYBR Premix Ex Taq™ kit (TaKaRa) on an ABI 7300 Real-Time PCR System. Three replicates were run for each sample. The soybean Actin11 gene (Glyma.18G290800) was used as the internal control (Cook et al. 2012). Transcript abundance for some GA mechanism-related genes in soybean was measured using primers listed in Table 2. The relative expression level against the Actin11 gene was quantified using the method (Livak and Schmittgen 2001).
Table 2.
GA mechanism-related genes in soybean and the primers for qPCR analysis
| Some identified GA mechanism-related genes in plants | Soybean homologs | Primer ID | Forward strand sequence (5′–3′) | Reverse strand sequence (5′–3′) |
|---|---|---|---|---|
| GAs biosynthesis-related genes from Arabidopsis and Medicago truncatula | ||||
| Copalyl pyrophosphate synthase (CPS) (AT4G02780) | Glyma.19G157000 | CPS-2 | ACTGCCACCTTCCCTCTTTC | TGTTTGTCGTTAGTCTCGGAC |
| GA-20 oxidase (AT4G25420) | Glyma.09G149200 | GA-1 | GATAGAGAGACCCTGTGCCT | TGAGAAGCAGAGCAAAACAGAG |
| Glyma.20G153400 | GA-2 | TGGCTGCAACGGAAAAGTAA | TAGCCCCATAGCCCTACTCA | |
| Ent-kaurene synthase (KS) (MTR_2G064295) | Glyma.08G163900 | RT08-5 | ATGTGCTGGCTTTGCGTATT | CCTTGCACTCTCTGGGAACT |
| GA-responsive genes isolated in Arabidopsis or Medicago truncatula | ||||
| GID1a (MTR_8G035520) | Glyma.20G230600 | GR-2 | AGTTCCTGTATCCCTGTGCC | TGGCAGGGAAAGAGAAGAGG |
| RGA (AT2G01570) | Glyma.05G140400 | GR-6 | CTGGCTCCAAACCATGCTTT | CCCCGGAATAGCCTTGAGAT |
| Glyma.11G216500 | GR-8 | TCCCCAGATCGTTACCATCG | TCCCAAGGTACAACTCGGAC | |
| Reference gene | ||||
| The soybean Actin11 gene | Glyma.18G290800 | Actin11 | ATCTTGACTGAGCGTGGTTATTCC | GCTGGTCCTGGCTGTCTCC |
Results
Characterization of dw mutants
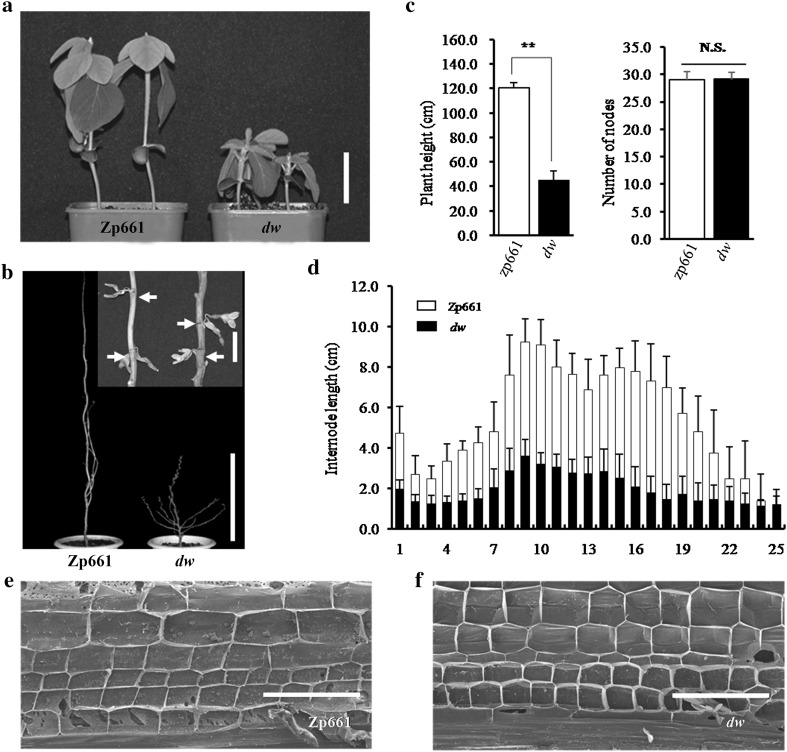
The dw mutant arose from an EMS-mutagenized M2 population as described by Li et al. (2017). From emergence to maturity stages, the mutant displayed a significant, constant decrease in the length of the main stem equivalent to 40% of the size of the mature wild-type Zp661 plants; the mutants also had dark green leaves (Fig. 1a, b). Compared with wild type, the dw plant was not significantly different in total node number on the main stem, but had a relatively consistent reduction in the internode length of 60% (Fig. 1c, d). The longitudinal sections of middle internodes at the seedling stage were observed with a microscope. In dw plants, cell width was similar to wild type, however, the cell length was much shorter than wild type (Fig. 1e, f). Therefore, both reduced plant height and shortened internodes in dw are mainly attributed to the longitudinally decreased cell length instead of a decrease in the number of cells.
Fig. 1.
Phenotypic characterization of the soybean dw mutant. a The plant height of the dw mutant and the parent Zp661 at the seedling stage (2 weeks after emergence). b Phenotype of wild type and dw at maturity. White arrows indicated the nodes bearing soybean pods. c The plant height and the number of nodes on the stem of the mutant and the parent at maturity. d Comparison of all internode lengths for dw and wild-type plants at maturity (n = 15 plants). Longitudinal sections of the first internodes on the stem of the mutant (f) and the parent (e) at the V2 stage (one fully expanded trifoliolate). Scale bar is 5 cm in a, 4 cm for local area magnification and 45 cm for overview in b, 200 µm in e–f, respectively. A Student’s t test indicated a significant difference (n = 15 plants) in c. **P < 0.01; NS not significant. All data are given as mean ± SD
The dwarf mutant is deficient in the GA biosynthesis pathway
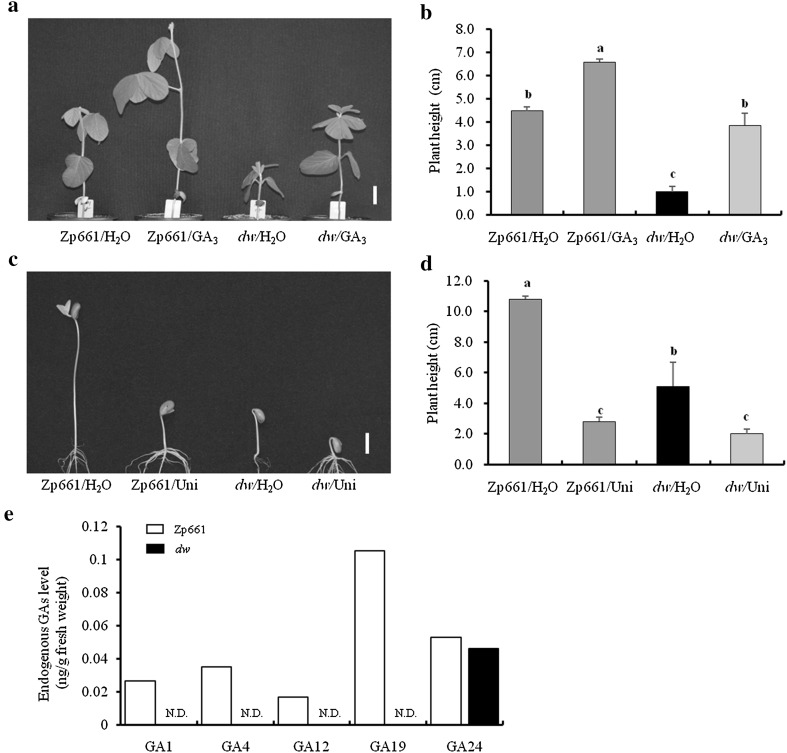
Various factors result in stunted stem growth (Hirano et al. 2010; Tong et al. 2009; Zhou et al. 2013). To determine possible reasons for the dw dwarf phenotype, a series of hormone treatments were performed. GA3 at concentrations of 0–1 mg/L promoted longitudinal stem internode extension in the dw plant, and restored the dwarf mutant to the wild-type phenotype (Fig. 2a, b, Supplemental Fig. S1), whereas BR and IAA showed no effect on stem elongation of dw (data not shown). Uni (Uniconazole), a GA biosynthesis inhibitor, was also used to treat the dw and Zp661 seedlings. In contrast to GA3, Uni treatment resulted in a greater reduction in the shoot length of wild-type seedlings compared to mutants (Fig. 2c, d). Endogenous GA levels in the stem internodes from both wild-type and dw plants were determined using gas chromatography–mass spectrometry (GC–MS). Bioactive GA1 and GA4, as well as their immediate precursors GA12, GA19, and GA24, were detected in wild-type plants, but only GA24 was detected in the dw mutant, suggesting that the dw phenotype was associated with substantially decreased levels of bioactive GA (Fig. 2e). Taken together, these results confirmed that dw has a lower active gibberellin level in the stem, and that it is a GA biosynthesis-deficient mutant.
Fig. 2.
dw is a GA-deficient soybean mutant. The morphological phenotypes (a) and the statistical data of plant height (b) of wild-type and dw plants 1 week past the exogenous GA3 (0.1 mg/L) application. Phenotypes (c) and the statistical data of plant height (d) of 1-week-old elongated dw and Zp661 seedlings after treatment with 0.6 mg/L Uni (uniconazole, a GA3 biosynthesis inhibitor). e Determination of endogenous GA levels in the first internodes of 14-day-old dw and Zp661 plants. The water treatment was used as the control and the scale bar is 2 cm for a and c. ND not detectable. Data for b and d are based on a growth chamber experiment using a randomized complete block design with three replications. The statistical significance of the phenotypic differences among different treatments was evaluated using one-way ANOVA. Bars superscripted by different small letters are significantly different at the 5% probability level
Genetic analysis of the dw mutant
Plant height in crop plants, such as wheat, maize, and soybean, is mostly determined by a set of quantitative trait loci (QTLs) (Singh et al. 2016; Teng et al. 2013; Zhang et al. 2004). To determine whether the extreme dwarf phenotype was controlled by a single gene or locus, the dw mutant was crossed with three varieties that had normal phenotypes, including the wild-type parent Zp661 and the cultivars JD12 and Zh13. All F1 plants displayed normal phenotypes resembling the wild-type parent. In the three F2 populations, mutant individuals were easy to distinguish because of the extreme dwarf stature and dark green leaves. Out of an F2 segregated population from dw × Zp661, plant height of 97 and 31 F2 plants were similar to the wild-type parent and mutant-type, respectively, corresponding to the expected 3:1 segregation ratio for a single recessive gene (χ2 = 0.04, P = 0.84) (Table 3). Similarly, a 3:1 ratio of wild-type individuals to mutant individuals was also detected in the F2 population derived from a cross between the dw mutant and JD12 (χ2 = 2.09, P = 0.15)/Zh13 (χ2 = 0.28, P = 0.59) (Table 3). These data indicated that the dw mutation behaved in a monogenic recessive manner.
Table 3.
Genetic analysis of the dw dwarf phenotype in F2 segregated populations from three crosses
| Cross | Phenotype of F1 plants | Wild type (plants) | Dwarfa (plants) | Total (plants) | P b | |
|---|---|---|---|---|---|---|
| dw × Zp661 | Wild type | 97 | 31 | 128 | 0.04 | 0.84 |
| dw × JD12 | Wild type | 231 | 92 | 323 | 2.09 | 0.15 |
| dw × Zh13 | Wild type | 146 | 53 | 199 | 0.28 | 0.59 |
aDwarf plants were identified by visual inspection based on a phenotype of reduced plant height, shortened internodes, and dark green leaves
bP > 0.05 is considered significant
Mapping of the GmDW1 gene by whole genome resequencing
Using next-generation sequencing (NGS), several strategies have been developed, and used to rapidly identify the causal mutations responsible for important traits induced by chemical mutagenesis (Deschamps et al. 2012). Here, an M3 plant carrying a heterozygous GmDW1 locus was selfed to form an isogenic M4 segregated population consisting of 53 dwarf and 161 wild-type individuals. The DNA pool was generated by bulking 45 mutants or 45 wild-type individuals, and was subsequently subjected to high-throughput whole genome resequencing (Illumina HiSeq 2500 platform), which yielded 454 million and 415 million 2 × 126 bp read pairs for the mutant- and wild-type pools, respectively. Over 94% of the total reads were properly and uniquely mapped to the Williams 82 reference genome, corresponding to average nucleic genome coverage of > 50-fold (Table 4). Based on alignment to the Williams 82 draft genome sequence, 283,626 SNPs were identified in the mutant pool, and 294,871 SNPs were present in the wild-type pool (Table 4). Between the two resequenced samples, a total of 47,535 high quality SNPs were detected for further analysis, indicating that a large number of SNP variations are present in the mutated dw genome.
Table 4.
Basic data for two DNA pools by whole genome resequencing
| Samples | No. of total clean reads | Mapped (%)a | Average depth (x) | Genomic coverage (%) | No. of SNPsb |
|---|---|---|---|---|---|
| Wild type | 415,681,226 | 95.89 | 50 | 99.39 | 294,871 |
| Mutant type | 454,898,978 | 94.01 | 53 | 99.15 | 283,626 |
aNumber of clean reads mapped to the Williams 82 reference genome divided by the total number of clean reads × 100
bThe number of base changes between the resequenced wild-type or mutant DNA pool and the Williams 82 reference genome
The Euclidean distance (ED) algorithm has been proven to be useful in obtaining the genetic distance to the associated genes or QTLs (Hill et al. 2013; Su et al. 2016). To map the GmDW1 gene controlling the dwarf phenotype of the dw mutant, we used the ED method to compute allele frequency differences for each SNP locus along the physical map of soybean between the two DNA pools (Hill et al. 2013; Su et al. 2016). ED value analysis, with a threshold of 0.259, revealed that a total of six intervals with a physical distance of 93 kb–5.7 Mb, were possibly linked to the dwarf trait in dw. Out of these candidate-mapping regions, Chr. 7 and Chr. 8 each had one interval (designated locus7-1 and locus8-1), and Chr. 14 and Chr. 15 each had two intervals (designated locus14-1, locus14-2, locus15-1, and locus15-2) (Table 5). However, just part of the identified SNPs in locus7-1, locus8-1, and locus14-1 result in nonsynonymous substitution of amino acids in the deduced protein sequence, suggesting that these three regions are associated with the dwarf phenotype in dw plants.
Table 5.
Mapping regions associated with the dwarf phenotype of dw mutants identified by whole genome resequencing of two bulked DNA pools
| The names of the linked regions | Chromosome ID | Physical position of candidate intervals | Locations of the identified SNPs in the corresponding mapping regions | ||||
|---|---|---|---|---|---|---|---|
| Interval start (bp) | Interval stop (bp) | Interval length (kb) | Intergenic regiona | Genea (nonsynoymous)b | Up- or downstreama | ||
| locus7-1 | Chr. 07 | 513,840 | 606,834 | 92 | 0 | 2 (1) | 0 |
| locus8-1 | Chr. 08 | 8,716,986 | 14,491,037 | 5774 | 12 | 68 (16) | 51 |
| locus14-1 | Chr. 14 | 8,075,271 | 9,355,387 | 1280 | 14 | 2 (1) | 8 |
| locus14-2 | Chr. 14 | 24,821,398 | 27,119,121 | 2297 | 5 | 0 | 1 |
| locus15-1 | Chr. 15 | 21,601,429 | 22,103,288 | 501 | 4 | 0 | 0 |
| locus15-2 | Chr. 15 | 35,344,325 | 36,214,614 | 870 | 6 | 1 (0) | 1 |
aThe number of identified SNPs located in the open reading frame, intergenic region, and up- or downstream. The number of SNPs resulting in nonsynonymous substitution of amino acids in the deduced protein sequence is identified by b
Validation of the candidate interval for GmDW1 by linkage mapping
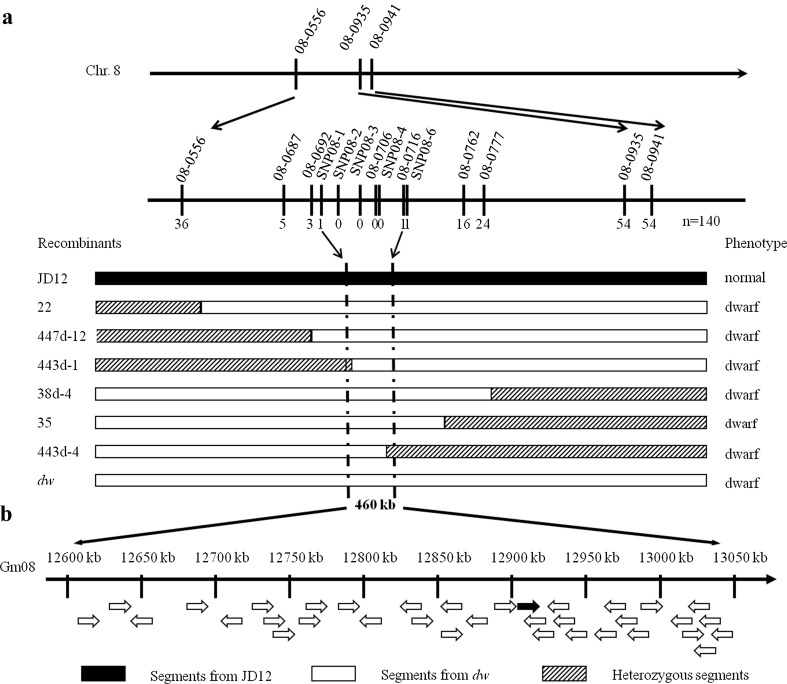
To further screen for the causal interval from those candidate regions, linkage analysis was also conducted based on the F2 population of dw × JD12. A total of 567 SSR markers (Song et al. 2010) evenly distributed on 20 chromosomes were used to screen the dw mutant, JD12, a wild-type pool HP (high plants), and a dwarf pool DP (dwarf plants). Bulked segregant analysis allowed for linkage of the dw locus to four SSR markers on Chr. 8: 08-0935 (BARCSOYSSR_08_0935), 08-0941 (BARCSOYSSR_08_0941), 08-0556 (GMENOD2B), and 08-0818 (Sat_129) (Fig. 3a). Thirty-seven dwarf F2 plants were individually genotyped using these linkage markers, and 3 or 12 recombinants were identified between SSR marker 08-0556 and/or 08-0818, or 08-0941 and the Gmdw1 gene, respectively. Thus, the dwarf gene was initially mapped within an interval of 6.7 Mb between the marker 08-0556 (GMENOD2B) and 08-0941 (BARCSOYSSR_08_0941). Interestingly, our linkage analysis showed good correspondence with the locus on Chr. 8 (locus8-1) resulting from resequencing the two bulked DNA pools, and confined the Gmdw1 gene to a 4.3-Mb physical interval.
Fig. 3.
Genetic and physical mapping of GmDW1. a Genetic mapping of the GmDW1 locus. Using some key recombinants screened from the F2 segregated population originating from dw × JD12, the location of the GmDW1 locus was narrowed down to a 460-kb region bounded by marker SNP08-1 and SSR marker 08-0716 on chromosome 8. The numerals below the markers indicate the number of identified recombinants. b Relative physical position of the GmDW1 locus. Thirty-six annotated ORFs (open reading frame) existed in the 460-kb fine-mapping interval according to the Williams 82 reference genome. Black arrow indicated the position of GmDW1 in b. 08-0716, BARCSOYSSR_08_0716; 08-0556, GMENOD2B; 08-0935, BARCSOYSSR_08_0935; 08-0941, BARCSOYSSR_08_0941; 08-0687, BARCSOYSSR_08_0687; 08-0692, BARCSOYSSR_08_0692; 08-0706, BARCSOYSSR_08_0706; 08-0762, BARCSOYSSR_08_0762; 08-0777, BARCSOYSSR_08_0777
Fine mapping of the GmDW1 gene
To further narrow down the candidate-mapping region, additional linkage markers on Chr. 8 were screened, and used to analyze a total of 140 F2 individuals with the dwarf phenotype from the F2 population of dw × JD12. Polymorphic markers 08-0692 (BARCSOYSSR_08_0692), 08-0706 (BARCSOYSSR_08_0706), and 08-0716 (BARCSOYSSR_08_0716) were applied to genotype the identified 90 recombinants between 08-0556 and 08-0941 from the 140 F2 dwarf individuals, and 3, 0, and 1 recombination events were detected, respectively, indicating that the candidate gene was mapped to the segment with a physical distance of approximately 500 kb between marker 08-0692 and 08-0716 (Fig. 3a). By analyzing the resequenced mutant genome, we found that there were only nine SNPs in the mapping region for dw (Table 6). Based on these SNP mutations, six SNP markers (SNP08-1-SNP08-6) were developed and used to genotype those four recombinant plants. Only one recombinant was detected between SNP08-1 or SNP08-6 and the GmDW1 gene, respectively, while the markers SNP08-2, SNP08-3, and SNP08-4 cosegregated with the dwarf phenotype in the F2 population, which was confirmed based on 50 F2 mutant plants from a cross of dw × Zh13. Accordingly, we were finally able to restrict the location of this gene to a 460-kb interval between markers SNP08-1 and 08-0716 (BARCSOYSSR_08_0716) on Chr. 8 (Fig. 3a). According to the Williams 82 reference genome, the fine-mapping region contains 36 predicted genes (Fig. 3b), which are listed in Table 7.
Table 6.
Nine identified SNPs in the 500-kb mapping region containing the GmDW1 allele in the dw genome and marker development
| The ID of developed markers based on the corresponding SNP locus | SNP | The wild-type pool | The mutant pool | SNP effect and target gene | ||||
|---|---|---|---|---|---|---|---|---|
| Physical position in Gm08 (bp) | Referencea | Variationb | Genotypec | Reads depthd | Genotypec | Reads depthd | ||
| 12,598,498 | G | A | G, A | 24, 15 | G, G | 28, 0 | Intergenic | |
| SNP08-1 | 12,613,790 | C | T | C, T | 18, 5 | T, T | 0, 26 | Nonsynonymous, Glyma.08G162100 |
| SNP08-2 | 12,686,354 | G | T | G, G | 26, 0 | G, T | 9, 6 | Upstream |
| SNP08-3 | 12,847,120 | T | A | T, A | 50, 6 | A, A | 0, 63 | Upstream |
| SNP08-4 | 12,903,104 | T | A | T, T | 43, 0 | A, A | 0, 41 | Nonsynonymous, Glyma.08G163900 |
| 12,969,978 | G | A | G, A | 10, 3 | G, G | 13, 0 | Downstream | |
| SNP08-5 | 13,012,790 | C | A | C, C | 37, 3 | C, A | 26, 5 | Nonsynonymous, Glyma.08G165100 |
| 13,055,558 | G | A | G, A | 32, 18 | G, G | 24, 0 | Upstream | |
| SNP08-6 | 13,077,287 | C | T | C, C | 34, 0 | T, T | 0, 37 | Nonsynonymous, Glyma.08G165800 |
aThe genotypes of the SNP locus in the Williams 82 reference genome, while b represents the genotypes of the corresponding mutated locus present in the mutant- or wild-type pool genome. c Homozygous or heterozygous genotype (e.g., “G,G” vs “G,A”) of the SNP locus was identified in the wild-type pool and mutant-type pool genome
dThe number of clean reads covering each of genotypes at the SNP locus
Table 7.
Thirty-six predicted genes in the 460-kb fine-mapping interval of GmDW1 in Gm08 according to the Williams 82 reference genome
| Gene ID | Functional annotation in the Phytozome database |
|---|---|
| Glyma.08G162200 | Methylated RNA-binding protein 1 |
| Glyma.08G162300 | Nucleoprotein TPR-related |
| Glyma.08G162400 | Aspartic protease CDR1-related |
| Glyma.08G162500 | DNA-3-methyladenine glycosylase I/DNA-3-methyladenine glycosidase |
| Glyma.08G162600 | 39S ribosomal protein L15, mitochondrial |
| Glyma.08G162700 | Peroxidase/Lactoperoxidase |
| Glyma.08G162800 | Zinc finger CCCH domain-containing protein 5 |
| Glyma.08G162900 | Metacaspase-5-like |
| Glyma.08G163000 | E3 ubiquitin-protein ligase RGLG2-like |
| Glyma.08G163100 | NAC domain-containing protein 20-related |
| Glyma.08G163200 | MYB-like DNA-binding protein |
| Glyma.08G163300 | Uncharacterized protein |
| Glyma.08G163400 | Uncharacterized protein |
| Glyma.08G163500 | MYB family transcription factor APL-like |
| Glyma.08G163600 | Uncharacterized protein |
| Glyma.08G163700 | Succinate-semialdehyde dehydrogenase, mitochondrial-like |
| Glyma.08G163800 | Cell cycle control protein 50 |
| Glyma.08G163900 | Ent-kaurene synthase, chloroplastic-like |
| Glyma.08G164000 | 50S ribosomal protein L7/L12-like, mitochondrial |
| Glyma.08G164100 | IMP dehydrogenase/Inosinic acid dehydrogenase |
| Glyma.08G164200 | Carbohydrate-binding X8 domain-containing protein |
| Glyma.08G164300 | Uncharacterized protein |
| Glyma.08G164400 | Zinc transporter 1-like |
| Glyma.08G164500 | G-protein coupled receptor |
| Glyma.08G164600 | Bifunctional L-3-cyanoalanine synthase/cysteine synthase D1-related |
| Glyma.08G164700 | Metal tolerance protein 10-like |
| Glyma.08G164800 | Metal tolerance protein 10-like |
| Glyma.08G164900 | Cullin binding (Cullin_binding)/UBA-like domain (UBA_4) |
| Glyma.08G165000 | Defense-like protein 1-related |
| Glyma.08G165100 | Transglycosylase SLT domain (SLT) |
| Glyma.08G165200 | Uncharacterized protein |
| Glyma.08G165300 | Uncharacterized protein |
| Glyma.08G165400 | Phosphoglycerate kinase, cytosolic-like |
| Glyma.08G165500 | Phosphoglycerate kinase 1, chloroplastic-like |
| Glyma.08G165600 | DEAD-box ATP-dependent RNA helicase 36-like |
| Glyma.08G165700 | Histone-like transcription factor CCAAT-related |
Candidate gene analysis of the dw dwarf mutant
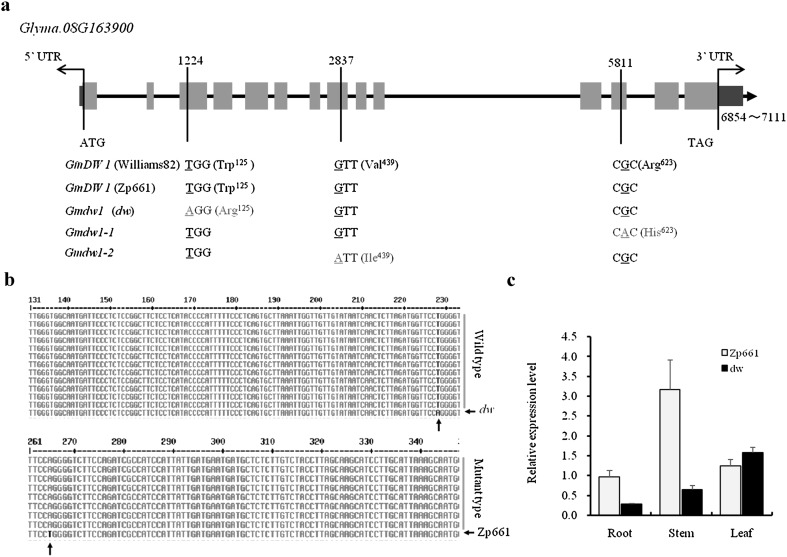
To rapidly isolate the causal mutation responsible for the dw mutant, we analyzed the two sequenced DNA samples. For the mutated dw genome, however, we only identified seven SNPs in the 460-kb candidate interval (Table 6). Of these SNPs, two were located in exons of Glyma.08G163900 (named GmDW1) and Glyma.08G165100, respectively, and formed missense substitutions of the amino acid sequence, while the others were distributed in genes downstream, upstream, or in the intergenic region (Table 6). The indels were also analyzed (data not shown); however, no variations were discovered in the 460-kb fine-mapping interval of the GmDW1 gene. Therefore, Glyma.08G165100 and GmDW1 were the main candidates for the dw mutant. According to gene function annotation in the Phytozome database, Glyma.08G165100 encodes a transglycosylase SLT domain-containing protein, and GmDW1 encodes KS in soybean. KS is an important enzyme in the upstream biosynthesis pathway of GA, and deficiency in endogenous gibberellin level always results in a dwarf phenotype. In addition, no other GA biosynthesis-related genes were predicted in the candidate-mapping region (Table 7). Further analysis of the clean reads covering the two mutations in the mutant pool revealed 26 wild-type reads out of 31 for the SNP mutation (C>A) in Glyma.08G165100. However, 41 reads with the mutation (T>A) in GmDW1 were detected, which was confirmed when genotyping every individual in the mutant pool (Table 6, Fig. 4b). Taken together with the observed result from GA3 treatment, GmDW1 is the candidate gene for the dw mutant.
Fig. 4.
Genetic correlations between the dwarf phenotype in dw and GmDW1 (Glyma.08G163900). a Genomic sequence of the GmDW1 allele among dw, Zp661, and the Williams 82 reference genome was compared; a T-to-A change in the third exon was detected in dw, and two additional allelic variations (Gmdw1-1 and Gmdw1-2) in GmDW1 were also screened from soybean accessions. b The SNP locus (T>A) in Glyma.08G163900 was linked to the mutant phenotype in dw, when genotyping each individual in the resequenced mutant or wild-type pool. c Relative expression level of GmDW1 was detected by qPCR, with data normalized to Actin11 levels (n = 3) (Cook et al. 2012), in different tissues including stem, leaf, and root from 2-week-old dw and Zp661 plants
GmDW1 contains 14 exons and 13 introns with a 2768-bp transcript encoding 834 amino acids. Analysis of the predicted protein sequence revealed several conserved domains. There is a terpene_cyclase_plant_C1 domain of 522 aa from aa 285–806. A terpene synthase family metal-binding domain is located at aa 501-756. The region of aa 34–815 contains an ent-kaurene-16 synthase domain.
In an attempt to identify sequence variations in the candidate gene, genomic sequence corresponding to the ORF and the promoter region of GmDW1 in the wild type and the dw mutant was amplified and sequenced. Besides a T-to-A change in the third exon from BSA (bulked segregant analysis) sequencing, a second SNP mutation (A2416G) and a 3-bp deletion (AAA) were also identified in the 6th intron of Gmdw1 in the dw mutant, but only the single missense point mutation (T1224A) in exon 3 caused an amino acid substitution at residue 125, from Trp to Arg in the Gmdw1 gene (Fig. 4a).
Tissue expression analysis of the parental line Zp661 at the seedling stage revealed that GmDW1 was expressed in various tissues including root, stem, and leaf, with the highest level in stem (Fig. 4c).
Allelic variation in GmDW1 associated with plant height
To identify the association between plant height and allelic variations in GmDW1, we analyzed the resequencing data from 57 wild and cultivated soybean accessions and identified two additional recessive alleles (Li et al. 2013; Lam et al. 2010; Kim et al. 2010). One allele, designated Gmdw1-1, had a G-to-A change at nucleotide 5811 that resulted in an amino acid substitution at residue 623 from Arg to His in the soybean cultivar ZDD23269 (Li et al. 2013) (Fig. 4a, Table 8). The second, designated Gmdw1-2, was also a G-to-A change at nucleotide 2837, leading to a substitution of Val with Ile at residue 439 (Fig. 4a). The two alleles were present simultaneously in the wild accession ZYD02878 (designated the Gmdw genotype) (Li et al. 2013) (Table 8).
To evaluate the plant height for the different GmDW1 genotypes, we grew the soybean accessions in the field in Hainan province (18.14°N, 109.31°E), or in Beijing (39.97°N, 116.33°E). Cultivars with GmDW1 and Gmdw1-1 genotypes had plant heights of 130.1–180.6, or 102.1 cm, respectively (Table 8), in the spring of 2012 in Beijing. A similar trend was observed when the wild soybean accessions with GmDW1 and Gmdw (having both the Gmdw1-1 and Gmdw1-2 allele) genotypes were grown in the winter of 2011 in Hainan province: the plant height was 34.5–54.2, or 7.9 cm (Table 8). The Gmdw1-1 and Gmdw genotypes showed consistent decreases in plant height phenotypes in both cultivars and wild accessions, despite different genetic backgrounds, suggesting that the sequence variations in the GmDW1 allele are associated with the dwarf trait in dw mutants. Taken together, the results obtained from map-based cloning, genetic, and phenotypic analysis of allelic variation in GmDW1 in the wild and cultivated soybean accessions strongly indicate that the GmDW1 gene is responsible for the dwarf phenotype of dw.
Expression analysis of GA metabolic pathway-related genes in soybean
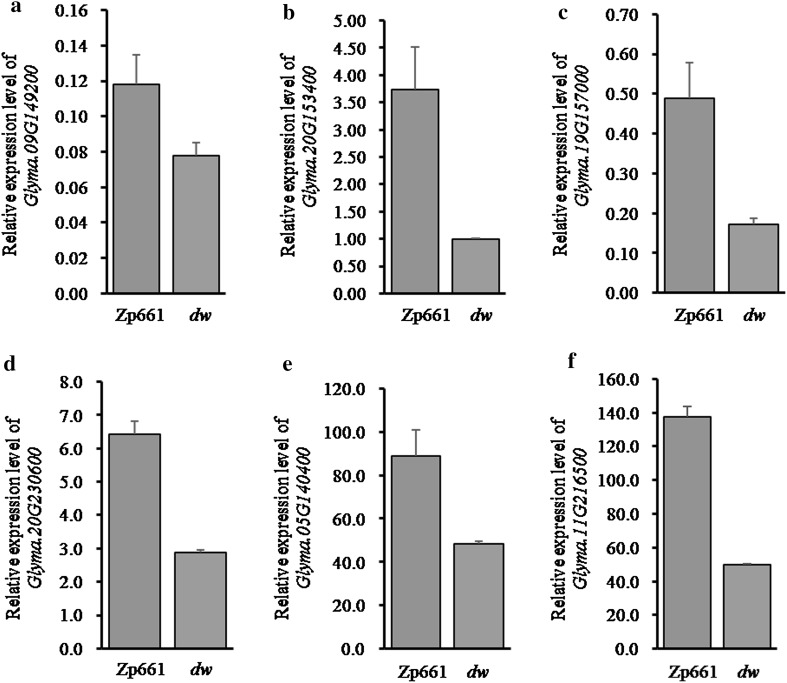
To assess whether relative expression level of GA biosynthesis pathway genes in the dw mutant changes, we downloaded the predicted amino acid sequences of Arabidopsis CPS (Copalyl pyrophosphate synthase, AT4G02780), and GA-20 oxidase (GA20oxs, AT4G25420) from the National Center for Biotechnology Information (NCBI). BLAST analysis against the current assembly of the Williams 82 genome was performed using the NCBI Protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The retrieved soybean genomic DNA sequences putatively encoding proteins with high identity to Arabidopsis homologs were predicted for GA biosynthesis pathway genes in soybean. As a result, five gene models, including three for CPS, and two for GA20oxs were selected. Of these, three soybean genes (Glyma.19G157000, Glyma.09G149200, and Glyma.20G153400) were expressed in young stem tissues (listed in Table 2). The expression levels of CPS and GA20oxs-encoding genes were lower in stems of dw than in the wild-type plant (Fig. 5a–c). Using the same method, we examined the relative expression of some GA response-related genes including Medicago truncatula GID1a (MTR_8G035520) homolog (Glyma.20G230600), and Arabidopsis RGA (AT2G01570) homologs (Glyma.05G140400 and Glyma.11G216500) in soybean (Table 2). Compared with the wild-type plant these genes also showed substantially decreased expression in stems of dw (Fig. 5d–f).
Fig. 5.
The relative expression of GA metabolic pathway-related genes in dw and the parental line Zp661. The expression level of GA biosynthesis-related genes GA-20 oxidase (a Glyma.09G149200, b Glyma.20G153400) and CPS (Copalyl pyrophosphate synthase) (c Glyma.19G157000) homologues in soybean was examined in stems of 14-day-old dw and Zp661 seedlings. d–f The relative expression of GA response-related genes GID1a (d Glyma.20G230600) and RGA (e Glyma.05G140400, f Glyma.11G216500) homologues in soybean in stems of two-week-old dw and Zp661 plants. The soybean Actin11 gene (Glyma.18G290800) was used as the internal control (Cook et al. 2012), and three replicates were performed for each of the genes in a–f. The relative transcript abundance for these genes in a-f against the soybean Actin11 gene (Glyma.18G290800) was quantified using the method (Livak and Schmittgen 2001). All the primers for qPCR in a–f are shown in Table 2
Discussion
dw is a new GA-deficient soybean mutant
There are many reasons for the dwarf phenotype in plants. In this study, we demonstrated that dw is a GA biosynthesis-deficient soybean mutant through exogenous application of GA3 and its synthesis inhibitor. The identity of dw was further confirmed by determination of endogenous GAs. Meanwhile, compared with the wild type, a relatively reduced inhibition effect of uniconazole on shoot growth was observed in the dw seedlings, which is likely related to the way uniconazole impairs GA biosynthesis as a competitive inhibitor of ent-kaurene oxidase (Izumi et al. 1985). Besides GAs, other phytohormones, such as BR, SLs, and IAA, may also be involved in regulating plant height (Tong et al. 2009; Lin et al. 2009; Zhou et al. 2013; Woodward and Bartel 2005). However, in the present study, the dwarf phenotype of dw was not rescued by BR or IAA treatment. The dw mutant is quite different from a few other soybean dwarf mutants that have been intensively studied recently. Of them, the Gmdwarf1 mutant slightly responded to GA3 treatment (Zhang et al. 2014), while dwarfism of Gmdwf1 could not be rescued by GA3 application (Cheng et al. 2016). The FN dwarf mutant, screened from an FN-mutagenized M4 population of Williams 82, began to display abnormal plant height after the V2 stage (Hwang et al. 2015), however, the dw mutant in our study showed a consistent dwarf phenotype from the cotyledon expansion stage to maturity. Plant height is generally controlled by node number on the main stem and internode length. We also found that the significantly decreased cell length contributed to the shortened internode, resulting in the sharp decrease in plant height of dw compared to wild type, while other dwarf soybean mutants mainly exhibited a substantial change in node number, internode length, or both (Hwang et al. 2015; Cheng et al. 2016). Furthermore, we mapped the GmDW1 gene to a 460-kb region on Chr. 8, in which no other dwarfing genes have been isolated in soybean.
The GA biosynthesis pathway has been studied extensively in the model organisms Arabidopsis thaliana and rice, and the majority of genes encoding key enzymes for each step have been identified (Hedden and Phillips 2000; Olszewski et al. 2002; Sun and Gubler 2004). On the contrary, few GA synthesis pathway-related genes have been isolated in soybean. Here, we determined that an ent-kaurene synthase (KS)-encoding gene, functioning at the early step of GA biosynthesis, is responsible for the dwarf phenotype in dw, suggesting a conserved function of KS genes in the GA biosynthesis pathway across different plant species.
Combination of NGS and linkage mapping accelerates the identification of target genes
Forward genetics, for instance, positional cloning is successful for isolating candidate genes of diverse traits, but is labor-intensive and time-consuming (Salvi and Tuberosa 2005). With increasingly high-throughput and decreasing cost, next-generation sequencing (NGS) technology coupled with the growing number of sequenced genomes, has been widely applied to biological research. NGS has been proven to be efficient in identification of candidate genes and SNP discovery in Arabidopsis (Ashelford et al. 2011; Leshchiner et al. 2012; Hartwig et al. 2012), rice (Abe et al. 2012), soybean (Zhou et al. 2015), barley (Mascher et al. 2014), and other plant species (Islam et al. 2016). Several mapping strategies based on NGS, such as mapping-by-sequencing or direct resequencing, have been developed, and enable rapid detection of causal mutations responsible for target traits differentiating the mutant from the wild type. However, this method always leads to a few false positive candidate intervals (Ashelford et al. 2011; Abe et al. 2012; Hwang et al. 2015), which also happened in the present study: six putative regions were identified to be linked to the dwarf phenotype of dw. Consequently, when NGS was combined with linkage mapping in our work, it was easier to exclude those false loci, and successfully anchor the causal mutation responsible for the dwarf phenotype of dw from 36 predicted genes in a short period, greatly reducing the input of labor and time. Our study provides an efficient strategy that has high potential for accelerating the identification of target genes located in a centromeric region in the model plant species such as rice, or in complex non-model genomes such as soybean with a relatively small number of recombinants generated from a segregated population, which will promote the development of functional genomics in crop plant species.
Author contribution statement
LQ supervised the experiment and revised the manuscript. ZFL, HH, and LO performed the research. ZFL and YG analyzed the data. ZFL wrote the draft manuscript. YG and JW assisted in editing the manuscript. HH, ZXL, BG, and LZ managed the field research and plant propagation. All authors read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0101005), the National Natural Science Foundation of China (31601326), and the National Transgenic Major Program of China (2016ZX08009003-003).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00122-017-3044-8) contains supplementary material, which is available to authorized users.
References
- Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, Matsumura H, Yoshida K, Mitsuoka C, Tamiru M, Innan H, Cano L, Kamoun S, Terauchi R. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat Biotechnol. 2012;30:174–178. doi: 10.1038/nbt.2095. [DOI] [PubMed] [Google Scholar]
- Asano K, Hirano K, Ueguchi-Tanaka M, Angeles-Shim RB, Komura T, Satoh H, Kitano H, Matsuoka M, Ashikari M. Isolation and characterization of dominant dwarf mutants, Slr1-d, in rice. Mol Genet Genomics. 2009;281:223–231. doi: 10.1007/s00438-008-0406-6. [DOI] [PubMed] [Google Scholar]
- Ashelford K, Eriksson ME, Allen CM, D-Amore R, Johansson M, Gould P, Kay S, Millar AJ, Hall N, Hall A. Full genome re-sequencing reveals a novel circadian clock mutation in Arabidopsis. Genome Biol. 2011;12:R28. doi: 10.1186/gb-2011-12-3-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Fu X, Liu J, Ye T, Hou S, Huang Y, Yuan B, Wu Y, Feng Y. Highly sensitive and quantitative profiling of acidic phytohormones using derivatization approach coupled with nano-LC-ESI-Q-TOF-MS analysis. J Chromatogr B. 2012;905:67–74. doi: 10.1016/j.jchromb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Cheng W, Gao J, Shao Q, Yang S, Feng X. Characterization of dwarf mutants and molecular mapping of a dwarf locus in soybean. J Integr Agric. 2016;15:2228–2236. doi: 10.1016/S2095-3119(15)61312-0. [DOI] [Google Scholar]
- Cook DE, Lee TG, Guo X, Melito S, Wang K, Bayless AM, Wang J, Hughes TJ, Willis DK, Clemente TE, Diers BW, Jiang J, Hudson ME, Bent AF. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science. 2012;338:1206–1209. doi: 10.1126/science.1228746. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Martin RJ, St. Martin SK, Calip-DuBois A, Fioritto RJ, Schmitthenner AF. Registration of ‘Charleston’ soybean. Crop Sci. 1995;35:593. doi: 10.2135/cropsci1995.0011183X003500020060x. [DOI] [Google Scholar]
- Cooper RL, Mendiola T, St. Martin SK, Fioritto RJ, Dorrance AE. Registration of ‘Apex’ soybean. Crop Sci. 2003;43:1563. doi: 10.2135/cropsci2003.1563. [DOI] [Google Scholar]
- Deschamps S, Llaca V, May GD. Genotyping-by-sequencing in plants. Biology. 2012;1:460–483. doi: 10.3390/biology1030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekih R, Takagi H, Tamiru M, Abe A, Natsume S, Yaegashi H, Sharma S, Kanzaki H, Matsumura H, Saitoh H, Mitsuoka C, Utsushi H, Uemura A, Kanzaki E, Kosugi S, Yoshida K, Cano L, Kamoun S, Terauchi R. MutMap+: genetic mapping and mutant identification without crossing in rice. PLoS One. 2013;8:e68529. doi: 10.1371/journal.pone.0068529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Katsumi M, Phinney BO, Gaskin P, MacMillan J, Takahashi N. The dominant non-gibberellin-responding dwarf mutant (D8) of maize accumulates native gibberellins. Proc Natl Acad Sci USA. 1988;85:9031–9035. doi: 10.1073/pnas.85.23.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP, Belfield E, Yasumura Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell. 2009;21:1328–1339. doi: 10.1105/tpc.109.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig B, James GV, Konrad K, Schneeberger K, Turck F. Fast isogenic mapping-by-sequencing of ethyl methanesulfonate-induced mutant bulks. Plant Physiol. 2012;160:591–600. doi: 10.1104/pp.112.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P. The genes of the green revolution. Trends Genet. 2003;19:5–9. doi: 10.1016/S0168-9525(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Hedden P, Phillips AL. Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci. 2000;5:1360–1385. doi: 10.1016/S1360-1385(00)01790-8. [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Sheldon CC, Olive MR, Walker AR, Zeevaart JA, Peacock WJ, Dennis ES. Cloning of the Arabidopsis ent-kaurene oxidase gene GA3. Proc Natl Acad Sci USA. 1998;95:9019–9024. doi: 10.1073/pnas.95.15.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JT, Demarest BL, Bisgrove BW, Gorsi B, Su Y, Yost HJ. MMAPPR: mutation mapping analysis pipeline for pooled RNA-seq. Genome Res. 2013;23:687–697. doi: 10.1101/gr.146936.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Asano K, Tsuji H, Kawamura M, Mori H, Kitano H, Ueguchi-Tanaka M, Matsuoka M. Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice. Plant Cell. 2010;22:2680–2696. doi: 10.1105/tpc.110.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell. 2003;15:2900–2910. doi: 10.1105/tpc.014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Kim M, Yang J, Kang Y, Shim S, Stacey MG, Stacey G, Lee S. Genome-wide analysis of mutations in a dwarf soybean mutant induced by fast neutron bombardment. Euphytica. 2015;203:399–408. doi: 10.1007/s10681-014-1295-x. [DOI] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J. Slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Zeng L, Thyssen GN, Delhom CD, Kim HJ, Li P, Fang DD. Mapping by sequencing in cotton (Gossypium hirsutum) line MD52ne identified candidate genes for fiber strength and its related quality attributes. Theor Appl Genet. 2016;129:1071–1086. doi: 10.1007/s00122-016-2684-4. [DOI] [PubMed] [Google Scholar]
- Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, Ashikari M, Ichihara S, Matsuoka M. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol. 2004;54:533–547. doi: 10.1023/B:PLAN.0000038261.21060.47. [DOI] [PubMed] [Google Scholar]
- Izumi K, Kamiya Y, Sakurai A, Oshio H, Takahashi N. Studies of sites of action of a new plant growth retardant (E)-1-(4-chloro-phenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)-1-penten-3-ol (S-3307) and comparative effects of its stereoisomers in a cell-free system from Cucurbita maxima. Plant Cell Physiol. 1985;26:821–827. [Google Scholar]
- Ji S, Gururani M, Lee J, Ahn B, Chun S. Isolation and characterisation of a dwarf rice mutant exhibiting defective gibberellins biosynthesis. Plant Biol. 2014;16:428–439. doi: 10.1111/plb.12069. [DOI] [PubMed] [Google Scholar]
- Khush GS. Green revolution: the way forward. Nat Rev Genet. 2001;2:815–822. doi: 10.1038/35093585. [DOI] [PubMed] [Google Scholar]
- Kim MY, Lee S, Van K, Kim TH, Jeong SC, Choi IY, Kim DS, Lee YS, Park D, Ma J, Kim WY, Kim BC, Park S, Lee KA, Kim DH, Kim KH, Shin JH, Jang YE, Kim KD, Liu W, Chaisan T, Kang YJ, Lee YH, Kim KH, Moon JK, Schmutz J, Jackson SA, Bhak J, Lee SH. Whole-genome sequencing and intensive analysis of the undomesticated soybean (Glycine soja Sieb. and Zucc.) genome. Proc Natl Acad Sci USA. 2010;107:22032–22037. doi: 10.1073/pnas.1009526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HM, Xu X, Liu X, Chen W, Yang G, Wong FL, Li MW, He W, Qin N, Wang B, Li J, Jian M, Wang J, Shao G, Wang J, Sun SS, Zhang G. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet. 2010;42:1053–1059. doi: 10.1038/ng.715. [DOI] [PubMed] [Google Scholar]
- Leshchiner I, Alexa K, Kelsey P, Adzhubei I, Austin-Tse CA, Cooney JD, Anderson H, King MJ, Stottmann RW, Garnaas MK, Ha S, Drummond IA, Paw BH, North TE, Beier DR, Goessling W, Sunyaev SR. Mutation mapping and identification by whole-genome sequencing. Genome Res. 2012;22:1541–1548. doi: 10.1101/gr.135541.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao S, Ma J, Li D, Yan L, Li J, Qi X, Guo X, Zhang L, He W, Chang R, Liang Q, Guo Y, Ye C, Wang X, Tao Y, Guan R, Wang J, Liu Y, Jin L, Zhang X, Liu Z, Zhang L, Chen J, Wang K, Nielsen R, Li R, Chen P, Li W, Reif JC, Purugganan M, Wang J, Zhang M, Wang J, Qiu L. Molecular footprints of domestication and improvement in soybean revealed by whole genome re-sequencing. BMC Genom. 2013;14:579. doi: 10.1186/1471-2164-14-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang L, Ma Y, Wei Z, Liu Z, Hong H, Liu Z, Lei J, Liu Y, Guan R, Guo Y, Jin L, Zhang L, Li Y, Ren Y, He W, Liu M, Htwe N, Liu L, Guo B, Song J, Tan B, Liu G, Li M, Zhang X, Liu B, Shi X, Han S, Hua S, Zhou F, Yu L, Li Y, Wang S, Wang J, Chang R, Qiu L. Development and utilization of a new chemically-induced soybean library with a high mutation density. J Integr Plant Biol. 2017;59:60–74. doi: 10.1111/jipb.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21:1512–1525. doi: 10.1105/tpc.109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K. Dwarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J. 2004;37:720–729. doi: 10.1111/j.1365-313X.2003.01998.x. [DOI] [PubMed] [Google Scholar]
- Mascher M, Jost M, Kuon JE, Himmelbach A, Aßfalg A, Beier S, Scholz U, Graner A, Stein N. Mapping-by-sequencing accelerates forward genetics in barley. Genome Biol. 2014;15:29–42. doi: 10.1186/gb-2014-15-6-r78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F. Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell. 2002;14:S61–S80. doi: 10.1105/tpc.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Gene Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP. ‘Green revolution’ genes encode mutant gibberellins response modulators. Nature. 1999;400:256–261. doi: 10.1038/22307. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Kuhlemeier C. Plant architecture. EMBO Rep. 2002;3:846–851. doi: 10.1093/embo-reports/kvf177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghaimaroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, Tuberosa R. To clone or not to clone plant QTLs: present and future challenges. Trends Plant Sci. 2005;10:297–304. doi: 10.1016/j.tplants.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M. A mutant gibberellins-synthesis gene in rice. Nature. 2002;416:701–702. doi: 10.1038/416701a. [DOI] [PubMed] [Google Scholar]
- Singh A, Knox RE, DePauw RM, Singh AK, Cuthbert RD, Kumar S, Campbell HL. Genetic mapping of common bunt resistance and plant height QTL in wheat. Theor Appl Genet. 2016;129:243–256. doi: 10.1007/s00122-015-2624-8. [DOI] [PubMed] [Google Scholar]
- Song Q, Jia G, Zhu Y, Grant D, Nelson RT, Hwang EY, Hyten DL, Cregan PB. Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in soybean. Crop Sci. 2010;50:1950–1960. doi: 10.2135/cropsci2009.10.0607. [DOI] [Google Scholar]
- Spielmeyer W, Ellis MH, Chandler PM. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA. 2002;99:9043–9048. doi: 10.1073/pnas.132266399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A, Song W, Xing J, Zhao Y, Zhang R, Li C, Duan M, Luo M, Shi Z, Zhao J. Identification of genes potentially associated with the fertility instability of S-type cytoplasmic male sterility in maize via bulked segregant RNA-Seq. PLoS One. 2016;11:e0163489. doi: 10.1371/journal.pone.0163489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol. 2004;55:197–223. doi: 10.1146/annurev.arplant.55.031903.141753. [DOI] [PubMed] [Google Scholar]
- Teng F, Zhai L, Liu R, Bai W, Wang L, Huo D, Tao Y, Zheng Y, Zhang Z. ZmGA3ox2, a candidate gene for a major QTL, qPH3.1, for plant height in maize. Plant J. 2013;73:405–416. doi: 10.1111/tpj.12038. [DOI] [PubMed] [Google Scholar]
- Tong H, Jin Y, Liu W, Li F, Fang J, Yin Y, Qian Q, Zhu L, Chu C. DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. 2009;58:803–816. doi: 10.1111/j.1365-313X.2009.03825.x. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow T, Hsing YC, Kitano H, Yamaguchi I, Matsuoka M. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Xiang H, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell. 2007;19:2140–2155. doi: 10.1105/tpc.106.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol. 2007;58:183–198. doi: 10.1146/annurev.arplant.58.032806.103830. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KS, Tanksley SD. Genetic and physical mapping of telomeres and macrosatellites of rice. Plant Mol Biol. 1993;22:861–872. doi: 10.1007/BF00027371. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang Y, Luo G, Zhang J, He C, Wu X, Gai J, Chen S. QTL mapping of ten agronomic traits on the soybean (Glycine max L. Merr) genetic map and their association with EST markers. Theor Appl Genet. 2004;108:1131–1139. doi: 10.1007/s00122-003-1527-2. [DOI] [PubMed] [Google Scholar]
- Zhang F, Shen Y, Sun S, Guo J, Li C, Wu C, Li Q, Nian H, Huang X, Tian Z, Han T. Genome-wide expression analysis in a dwarf soybean mutant. Plant Genet Resour. 2014;12:S70–S73. doi: 10.1017/S1479262114000306. [DOI] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, Ma W, Gao H, Chen J, Yang C, Wang D, Tan J, Zhang X, Guo X, Wang J, Jiang L, Liu X, Chen W, Chu J, Yan C, Ueno K, Ito S, Asami T, Cheng Z, Wang J, Lei C, Zhai H, Wu C, Wang H, Zheng N, Wan J. D14-SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504:406–410. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Jiang Y, Wang Z, Gou Z, Lyu J, Li W, Yu Y, Shu L, Zhao Y, Ma Y, Fang C, Shen Y, Liu T, Li C, Li Q, Wu M, Wang M, Wu Y, Dong Y, Wan W, Wang X, Ding Z, Gao Y, Xiang H, Zhu B, Lee SH, Wang W, Tian Z. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat Biotechnol. 2015;33:408–414. doi: 10.1038/nbt.3096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.