Abstract
Background
Limited valid data are available regarding the association of fructose-induced symptoms, fructose malabsorption, and clinical symptoms.
Aim
To develop a questionnaire for valid symptom assessment before and during a carbohydrate breath test and to correlate symptoms with fructose breath test results in children/adolescents with functional abdominal pain.
Methods
A Likert-type questionnaire assessing symptoms considered relevant for hydrogen breath test in children was developed and underwent initial validation. Fructose malabsorption was determined by increased breath hydrogen in 82 pediatric patients with functional abdominal pain disorders; fructose-induced symptoms were quantified by symptom score ≥2 and relevant symptom increase over baseline. The results were correlated with clinical symptoms. The time course of symptoms during the breath test was assessed.
Results
The questionnaire exhibited good psychometric properties in a standardized assessment of the severity of carbohydrate-related symptoms. A total of 40 % (n = 33) had malabsorption; symptoms were induced in 38 % (n = 31), but only 46 % (n = 15) with malabsorption were symptomatic. There was no significant correlation between fructose malabsorption and fructose-induced symptoms. Clinical symptoms correlated with symptoms evoked during the breath test (p < 0.001, r2 = 0.21) but not with malabsorption (NS). Malabsorbers did not differ from non-malabsorbers in terms of symptoms during breath test. Symptomatic patients had significantly higher pain and flatulence scores over the 9-h observation period (p < 0.01) than did nonsymptomatic patients; the meteorism score was higher after 90 min.
Conclusions
Fructose-induced symptoms but not fructose malabsorption are related to increased abdominal symptoms and have distinct timing patterns.
Keywords: Fructose malabsorption, Hypersensitivity, Functional abdominal pain, Children, Adolescents
Key Points
Fructose ingestion has been implicated in the pathophysiology of functional abdominal pain in children, but symptoms correlate poorly with the degree of fructose malabsorption. Symptom recording during fructose breath test has been advised, but validated instruments do not exist.
A symptom questionnaire for use in hydrogen breath test was developed and initially validated.
Severity of clinical symptoms did not correlate with presence of fructose malabsorption but significantly correlated with “sensitivity to fructose” (fructose intolerance). The time course of gastrointestinal symptoms varied considerably after fructose challenge.
Evaluation of sensitivity to fructose, rather than fructose malabsorption, is clinically relevant.
Introduction
Chronic abdominal pain is a common problem seen by pediatricians [1]. The majority of abdominal pain in children is classified as functional [2] and includes diagnoses of functional dyspepsia, irritable bowel syndrome, abdominal migraine or functional abdominal pain—not otherwise specified (NOS) [3]. Although these disorders do not have an identifiable cause, children with functional abdominal pain disorders (FAPD) have decreased quality of life and increased absence from school compared with their peers [4]. The pathophysiology of functional abdominal pain disorders is multifactorial and not completely understood; the underlying mechanisms remain the subject of investigation. The current conceptual model proposes gastrointestinal symptoms resulting from a combination of early life events, psychological factors, and physiological factors [5].
In children with FAPD, several carbohydrates, including fructose, have been implicated in exacerbating gastrointestinal (GI) symptoms [6, 7]. Fructose is a poorly absorbable monosaccharide. Fructose ingestion leads to profound increase in small bowel water content and increase in colonic gas compared with glucose, an easily absorbable monosaccharide [8, 9]. These physiological effects of fructose ingestion may explain gastrointestinal symptoms after intake of fructose-containing food, especially if large amounts are ingested and not completely absorbed in the small intestine, enabling fructose to reach the colon, where it is quickly metabolized by the colonic bacterial flora [10].
Fructose malabsorption can be determined clinically by hydrogen breath test [11]. Despite widespread use of fructose hydrogen breath testing, its clinical utility remains disputed [12–14]. Assessment of symptoms in addition to the hydrogen breath test is recommended [15], but no appropriately validated instruments have been developed. Symptoms are usually reported as either present or absent in conjunction with breath test outcomes in clinical practice. A number of clinicians use questionnaires that were validated for other diseases, rather than specifically designed for the pediatric population undergoing hydrogen breath test [16].
Since the first description of fructose malabsorption [17], a consistent observation has been a higher proportion of malabsorbers compared with non-malabsorbers reporting abdominal symptoms after fructose challenge. As such, fructose malabsorption is thought to be a relevant pathomechanism in functional bowel disorders [18]. However, symptoms do not appear to correlate well with the degree of hydrogen production [19, 20]. Moreover, patients with functional bowel disorders benefit from a fructose-restricted diet, regardless of whether fructose malabsorption is present [4, 21, 22]. Thus, whether symptoms following provocation by fructose provide a better indication of the role of fructose in the genesis of a patient’s symptoms is debatable [23]. A potential relationship between clinical symptoms and fructose challenge test has rarely been explored but is of potential interest to provide indirect evidence of the role of malabsorption versus fructose intolerance (or sensitivity to fructose) in the pathogenesis of functional abdominal pain disorders [24].
One aim of the current study is to investigate whether clinical symptoms generally considered to be related with carbohydrate malabsorption correlate with two outcome parameters of fructose hydrogen breath test, viz. malabsorption and sensitivity to fructose. We use the term “sensitivity to fructose” instead of “fructose intolerance” to indicate a proposed relationship to “visceral sensitivity,” a term often used in the context of the pathophysiology of functional abdominal pain disorders, and to avoid confusion with hereditary fructose intolerance, a genetic disease resulting from an enzyme defect. Because a validated scale for assessing abdominal symptoms during breath hydrogen test does not exist, we developed and initially validated a questionnaire for use in the pediatric population undergoing such tests. This scale also enabled us to address our second aim, viz. to obtain data on the time course of specific abdominal symptoms during and after fructose breath hydrogen test.
Patients and Methods
Patients
In this prospective trial, we examined a pediatric cohort of patients who underwent fructose breath testing as diagnostic workup for functional abdominal pain disorders as defined by recurrent or continuous abdominal pain of at least 2 months’ duration. The trial ran from February 2012 to December 2016. Functional nausea and vomiting and functional defecation disorders [25] were ruled out by clinical evaluation, as was abdominal migraine. Clinical evaluation of patients referred to the gastrointestinal unit of St. Anna Kinderspital, Vienna, Austria was based on history, physical examination, pain diary, and stool testing for occult blood to identify potential indications of organic etiology. Additional investigations [26] (including other laboratory tests, radiologic evaluation, and/or endoscopy) were performed as considered appropriate [27] by Kar.H., an experienced board-certified pediatric gastroenterologist at St. Anna Kinderspital, Vienna, Austria. Fructose breath test was considered indicated if patient history could not exclude an association of abdominal symptoms with ingestion of fructose-containing food products. Indications for the breath test were assessed by Kar.H., who also evaluated the ability of the patient to cooperate. The study was approved by the institutional Ethics Committee of the Medical University of Vienna (EK no. 1149/2012).
Fructose–Hydrogen (H2) Breath Test
Patients and their parents received routine instructions to abstain from eating for 12 h prior to the test; only water was allowed to be consumed during this period. Additionally, patients and their parents were instructed to avoid certain foods 12 h prior to the nothing per mouth (nil per os, NPO) request. Smoking and exposure to secondhand smoke was disallowed for at least 1 h before and at any time during the test; sleeping during the test was also disallowed. No other preparation was performed. The breath test was scheduled such that antibiotic treatment had been stopped at least 7 days before the test.
Breath samples were collected and analyzed for hydrogen (H2) using a Gastrolyzer Gastro+ (Bedfont® Scientific Ltd., Station Road, Harrietsham, Maidstone, Kent, ME17 1JA, Great Britain). The first alveolar breath sample was collected at baseline before fructose was ingested. After ingesting 250 ml of fructose in water solution (Kwizda Pharma, Effingergasse 21, 1060 Vienna, Austria) (1 g/kg body weight up to maximum of 25 g), alveolar breath samples were taken at 30-min intervals for 3 h. Breath samples were collected by a maximal inspiration followed by a short period of breath-holding, and then a prolonged expiration through a mouthpiece. Hydrogen levels of end-expiratory breath samples were analyzed using the handheld Gastrolyzer and recorded by a trained technician.
If baseline H2 exhalation was ≥15 ppm, patients were asked to rinse their mouth with tap water and measurement of H2 exhalation was repeated. If the H2 exhalation level remained ≥15 ppm, the breath test was discontinued, and patients received a pro- or antibiotic trial.
Symptom Assessment
Development of the Questionnaire During the Breath Test (BT-Q)
There is a lack of validated symptom scales to be used during breath hydrogen test [10]. To overcome this shortage, we developed a clinical tool to assess the severity of gastrointestinal symptoms that are considered relevant for hydrogen breath test in children. After a literature search and initial focus-group-style interviews of parents and children who underwent breath hydrogen test and of two pediatric physicians experienced in breath hydrogen testing, five relevant complaints—pain, nausea, meteorism, flatulence, and diarrhea—were identified, and a Likert-type faces questionnaire was constructed. The symptoms were assessed using child-appropriate language. Responses were given on a six-face scale, with a happy face and the words “not at all” on the left and a sad face and the words “particularly bad” at the right. At patients’ and parents’ request, half-points between faces were added; thus, the final version undergoing further validation was an 11-point scale. The timeframe of symptoms was given as “current” (for baseline symptom assessment) and “since filling out the last questionnaire” (for the following symptom assessments during the breath test).
Face validity was determined by four children and five parents, and content validity was assessed by four pediatricians and one gastroenterologist. Content validity was determined after administering the questionnaire to 90 consecutive pediatric patients undergoing lactose breath hydrogen testing. This sample was used to suggest a grouping of symptom items into domains of symptom burden. A data-driven approach was adopted using principal components analysis followed by varimax rotation. Components were extracted based on a fixed number of 3. Based on these findings, convergent and discriminant validity was determined by the multitrait–multimethod matrix method described by Campbell and Fiske [28]. Cronbach’s alpha was calculated as a measure of internal consistency. Additionally, the questionnaire was administered to 19 patients undergoing lactose or fructose hydrogen breath testing; these patients were not part of the main study reported here. A pediatrician blinded to the results of the questionnaire determined the presence of symptoms by medical interview after the breath test. Fisher’s exact test was used to determine the correlation between the questionnaire (symptom score <2 versus ≥2 during the breath test) and physician interview.
Symptoms During the Breath Test
Patients filled out the BT-Q with or without their parents’ assistance (at the patients’ discretion) at baseline and every 30 min concomitantly with collection of breath samples for 3 h. Additional questionnaires were completed 3 and 6 h after the breath test was terminated, and the patients had left the clinic and resumed their daily routine. These two additional questionnaires were delivered by mail or at the next visit.
Development of the Questionnaire for Clinical Symptoms in the Preceding 4 Weeks
After the development of the aforementioned questionnaire (BT-Q), an identical questionnaire was developed to obtain information on clinical symptoms in the 4 weeks preceding the breath test. The only difference from the BT-Q was the timeframe mentioned in the questionnaire (“in the last 4 weeks”) and accordingly the instruction given to the patient before completion of this questionnaire. Internal consistency was calculated (Cronbach’s alpha) in a sample of 71 pediatric patients undergoing evaluation for gastrointestinal symptoms in the outpatient unit of St. Anna Kinderspital. Additionally, the results of this questionnaire were correlated with a physician’s interview (16 patients with functional abdominal pain disorders). The interviewing physician was unaware of the results of the questionnaire. Spearman correlation ρ was calculated.
Clinical Symptoms in the Preceding 4 Weeks
Clinical symptoms in the preceding 4 weeks were assessed with the Likert-type faces questionnaire. Patients filled out the questionnaires before the test commenced with or without their parents’ assistance (at the patients’ discretion) after receiving routine instructions on the day of the breath test.
End Points
Two primary end points were considered: (1) To assess whether clinical symptoms correlate with fructose malabsorption and/or sensitivity to fructose. A significant correlation may be interpreted as relating to a role of the respective outcome of the fructose challenge in symptom generation. (2) The other primary endpoint addressed the time course of symptoms during the breath test; we compared symptoms during the test with baseline symptoms before fructose ingestion (at time 0 min). Significant increase in symptoms may be interpreted as being induced by fructose ingestion, and the time course of symptoms may allow inference regarding the underlying pathomechanism of these symptoms.
Secondary end points we assessed were (1) a possible association of fructose malabsorption and sensitivity to fructose as determined by fructose H2 breath test, (2) a comparison of individual symptom scores in the preceding 4 weeks in the malabsorption/non-malabsorption and sensitivity/no sensitivity groups, and (3) a possible correlation of the time of peak symptoms with the time of H2 exhalation peaks during the breath test.
Data and Statistical Analysis
Breath tests were interpreted by an experienced pediatric gastroenterologist blinded to subjects’ diagnosis and symptoms. Increase in production of hydrogen ≥20 parts per million (ppm) over baseline was considered positive, i.e., an indicator of fructose malabsorption.
Relevant symptom severity during the breath test was arbitrarily defined as score ≥2 after validation of the instrument. The diagnosis of sensitivity to fructose was established if an increase in symptom score of more than 1 point over baseline was observed after fructose ingestion, when the resulting absolute score was two or higher. Clinical symptoms in the 4 weeks preceding the breath test were arbitrarily considered to be relevant when score ≥2 was reported.
Statistical analysis was performed using SPSS 24 (IBM SPSS Statistics for Windows, version 24.0, released 2016, Armonk, NY: IBM Corp). The statistic to address the first primary end point was a multiple regression of global symptoms scores (in the 4 weeks preceding the breath test) on fructose malabsorption and sensitivity while adjusting for age and gender. Analysis of variance (ANOVA, F-test) and individual p values and r2 values for the predictor variables are reported. Following this analysis, global symptom scores were compared between malabsorbers and non-malabsorbers as well as between sensitive versus nonsensitive patients. For the second primary end point, the difference in individual symptom scores during the breath test from the respective baseline score was assessed by Mann–Whitney U test.
For secondary end point analysis, normality of data was assessed using Shapiro–Wilk test, confirmation of which permitted parametric statistical analysis (Student t test for unpaired observations) (comparing H2 peaks). Categorical data required nonparametric test statistics (Mann–Whitney U test). Significance of correlation was assessed by chi-squared correlation analysis. Furthermore, in patients with fructose malabsorption, a multiple regression of the time of H2 peak on the time of symptom peak (for significant symptoms determined during primary end point analysis) was performed in patients with fructose malabsorption; ANOVA (F-test) and individual p values and r2 values for significant predictor variables are reported, adjusted for age and gender.
P values <0.05 were considered significant in single comparisons and in ANOVA analysis. In instances of multiple comparisons (individual clinical symptoms in the preceding 4 weeks, time course of symptoms), a more conservative p value of <0.01 was considered to be significant. p-Values are reported when <0.05.
No sample size calculation was performed for this study, as it was based on an opportunistic sample of patients in the outpatient gastroenterology unit of St. Anna Kinderspital.
Results
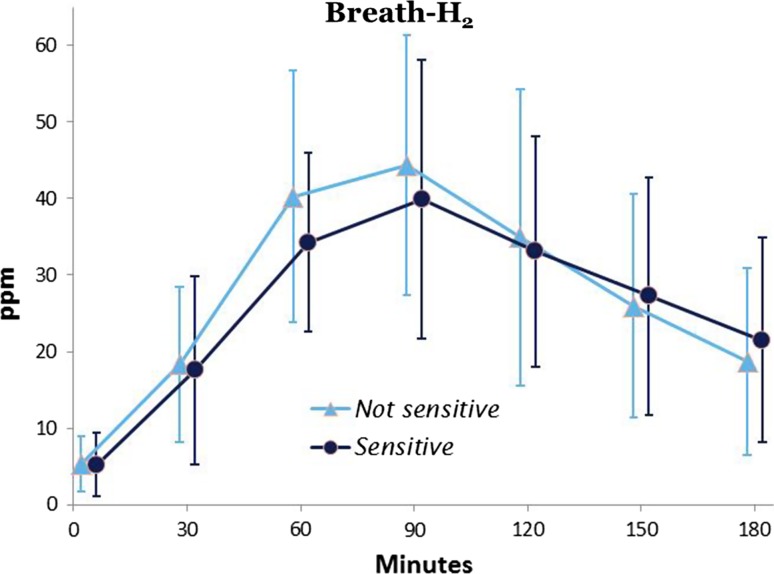
Patient characteristics are presented in Table 1. Mean breath H2 concentration at baseline was 5.89 ± 5.6 ppm. Baseline hydrogen value ≥10 ppm was observed in 17 breath tests, and consecutive test results were positive (malabsorption) in 6 (35 %) of these cases. Overall, 33 (40 %) children were classified as fructose malabsorbers; the mean H2-peak in the exhaled air reached 81.8 ± 4.6 min after fructose ingestion. Thirty-one (38 %) patients were classified as hypersensitive, of whom 15 (48 %) had fructose malabsorption (Table 2). The time course of breath H2 concentration during breath test was almost identical between fructose malabsorbers who were hypersensitive and malabsorbers with normal sensitivity (n = 16; Table 2; Fig. 1). There was no significant correlation between fructose malabsorption and hypersensitivity (NS).
Table 1.
Patient characteristics
| Female | Male | Overall | |
|---|---|---|---|
| Patients, n (%) | 47 (57 %) | 35 (43 %) | 82 (100 %) |
| Age, years (mean ± SEM) | 10.8 ± 3.3 | 10.1 ± 3.2 | 10.7 ± 3.5 |
| Minimum | 5.0 | 5.6 | 5.0 |
| Maximum | 17.6 | 17.1 | 17.6 |
| Clinical symptoms in 4 weeks preceding test (score ≥2) | |||
| Abdominal pain, n (%) | 20 (43 %) | 18 (51 %) | 38 (46 %) |
| Nausea, n (%) | 13 (28 %) | 12 (34 %) | 25 (31 %) |
| Meteorism, n (%) | 12 (26 %) | 6 (17 %) | 18 (22 %) |
| Flatulence, n (%) | 16 (34 %) | 13 (37 %) | 29 (35 %) |
| Diarrhea, n (%) | 8 (17 %) | 9 (26 %) | 17 (21 %) |
| Relevant symptoms on day of test (score ≥2) | |||
| Before fructose ingestion, n (%) | |||
| Abdominal pain, n (%) | 8 (17 %) | 3 (9 %) | 11 (13 %) |
| Nausea, n (%) | 5 (11 %) | 5 (14 %) | 10 (12 %) |
| Meteorism, n (%) | 9 (19 %) | 1 (3 %) | 10 (12 %) |
| Flatulence, n (%) | 4 (9 %) | 1 (3 %) | 5 (6 %) |
| Diarrhea, n (%) | 0 (0 %) | 1 (3 %) | 1 (1 %) |
| After fructose ingestion, n (%) | |||
| Abdominal pain, n (%) | 17 (36 %) | 17 (49 %) | 34 (41 %) |
| Nausea, n (%) | 10 (21 %) | 10 (29 %) | 20 (24 %) |
| Meteorism, n (%) | 14 (30 %) | 8 (23 %) | 22 (27 %) |
| Flatulence, n (%) | 5 (11 %) | 9 (26 %) | 14 (17 %) |
| Diarrhea, n (%) | 3 (6 %) | 4 (11 %) | 7 (9 %) |
Table 2.
Correlation of fructose malabsorption and fructose sensitivity
| Hypersensitivity | Normal sensitivity | Total | |
|---|---|---|---|
| Malabsorption | 15 | 18 | 33 |
| No malabsorption | 16 | 33 | 49 |
| Total | 31 | 51 | 82 |
Fig. 1.
End-expiratory breath hydrogen (ppm) over time in fructose malabsorbers who were fructose sensitive and not sensitive to fructose, respectively. Mean ± SEM values are shown
Questionnaire Validity
The BT-Q appeared to have strong face validity in that it was simple, easy to understand, and brief. The content validity ratio according to Lawshe [29] equaled 1. The three factors obtained were grouped as (A) intestinal gas (two variables: meteorism and flatulence; average loading: 0.59), (B) pain/nausea (two variables: pain and nausea: average loading 0.68), and (C) diarrhea (one variable; loading: 0.65). The significance according to Bartlett’s test of sphericity [30] was calculated to be <0.001. Correlation of items within factors was highly significant (p < 0.001) for both meteorism and flatulence (correlation coefficient 0.46) as well as for pain and nausea (0.53), hence convergent validity was supported. No item correlated more strongly with items of other factors than with items of its own factor, hence discriminant validity was supported [26]. Cronbach’s alpha was 0.71, indicating acceptable internal consistency. Additionally, the results obtained by the questionnaire (symptomatic versus not symptomatic) highly correlated with the results of physician’s interview in 19 pediatric subjects (p < 0.001; Fisher exact test).
The questionnaire used for determination of symptom scores in the preceding 4 weeks had acceptable internal consistency (Cronbach’s alpha: 0.74). The questionnaire correlated significantly with the result of physician’s interview in 16 pediatric subjects (p < 0.001; ρ = 0.45; Spearman correlation).
Clinical Symptoms
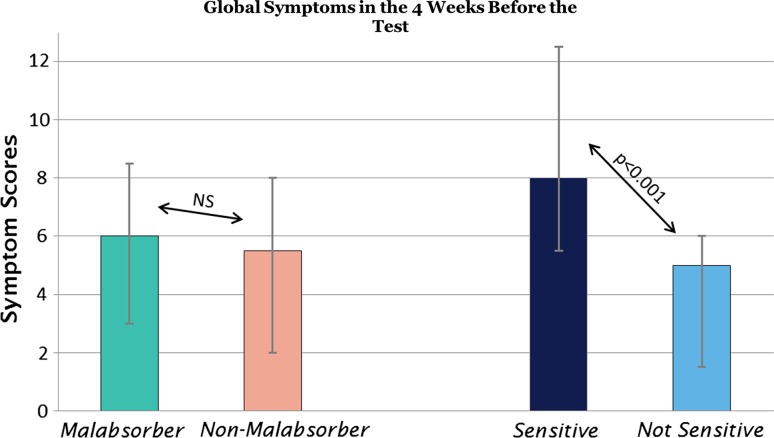
Gastrointestinal symptoms in the 4 weeks before the breath test are summarized in Table 1. Primary end point 1 Median global symptom scores in the 4 weeks before the breath test were 6.0 (25 %/75 %: 3.0/8.5) in fructose malabsorbers and 5.5 (2.0/8.0) in fructose absorbers (NS) (Fig. 2). In contrast, patients with sensitivity to fructose had a significantly higher total symptom score in the 4 weeks before the breath test (8.0; 5.5/12.5) compared with nonsensitive children (5.0; 1.5/6.0 p < 0.001; Fig. 2).
Fig. 2.
Global symptom scores in preceding 4 weeks reported by children and adolescents with functional abdominal pain disorders. Median ± 25 and 75 % quartiles are shown. NS, not significant
In a multiple regression analysis of global symptom scores on fructose malabsorption and sensitivity, overall a p value of <0.001 (ANOVA) (r2 = 0.25) was obtained. The effect of sensitivity on global symptom scores was highly significant (p < 0.001; r2 = 0.24), while there was no significant effect of malabsorption on global symptom scores (p = 0.51; r2 = 0.03).
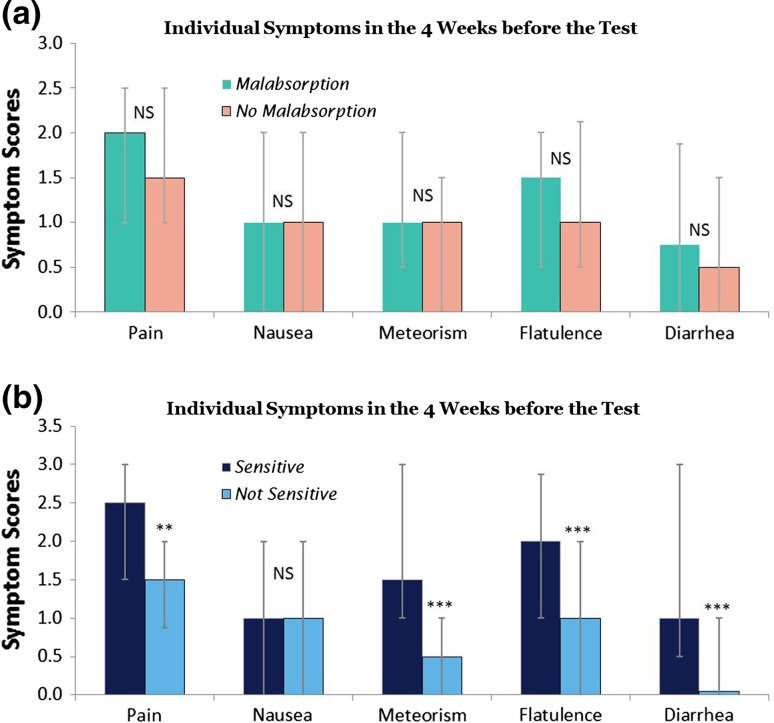
Secondary end point 1 No individual symptom score in the 4 weeks before the breath test was observed to significantly differ among fructose absorbers and malabsorbers (Fig. 3a). In contrast, pain (p < 0.01), meteorism (p < 0.001), flatulence (p < 0.001), and diarrhea (p < 0.001) were significantly more severe in fructose-sensitive patients compared with nonsensitive subjects, while nausea was not (NS) (Fig. 3b).
Fig. 3.
Severity of abdominal symptoms in preceding 4 weeks reported by children and adolescents with functional abdominal pain disorders, comparing a patients with and without fructose malabsorption, and b patients with or without sensitivity for fructose. Median ± 25 and 75 % quartiles are shown. p-value <0.01 was considered significant considering multiple comparisons
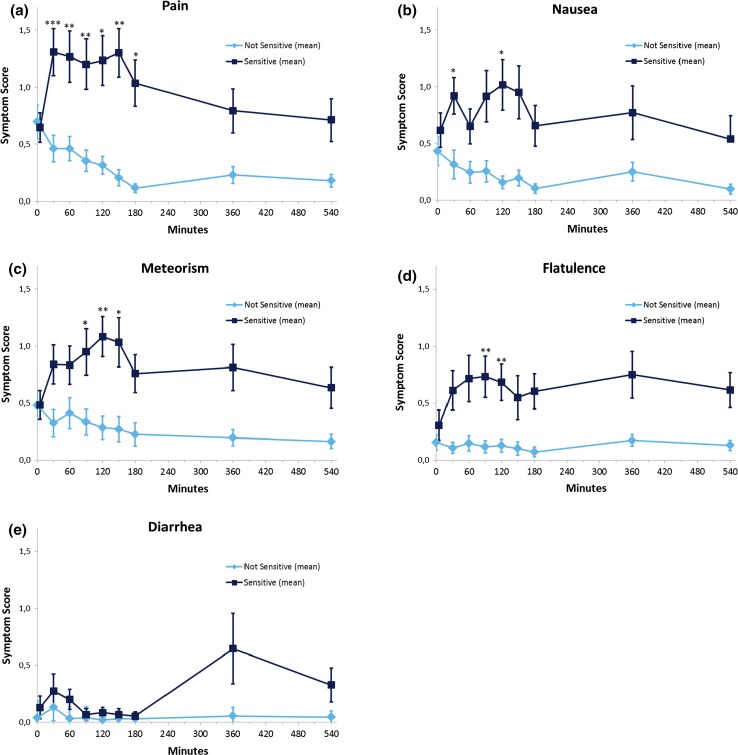
Timing of Symptoms After Fructose Ingestion (Fig. 4a–e)
Fig. 4.
Time course of individual symptoms during fructose breath test in children and adolescents with functional abdominal pain disorders with or without sensitivity to fructose. Mean ± SEM values are shown. p-Value <0.01 [difference of symptoms compared with baseline (0 min)] was considered significant considering multiple comparisons. Asterisks denote p value <0.05 (*), <0.01 (**), and <0.001 (***)
Before fructose ingestion, scores of each individual symptom were comparable in sensitive and nonsensitive patients (NS for all symptoms).
Primary end point 2 in sensitive patients: Compared with the pain scores at baseline, pain scores increased significantly (p values <0.01) at 30 min and stayed high until minute 150 (Fig. 4a). Pain resolved thereafter and did not recur. In contrast, meteorism scores started to rise over baseline at minute 90 and reached a significant level 120 min after fructose ingestion (Fig. 4c). Significant flatulence was reported 90–120 min after fructose ingestion (Fig. 4d). Nausea and diarrhea scores were not significantly different from baseline (p values >0.01) during the 9-h observation period (Fig. 4e).
The same analysis in the group of nonsensitive as well as malabsorbers and non-malabsorbers did not demonstrate any significant rise in symptoms over baseline during the whole observation period (data not shown).
Comparing each individual symptom in the sensitive and nonsensitive groups, pain and flatulence remained significantly higher (p < 0.01) in the sensitive group during the observation period starting at 30 min to 9 h after fructose ingestion, meteorism remained significant (p < 0.01) starting at 90 min, and nausea was significantly higher during the whole study period except at 60 min. Diarrhea did not reach significance during the whole study period.
No significant difference in symptom scores was found in malabsorbers and non-malabsorbers at any point in time (data not shown).
Secondary end point 2 In patients with malabsorption (no matter whether sensitive or nonsensitive), the time of peak H2 exhalation correlated significantly with the time of flatulence peak (p = 0.009, r2 = 0.28) but not with other symptom peaks.
Discussion
Clinical experience indicates a sometimes dramatic improvement of abdominal symptoms in patients with functional abdominal symptoms after changing the diet to eliminate fructose-containing food [4]. This improvement has been interpreted by a number of researchers to indicate a role of fructose malabsorption in the genesis of abdominal symptoms similar to the role of lactose malabsorption in abdominal symptom provocation [31]. However, a fructose-restricted diet improves abdominal symptoms independent of presence or absence of fructose malabsorption [4, 22], and the association between abdominal symptoms after fructose challenge and malabsorption is poor [21]. Although our results strongly confirm previous observations of a higher rate of symptoms in fructose malabsorbers than in non-malabsorbers after fructose challenge, there was no discernible association between malabsorption and sensitivity to fructose. Malabsorption was also not associated with clinical symptoms. In contrast, clinical symptoms correlated significantly with sensitivity to fructose challenge. To obtain valid information on abdominal symptoms in a standardized manner, we developed a specific questionnaire suitable for children that also allowed a demonstration of the time course of individual symptoms after fructose challenge and revealed pain, meteorism, and flatulence as relevant symptoms after fructose ingestion.
The study population was a selected group of patients with functional abdominal pain disorders [3] whose history was considered indicative of a possible association of abdominal symptoms with ingestion of fructose. This is a distinct patient group that constitutes a large proportion of patients undergoing fructose breath test in clinical practice but that does not allow generalization of the results to all patients with idiopathic functional abdominal pain. Evaluation of fructose malabsorption has been established in many gastrointestinal function laboratories despite its controversial relevance in clinical practice [10]. In our sample, 40 % malabsorbed fructose and an equal number (38 %) were symptomatic after fructose ingestion; however, less than half of malabsorbers (46 %) were sensitive to fructose, and less than half of sensitive patients (48 %) malabsorbed fructose. Overall, there was no relationship between malabsorption and sensitivity to fructose, thus confirming previous studies showing poor (or missing) correlation between fructose malabsorption and symptom induction by fructose [4, 21].
Our findings also confirm a higher proportion of malabsorbers reporting fructose-induced symptoms than non-malabsorbers [10, 21]. However, this cannot be considered as evidence for clinical relevance of fructose malabsorption per se. Global symptom scores and individual symptoms were equal in malabsorbers and absorbers; in contrast, when global symptoms were compared in patients with sensitivity to fructose versus patients without substantiated sensitivity, fructose-sensitive patients stood out with significantly higher symptom scores. Pain, meteorism, flatulence, and diarrhea were significantly more pronounced in fructose-sensitive patients. Thus, while fructose ingestion indeed was correlated with clinical symptoms, it appears that induction of symptoms is clinically more relevant than malabsorption as such. Furthermore, fructose malabsorption was equally common in irritable bowel syndrome (IBS) and controls, but symptom induction was considerably higher in IBS [16, 32].
We use the term “sensitivity to fructose” instead of the commonly used term “fructose intolerance.” There is confusion regarding the term “fructose intolerance,” since it has often been used indiscriminately in the context of fructose malabsorption, encompassing both malabsorption and fructose-induced symptoms. Moreover, a disease called hereditary fructose intolerance, which has a completely different pathophysiological basis, adds to confusion regarding the term “fructose intolerance.” As data accumulate that suggest that symptoms induced by fructose ingestion are mainly due to visceral hypersensitivity, we consider “sensitivity to fructose” appropriate. “Fructose hypersensitivity” might be a somewhat less cumbersome alternative that also expresses the link to visceral hypersensitivity, which is an established and well-defined term in functional gastrointestinal research [33].
Recording abdominal symptoms related to carbohydrate consumption during breath test is considered important [13]. Because no valid instrument existed to quantify symptoms relevant for carbohydrate malabsorption before and during carbohydrate challenge in children/adolescents, we developed a child-oriented questionnaire for evaluation of specific symptoms during breath test. The initial validation affirmed the questionnaire to be a valid measure for standard assessment of the type and severity of gastrointestinal symptoms during breath test. Moving forward, data on larger samples will be necessary to allow final evaluation of this questionnaire. A related questionnaire that assessed clinical symptoms in the month before breath test also had good test statistics in this initial validation.
Pain was reported early after fructose ingestion and continued for nearly the entire 3-h breath test. During the rest of the day, pain was not a significant problem over baseline. Fructose ingestion is known to induce early water inflow into the small intestine [6]. Arrival of up to 25 g fructose (corresponding to 139 mmol) in the small intestine may cause inflow of up to 479 ml water into the intestinal lumen to ensure osmotic equilibrium between the bloodstream and intestinal lumen. This fluid load may result in bowel distension that may excite mechanoreceptors in hypersensitive patients. Mechanical distension of the upper gastrointestinal tract in response to a meal has long been implicated in the induction of symptoms [34]. Although a recent study did not find evidence that fructose generated symptoms directly through small-bowel distension, that study was underpowered and thus did not allow a sound conclusion [6].
Meteorism and flatulence were reported during a fraction of the observation time after peak H2 excretion, (90 min to) 120 min after fructose ingestion. If we assume that both meteorism and flatulence result from gas production, the time of symptom occurrence may be explained by human physiology: gas production arises after unabsorbed fructose is fermented by colonic bacteria, a process that results in production of gases such as H2, CH4, and CO2. Our data suggest a strong association between the time of flatulence and colonic arrival of unabsorbed fructose, although malabsorbers did not experience higher flatulence scores than non-malabsorbers. It cannot be deduced from our results whether this absent relation reflects failed detection of malabsorption by measuring breath H2 concentration only. From a physiological standpoint, we would have expected that development of meteorism would precede flatulence; instead, meteorism and flatulence were almost concurrent. As the commencement of flatulence correlated with the peak H2 exhalation, it might be argued that colonic gas clearance in the distal colon may be initiated by the volume load arriving in the proximal colon. Noteworthily, excessive colonic clearance of stool (in the form of diarrhea) was not observed, as significant diarrhea was not detected during the study period.
Nausea is a common symptom in the pediatric population [35]. Despite nausea not being regularly considered a symptom of carbohydrate malabsorption [13], pediatricians and patients/parents requested that nausea be incorporated in the symptom questionnaire, and nearly one-third of patients complained of nausea before the test. However, nausea as a clinical symptom in the preceding month did not differ between malabsorbers/non-malabsorbers or between sensitive/nonsensitive patient groups. Nausea did not significantly increase over baseline after fructose challenge in all patient groups, although fructose-sensitive patients had significantly higher nausea scores than nonsensitive patients during nearly the entire observation period.
While the diagnosis of malabsorption might have been impaired in the fraction of patients with high baseline hydrogen exhalation (≥10 ppm), the H2 analysis was based on widely accepted standards [12]; patients with baseline H2 exhalation above a preset level (≥15 ppm) were not tested, to exclude patients with small intestinal bacterial overgrowth. We did not measure methane (CH4) in the exhaled air to detect non-hydrogen-producing malabsorbers [36]. Quantification of this additional gas in exhaled breath may have increased the number of malabsorbers detected. Thus, we cannot exclude that CH4 measurement of these parameters would have changed our conclusion regarding the correlation between symptoms and malabsorption. The lack of an association of symptoms of gas production (meteorism, flatulence) and malabsorption may argue for missing patients with malabsorption. However, the proportion of patients with isolated elevation of CH4 is small, and a combined measurement of H2 and CH4 showed comparable results in an adult population with respect to a poor association between malabsorption and clinical symptoms [10, 22]. In our experience in 36 unselected pediatric patients (61 % malabsorbers), no additional malabsorbers were detected by isolated CH4 elevation [unpublished data].
Evaluation of a fructose elimination diet in patients with fructose malabsorption versus patients with sensitivity to fructose was not part of the present study. A high proportion of patients with functional bowel disorders benefit from fructose restriction, independent of presence or absence of malabsorption [4]. A short-term diet that contains no fructose and low amounts of other indigestible or poorly absorbable carbohydrates [low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet] decreases abdominal pain frequency in a pediatric population. However, such a restricted diet may lead to nutritional deficits in children and has not been studied long term. Follow-up studies comparing the effect of a fructose-free diet in malabsorbers and sensitive patients will require large patient samples to substantiate differences between groups.
In summary, we developed a novel instrument to assess symptoms before and during carbohydrate breath test in a pediatric population. Our data suggest that visceral hypersensitivity, rather than malabsorption per se, is correlated with symptoms. Obtaining information on fructose malabsorption using the H2 breath test did not provide any valuable clinical information regarding the role of fructose ingestion in clinical symptom severity. Careful assessment of symptoms after fructose challenge using a validated instrument may enable assessment of the pathophysiological role of fructose in individual patients. In this context, a fructose challenge may be considered another test for visceral hypersensitivity, like balloon distension studies [37], nutrient challenge [38], or capsaicin ingestion [39].
Acknowledgments
Open access funding provided by Medical University of Vienna.
Author’s contributions
K.H., V.H.—acquisition, administration, and analysis of data; administrative support; revision of the manuscript. N.M., W.-D.H.—acquisition of data; administrative, technical, or material support; revision of the manuscript. K.H.—study design; study supervision; acquisition of data; administrative, technical, or material support; critical revision of the manuscript for important intellectual content. J.H.—study concept and design; study supervision; statistical analysis; interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Compliance with ethical standards
Conflict of interest
No conflicts of interest exist.
Provision of questionnaires
For studies without financial support from industrial sponsors, we provide the questionnaires free of charge.
Footnotes
Veronika Hammer and Katharina Hammer contributed equally to this work.
A correction to this article is available online at https://doi.org/10.1007/s10620-018-5046-z.
References
- 1.Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol. 2005;100:1868–1875. doi: 10.1111/j.1572-0241.2005.41893.x. [DOI] [PubMed] [Google Scholar]
- 2.Lewis ML, Palsson OS, Whitehead WE, et al. Prevalence of functional gastrointestinal disorders in children and adolescents. J Pediatr. 2016;177:e3. doi: 10.1016/j.jpeds.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 3.DiLorenzo C, Hyams J, Saps M, et al. et al. Childhood functional gastrointestinal disorders, child/adolescent. In: Drossman D, Chang L, Chey W, et al.et al., editors. Rome IV, Functional Gastrointestinal Disorders, Disorders of Gut-Brain Interaction. Raleigh, NC: The Rome Foundation; 2016. pp. 1297–1371. [Google Scholar]
- 4.Youssef NN, Murphy TG, Langseder AL, Rosh JR. Quality of life for children with functional abdominal pain: a comparison study of patients’ and parents’ perceptions. Pediatrics. 2006;117:54–59. doi: 10.1542/peds.2005-0114. [DOI] [PubMed] [Google Scholar]
- 5.Zeiter DK. Abdominal pain in children: from the eternal city to the examination room. Pediatr Clin North Am. 2017;64:525–541. doi: 10.1016/j.pcl.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Chumpitazi BP, Shulman RJ. Dietary carbohydrates and childhood functional abdominal pain. Ann Nutr Metab. 2016;68:8–17. doi: 10.1159/000445390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomara RE, Halata MS, Newman LJ, et al. Fructose intolerance in children presenting with abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:303–308. doi: 10.1097/MPG.0b013e318166cbe4. [DOI] [PubMed] [Google Scholar]
- 8.Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110–119. doi: 10.1038/ajg.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Major G, Pritchard S, Murray K, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology. 2017;152:124–133. doi: 10.1053/j.gastro.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 10.Hammer HF, Hammer J. Diarrhoea caused by carbohydrate malabsorption. Gastroenterol Clin North Am. 2012;41:611–627. doi: 10.1016/j.gtc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Braden B. Methods and functions: breath tests. Best Pract Res Clin Gastroenterol. 2009;23:337–352. doi: 10.1016/j.bpg.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29:1–49. doi: 10.1111/j.1365-2036.2009.03951.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones HF, Butler RN, Moore DJ, Brooks DA. Developmental changes and fructose absorption in children: effect on malabsorption testing and dietary management. Nutr Rev. 2013;71:300–309. doi: 10.1111/nure.12020. [DOI] [PubMed] [Google Scholar]
- 14.Yao CK, Tuck CJ, Barrett JS, Canale KE, Philpott HL, Gibson PR. Poor reproducibility of breath hydrogen testing: implications for its application in functional bowel disorders. United Eur Gastroenterol J. 2017;5:284–292. doi: 10.1177/2050640616657978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usai Satta P, Anania C, Astegiano M, Miceli E, Montalto M, Tursi A. H2-breath testing for carbohydrate malabsorption. Aliment Pharmacol Ther. 2009;29:14–18. doi: 10.1111/j.1365-2036.2009.03951.x. [DOI] [PubMed] [Google Scholar]
- 16.Glatstein M, Reif S, Scolnik D, Rom L, Yerushalmy-Feler A, Dali-Levy M, et al. Lactose breath test in children: relationship between symptoms during the test and test results. Am J Ther. 2016; [Epub ahead of print]. [DOI] [PubMed]
- 17.Hoekstra JH, van-Kempen AA, Kneepkens CM. Apple juice malabsorption: Fructose or sorbitol. J Pediatr Gastroenterol Nutr. 1993;16:39–42. doi: 10.1097/00005176-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Rumessen JJ, Gudmand-Hoyer E. Functional bowel disease: malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology. 1988;95:694–700. doi: 10.1016/S0016-5085(88)80016-7. [DOI] [PubMed] [Google Scholar]
- 19.Hoekstra JH, van Kempen AA, Bijl SB, Kneepkens CM. Fructose breath hydrogen tests. Arch Dis Child. 1993;68:136–138. doi: 10.1136/adc.68.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kneepkens CM, Vonk RJ, Fernandes J. Incomplete intestinal absorption of fructose. Arch Dis Child. 1984;59:735–738. doi: 10.1136/adc.59.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg LK, Fagerli E, Martinussen M, Myhre AO, Florholmen J, Goll R. Effect of fructose-reduced diet in patients with irritable bowel syndrome, and its correlation to a standard fructose breath test. Scand J Gastroenterol. 2013;48:936–943. doi: 10.3109/00365521.2013.812139. [DOI] [PubMed] [Google Scholar]
- 22.Wirth S, Klodt C, Wintermeyer P, et al. Positive or negative fructose breath test results do not predict response to fructose restricted diet in children with recurrent abdominal pain: results from a prospective randomized trial. Klin Padiatr. 2014;226:268–273. doi: 10.1055/s-0034-1383653. [DOI] [PubMed] [Google Scholar]
- 23.Gibson PR, Newnham E, Barrett JS, Shepherd SJ, Muir JG. Review article: fructose malabsorption and the bigger picture. Aliment Pharmacol Ther. 2007;25:349–363. doi: 10.1111/j.1365-2036.2006.03186.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37:1074–1083. doi: 10.1111/apt.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyams JS, DiLorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2016;150:1456–1468. doi: 10.1053/j.gastro.2016.02.015. [DOI] [Google Scholar]
- 26.Di Lorenzo C, Colletti RB, Lehmann HP, et al. Chronic abdominal pain in children: a technical report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:249–261. doi: 10.1097/01.MPG.0000154661.39488.AC. [DOI] [PubMed] [Google Scholar]
- 27.Di Lorenzo C, Colletti RB, Lehmann HP, et al. Chronic abdominal pain in children: a clinical report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:245–248. doi: 10.1097/01.MPG.0000155367.44628.21. [DOI] [PubMed] [Google Scholar]
- 28.Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull. 1959;56:81–105. doi: 10.1037/h0046016. [DOI] [PubMed] [Google Scholar]
- 29.Lawshe CH. A quantitative approach to content validity. Pers Psychol. 1975;28:563–575. doi: 10.1111/j.1744-6570.1975.tb01393.x. [DOI] [Google Scholar]
- 30.Field A. Discovering Statistics using SPSS for Windows. London: Sage; 2000. [Google Scholar]
- 31.Heyman MB. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118:1279–1286. doi: 10.1542/peds.2006-1721. [DOI] [PubMed] [Google Scholar]
- 32.Rao SS, Attaluri A, Anderson L, Stumbo P. Ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol. 2007;5:959–963. doi: 10.1016/j.cgh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado-Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39:S194–S203. doi: 10.1097/01.mcg.0000156114.22598.1b. [DOI] [PubMed] [Google Scholar]
- 34.Cecil JE, Francis J, Read NW. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behavior. Physiol Behav. 1999;67:299–306. doi: 10.1016/S0031-9384(99)00069-4. [DOI] [PubMed] [Google Scholar]
- 35.Kovacic K, Di Lorenzo C. Functional nausea in children. J Pediatr Gastroenterol Nutr. 2016;62:365–371. doi: 10.1097/MPG.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 36.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corsetti M, Gevers AM, Caenepeel P, Tack J. The role of tension receptors in colonic mechanosensitivity in humans. Gut. 2004;53:1787–1793. doi: 10.1136/gut.2004.038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53:1445–1451. doi: 10.1136/gut.2003.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Führer M, Vogelsang H, Hammer J. A placebo-controlled trial of an oral capsaicin load in patients with functional dyspepsia. Neurogastroenterol Motil. 2011;23:918-e397. doi: 10.1111/j.1365-2982.2011.01766.x. [DOI] [PubMed] [Google Scholar]