Abstract
Extracts prepared from young leaves of Pea (Pisum sativum), tobacco (Nicotiana tabacum), rape (Brassica napus), and spinach (Spinacia oleracea) all contained ATP:citrate lyase (ACL) activity, which was most active in rape leaflets (130 nmol min−1 g fresh weight). In rape and spinach, ACL activity was predominantly localized in the plastids (between about 78% and 90% of the total activity), whereas in pea and tobacco, distribution was mainly cytosolic (about 85% and 78%, respectively, of the total). These distributions were calculated from the relative distributions of plastid and cytosol marker enzymes. Cross-reactivity between plant and rat ACL antibody was carried out by immunoblot analysis and, in rape and spinach, showed that a 120-kD protein, presumably indicating homomeric ACL proteins, was present in both cytosolic and plastidic fractions. In pea, two cross-reacting proteins were detected, the major material being in the cytosol fraction. Therefore, ACL occurs both in the cytosol and plastids of higher plants, but the distribution of activity changes according to the species. The plastidic ACL is proposed to function for the supply of acetyl-coenzyme A for lipid biosynthesis de novo, whereas the cytosolic ACL may provide acetyl-coenzyme A for the mevalonate pathway or fatty acid elongation.
The plastid is considered to be the major site of fatty acid synthesis de novo in plant cells. All of the carbon atoms found in a fatty acid are derived from acetyl-coenzyme A (acetyl-CoA). The concentration of acetyl-CoA in plastids has reported to be only 30 to 50 μm, which is sufficient to supply the needs of fatty acid synthesis for only a few seconds (Post-Beittenmiller et al., 1992). Thus, cells must have systems that rapidly synthesize acetyl-CoA for metabolic pathways. The conventional view is that the action of acetyl-CoA synthetase acting on free acetate (Stumpf, 1984) and/or plastidial pyruvate dehydrogenase on pyruvate (Camp and Randall, 1985) produces within the plastid sufficient acetyl-CoA for fatty acid synthesis. In addition, malate and Glc-6-P have also been proposed as precursors of the plastid acetyl-CoA pool (Smith et al., 1992; Kang and Rawsthorne, 1994). Whether these pathways produce sufficient acetyl-CoA to meet the demand of fatty acid synthesis has been questioned on several grounds (Ohlrogge et al., 1993). A consensus has not yet been reached about the sources of acetyl-CoA. What is clear at this point is that the acetyl-CoA almost certainly is synthesized in the plastids, since current opinion would suggest that acetyl-CoA cannot move between subcellular compartments because of its size (Kohlhaw and Tan-Wilson, 1977; Patel and Clark, 1980).
In animals (Elshourbagy et al., 1990) and oleaginous yeast and fungi (Ratledge and Evans, 1989), citrate is considered to be the precursor of acetyl-CoA by the action of ATP:citrate lyase (ACL; EC 4.1.3.8) in the cytosol. In addition, inhibition of this enzyme by (−)-hydroxycitrate, a specific inhibitor of ACL, decreases fatty acid synthesis dramatically in a variety of tissues (Sullivan et al., 1973). ACL catalyzes the reaction:
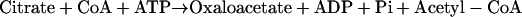 |
In lipid-accumulating yeasts, ACL has been regarded as the rate-limiting reaction in lipid biosynthesis (Boulton and Ratledge, 1983; Evans and Ratledge, 1985a) because: (a) its activity parallels the rate of fatty acid synthesis, and (b) the substrate for ACL, i.e. citrate, physically accumulates in the cytosol during lipogenesis. This buildup of citrate might then initiate “secondary controls” of lipid accumulation by restrictive flow of carbon (from Glc) to pyruvate through glycolysis (Ratledge and Evans, 1989). In addition, the activation of acetyl-CoA carboxylase by citrate ensures that acetyl-CoA generated in the ACL reaction is efficiently utilized for lipid synthesis (Evans and Ratledge, 1985b).
Our interest in ACL in plants has arisen as a consequence of biochemical studies of lipid accumulation in oleaginous microorganisms (Ratledge and Evans, 1989; Ratledge, 1997). Previous work in our laboratory (Ratledge et al., 1997) demonstrated that the increased activity of ACL in rape (Brassica napus) correlated positively with the increased rate of lipid synthesis in developing seeds. However, the occurrence, subcellular location, and role of ACL in plants is less well understood than in animals or yeast. Thus, with the aim of determining the location of ACL, we used rat ACL antibody with different plant tissues and compared four different plant species for their subcellular distribution of ACL. All of the experiments were performed on leaves rather than other parts of plants to minimize differences in activities in different cell types. We describe the subcellular compartmentalization of ACL in plant tissues.
MATERIALS AND METHODS
Materials
Pea (Pisum sativum L. cv Hurst Greenshaft), tobacco (Nicotiana tabacum L. cv White Burley), rape (Brassica napus L. cv Weber), and spinach (Spinacia oleracea L. cv Tiradea) were grown at 20°C in a soil-based compost under natural light supplemented with artificial light to give a minimum photon flux of 100 μmol m−2 s−1 for the 16-h photoperiod. All of the experiments were done with newly emerging, uppermost, still-expanding leaves from 14- to 16-d-old plants.
Preparation of Extracts
Crude extracts were prepared as detailed by Ratledge et al. (1997): Fresh tissues, approximately 1 g, were homogenized in 5 mL of 0.1 m KH2PO4, pH 7.2, 50 mm NaF, 1 mm MgCl2, 2 mm 1,1,1,-trichloro-2,2-bis(p-chlorophenyl)ethane (DTT), 1 mm phenylmethylsulfonyl fluoride (PMSF), and 2 mm p-aminobenzamidine as protease inhibitors. Cell debris were removed by centrifugation at 2,400g for 4 min, and the resulting supernatant was re-centrifuged at 30,000g for 15 min. The final supernatant was dialyzed twice for 1 h at 4°C against 500 mL of 5% (v/v) glycerol in distilled water. DTT and p-aminobenzamidine were immediately added to the retentate to give 2 and 1 mm, respectively. The enzyme extract was either used directly or stored for no longer than overnight at −20°C before use.
Subcellular Fractionation
Leaves, approximately 2 g, were cut into small pieces and gently homogenized in 10 mL of buffer containing 0.33 m Suc, 25mm 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES), and 2 mm EDTA, pH 7.6. For pea leaves, the grinding buffer containing 0.33 m Suc, 25 mm HEPES, 0.2% (v/v) polyvinylpyrrolidone, 0.06% (w/v) bovine serum albumin (BSA), and 5 mm EDTA to minimize adhesion of mitochondria and chloroplasts after cell breakage (Lernmark and Gardeström, 1994). All leaf homogenates were filtered through three layers of Miracloth (Calbiochem, San Diego) and centrifuged at 200g for 2 min at 4°C to remove cell debris. The supernatant was re-centrifuged at 3,000g for 2 min to give an enriched chloroplast pellet. The resulting supernatant, after re-centrifugation for 3 min at 13,000g to pellet the mitochondria, was used as the cytosolic fraction. The intact chloroplasts were further purified from an enriched chloroplast pellet using 10% to 80% (v/v) Percoll gradient centrifugation as described by Robinson and Barnett (1988). The purified fractions were resuspended in 50 mm Tris/HCl, pH 7.8, 0.1 mm EDTA, 1 mm MgCl2, 2 mm DTT, and 1 mm p-aminobenzamidine, freeze-thawed once in liquid N2, and centrifuged briefly before being assayed. In some cases, crude extract was de-salted through a PD-10 column (Pharmacia Biotech, Piscataway, NJ) before assay. Protein content was determined by the Bradford (1976) method using γ-globulin as a standard.
Enzyme Assays
Marker enzyme activities were assayed at 30°C: phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31) was measured as a cytosolic marker according to the method of Wedding and Kline (1994); NADP-glyceraldehyde-3-P dehydrogenase (GAPDH; EC 1.2.1.13) (Entwistle and ap Rees, 1988), and ribulose-1,5-bisphosphate (RuBP) carboxylase (EC 4.1.1.39) (Anderson, 1975) were assayed as chloroplast markers. Fumarase (EC 4.2.1.2) was measured as a mitochondrial marker, as described by Hatch (1978). Intactness of the chloroplasts was estimated by measuring the latent activity of the stroma marker enzyme, NADP-GAPDH, in an intact sample (with the addition of 330 mm sorbitol to the assay buffer) and in a lysed sample in which the plastids had been disintegrated by brief ultrasonication prior to measurement (Entwistle and ap Rees, 1988).
ACL activity was measured in both the cytosolic and plastidic fractions as described by Elshourbagy et al. (1990) using the malate-dehydrogenase-dependent coupled spectrophotometric method. The assay mixture contained 50 mm Tris/HCl, 0.2 mm NADH, 10 mm MgCl2, 10 mm KCl, 5 mm dithiothreitol, 200 μm CoA, 5 mm ATP, and 1, 2, or 3 mg of protein from the cytosolic or plastid fraction. The enzyme was pre-incubated with 10 mm mercaptoethanol for 5 to 10 min before assay. Prior to the start of reaction, the background activity was monitored for 5 min until it was close to zero. The assay was usually initiated by the addition of CoA. In the case of crude extract of enzymes, the hydroxylamine method of Kaethner and ap Rees (1985) was preferred due to the interference of crude extracts with the coupled spectrophotometric assay. All assays were carried out at 30°C.
Immunoblot Analysis
For immunoblot analysis, proteins were extracted as detailed above. Protein solutions were mixed with SDS sample buffer, resolved by 8% (w/v) SDS-PAGE as described by Laemmli (1970), and then transferred to nitrocellulose, using the Transblot system (Bio-Rad Laboratories, Hercules, CA), in 25 mm Tris/HCl, pH 8.3, 192 mm Gly in 20% (v/v) methanol. Transferred protein was confirmed by reversibly staining with Ponceau S solution (Sigma, St. Louis). The filter was then blocked in Tris/buffered saline (10 mm Tris and 0.9% [w/v] NaCl) supplemented with 0.05% (w/v) Tween 20 (TBST) and 2.5% (w/v) BSA for 16 h at 4°C. Blotted protein was detected with rat anti-ACL polyclonal antibody in a dilution of 1:1,000 in TBST containing 1% (w/v) BSA for 1 h at room temperature. After washing the filter four times in TBST, 10 min each, it was incubated with alkaline phosphatase-conjugated secondary antibody (1:10,000) in TBST and 1% (w/v) BSA for 1 h. The filter was then washed four times in TBST and visualization was performed with 5-bromo-4-chloro-3-indoylphosphate/nitroblue tetrazolium (FAST, Sigma) until color formation.
The rat anti-ACL antibody was a kind gift from Dr. Elshourbagy (SmithKline Beecham, Philadelphia). This antibody had been raised against purified rat ACL, and western blots carried out in our laboratory confirmed that it reacted only with ACL expressed in Escherichia coli, not with E. coli extracts without ACL (E. coli itself does not produce ACL). The E. coli strain BL21 harboring the rat ACL gene was kindly supplied by Dr. C. Southern (SmithKline Beecham, Stevenage, UK).
RESULTS AND DISCUSSION
Occurrence of ACL
Because of the presence of high NADH oxidase activity in the crude extracts, which interfered with the coupled spectrophotometric assay, ACL activity was initially measured in the crude cell-free extracts prepared from expanding leaflets of 14-d-old seedlings using the hydroxylamine assay (Kaethner and ap Rees, 1985) Plant ACL was highly unstable, similar to mammalian or yeast ACL enzymes (Boulton and Ratledge, 1983), and an almost complete loss of activity occurred at 4°C within 24 h of extraction, even in the presence of stabilizers such as glycerol, DTT, and citrate (Fritsch and Beevers, 1979).
ACL activity was dependent upon each of its substrates: CoA, ATP, MgCl2, and citrate (Table I). Crude extracts of rape appeared to have a small amount of a bacterial-type citrate lyase enzyme that was independent of ATP and CoA (Table I). A similar observation was reported by Nelson and Rinne (1977) for developing soybean and for sweet potato by Takeuchi et al. (1981). Maximum ACL activity was in young leaves of rape and was approximately 2- to 3-fold higher than the ACL activity in tobacco and spinach leaves (Table II). Following this demonstration of ACL activity in a range of plants, we investigated further the intracellular location of ACL in cell extracts of the different species.
Table I.
Substrate dependency of ACL from rape leaflets
| System | NADH Oxidation
|
|
|---|---|---|
| Pre-incubationa | After additionb | |
| nmol min−1 mg protein | ||
| Complete | 6.8 | 23.6 |
| Citrate | No activity | 22.1 |
| ATP | 5.4 | 17.7 |
| MgCl2 | No activity | 18.6 |
| MDHc | No activity | 16.4 |
A crude enzyme extract from rape leaflets was prepared using homogenization buffer without citrate. Enzyme activity was assayed by the coupled spectrophotometric method using malate dehydrogenase (see “Materials and Methods”).
Pre-incubation was without CoA and without each of the components indicated. After the addition of CoA, the amount of NADH oxidized (from conversion of oxaloacetate to malate) without each substrate was monitored spectrophotometrically.
The component omitted from the complete system was added and the reaction started by the addition of CoA.
MDH, Malate dehydrogenase.
Table II.
Occurrence of ACL in extracts of expanding young leaves of different plants
| Plant | Specific Activity | Total Activitya |
|---|---|---|
| nmol min−1 mg protein | nmol min−1 g fresh wt | |
| Pea | 11.2 | 94 |
| Tobacco | 5.4 | 19 |
| Spinach | 19 | 68 |
| Rape | 28 | 130 |
Total ACL activity was calculated by multiplying the specific activity of ACL with the amount of total protein extracted per gram fresh weight of each leaf sample.
Chloroplast Preparation and Fractionation
Percoll density gradient centrifugation was used to isolate chloroplasts that, judging from the activities of the stroma marker enzyme NADP-GADPH in the intact and lysed (cytosolic) fractions (see Table III), were typically between 88% and 80% intact. Activities of the other stroma marker enzyme, RuBP carboxylase, indicated that a somewhat higher breakage of plastids had occurred. The degree of cross-contamination between the chloroplasts and cytosol fractions was indicated from the distribution of PEPC activity, which should have been wholly cytosolic. Pea and tobacco preparations of plastids picked up very little (approximately 6%) PEPC activity, but spinach and rape had more contamination (12% and 20%; see Table III). The purity of the fractions could not be improved even after repeating the experiments at least three times or following the improved methods developed by Quick et al. (1995). Treatment of the isolated chloroplasts with thermolysin (see Rangasamy and Ratledge, 2000) did not affect ACL activity, indicating that the enzyme had not spuriously bound to the plastids during isolation.
Table III.
Distribution of ACL and marker enzymes in cytosol and plastids of different species
| Species | Organelle | Protein | Enzyme Activitya
|
|||
|---|---|---|---|---|---|---|
| RuBC | NADP-GAPDH | PEPC | ACL | |||
| mg−1 fresh wt leafb | nmol min−1 mg−1 organelle protein (% distribution) | |||||
| Pea | Cytosol | 3.2 | 5 ± 3 (24) | 30 ± 8 (12) | 42 ± 16 (94) | 21.4 ± 2 (79) |
| Plastid | 2.0 | 25 ± 3 (76) | 358 ± 42 (88) | 4.4 ± 1 (6) | 9.3 ± 1 (21) | |
| Tobacco | Cytosol | 3.8 | 12 ± 8 (19) | 34 ± 7 (21) | 38 ± 9 (94) | 5.3 ± 0.8 (72) |
| Plastid | 2.2 | 87 ± 14 (81) | 225 ± 18 (79) | 4.2 ± 1 (6) | 3.5 ± 1.2 (28) | |
| Spinach | Cytosol | 3.3 | 17 ± 5 (33) | 123 ± 27 (22) | 14.2 ± 2 (88) | 15.4 ± 1.6 (39) |
| Plastid | 2.3 | 49 ± 11 (67) | 643 ± 52 (78) | 2.8 ± 0.3 (12) | 34.6 ± 9 (61) | |
| Rape | Cytosol | 5.6 | 12 ± 2 (29) | 18.9 ± 6 (20) | 21.3 ± 4.6 (80) | 13.6 ± 3 (37) |
| Plastid | 3.6 | 46 ± 9 (71) | 114 ± 21 (80) | 8.1 ± 2 (20) | 36.5 ± 4 (63) | |
Values represents the mean ± se of two different fractionations with each enzyme assayed in triplicate. All activities were measured on the same day as preparing the extracts.
Amount of total proteins extracted from each fractions based on gram fresh weight of leaf.
ACL Distribution in Different Species
The distributions of ACL and the marker enzymes between the chloroplasts and cytosol preparations of the four plants are shown in Table III. Although there were significant differences in the amount of ACL activity in the species tested, with different distributions between the two fractions, in each case, ACL activity was found in both the cytosolic and plastid fractions. The distribution in pea and tobacco was similar, with a higher proportion of ACL activity in the cytosol than in the plastid, whereas in rape and spinach the reverse was found.
In pea, ACL was predominantly in the cytosol, with a 4-fold higher activity (79%) than in the plastids (21%). The cytosolic nature of pea ACL is consistent with the previous report of Kaethner and ap Rees (1985), although these authors did not rule out the possible presence of additional ACL activity in the plastids. This proposal is in keeping with our findings that there was only a 6% contamination of the cytosol marker enzyme (PEPC) in the plastid fraction, so only a correspondingly small part of the ACL activity in the plastid (i.e. 6%) could have arisen by contamination and ACL must have been present initially in both fractions to give the results shown in Table III. Allowing for this cross-contamination, the values given in Table III for the distribution of ACL between cytosol and plastid could be adjusted to 85% and 15%, respectively.
With tobacco, a similar dual location for ACL was indicated, with a slightly lower proportion within the cytosol (72%, adjusted to 78% for cross-contamination) than within the pea cytosol.
In rape and spinach, the majority of ACL activity was localized in the plastid fractions (63% and 61%, respectively), but in both the extent of plastid breakage was between 20% and 33% (see above for the data with the two plastid marker enzymes RuBP carboxylase and NADP-linked GAPDH), the original ACL activity in the plastids could have been between 90% and 78% depending on whether the comparison is made with the distribution of NADP-GAPDH or RuBP carboxylase, respectively. However, in both these tissues, there would undoubtedly be ACL activity both in the plastids and in the cytosol, suggesting a consistent dual location for this enzyme, albeit with different distributions in all four plant tissues studied here.
Immunolocation of ACL Proteins
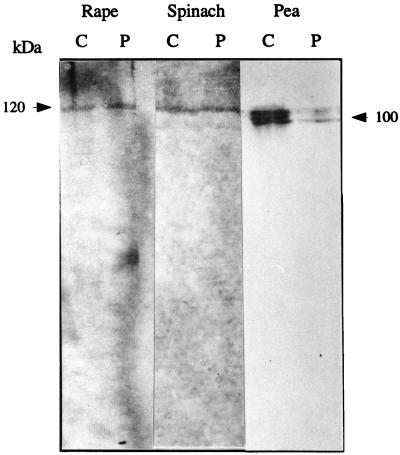
The spatial distribution of ACL proteins in organelle-enriched fractions of different species was confirmed by immunoblotting using rat-ACL antibody. There was considerable cross-reactivity between rat and plant ACL enzymes (Fig. 1), but there was no cross-reactivity between plant ACL and preimmune serum (data not shown). A single band of 120 kD was recognized in rape and spinach cytosolic and plastidic fractions by rat ACL antibody. The ACL from spinach was unstable: when stored for 16 h at 4°C, it started to degrade into the minor 60-kD proteins presumably arising by nicking by an endogenous trypsin-like enzyme (data not shown). Similar nicking processes have previously been observed in mammalian ACL (Singh et al., 1976; Alexander et al., 1979) and in ACL from an oleaginous yeast, Rhodotorula gracilis (Shashi et al., 1990), and could be a reason why this enzyme is unstable in vitro.
Figure 1.
Immunolocation of ACL. The spatial distribution of ACL proteins in cytosol and plastids of different species was confirmed by western-blot analysis using rat-ACL antibody. About 100 μg of proteins was loaded in each lane. (No cross-reactivity with ACL from tobacco was noted; see text.) Control western blots carried out with preimmune rabbit serum gave no interaction with ACL or any plant protein above 100 kD. C, Cytosol; P, plastids.
In tobacco, no cross-reactivity was observed between the tobacco and rat ACL proteins, which was not surprising because tobacco has only a very low activity of ACL in both cytosolic and plastidic fractions compared with the other tested species (see Tables II and III). This absence of cross-reactivity was further evidence that the cross-reactivities that were observed with the other three plant tissues were due to ACL.
In pea, ACL appeared to have two or three isoforms, each with a molecular mass of approximately 100 kD (Fig. 1). Data from previous studies on ACL from castor bean (Fritsch and Beevers, 1979) and sweet potato (Takeuchi et al., 1981) suggest that two forms of ACL may exist in plant cells. Form I refers to an ACL with a higher molecular mass and slightly higher total activity than form II, whose molecular mass is approximately 100 kD (Fritsch and Beevers, 1979; Takeuchi et al., 1981). The enzymatic nature of the two forms of ACL and their physiological roles in the supply of acetyl-CoA are not yet established in plant cells, nor is it known whether both forms of ACL are encoded by the same or different genes. However, further speculation is unwarranted at this time because the two (or even three) isoforms might have arisen by protease nicking during the isolation procedures.
The factors limiting plant fatty acid synthesis and oil content of seeds are not well understood. Although plastid pyruvate dehydrogenase and plastid acetyl-CoA synthetase have been suggested as a possible suppliers of acetyl-CoA, the cells might have other sources of acetyl-CoA in the plastids. There is uncertainty in the literature whether the plastid in all species is fully self-providing with respect to acetyl-CoA by its own pyruvate dehydrogenase and acetyl-CoA synthetase, or if acetyl-CoA is supplied from other sources in the cell (Lernmark and Gardeström, 1994). The present study suggests that ACL is involved in supplying acetyl-CoA either as an alternative pathway to existing routes or whenever the demand for acetyl-CoA increases, as, for example, during lipid synthesis. Studies with castor bean (Fritsch and Beevers, 1979) and rape (Ratledge et al., 1997) have provided evidence that ACL in plastids functions to supply acetyl-CoA for fatty acid synthesis, and that its activity parallels that of lipid accumulation in rape and lipid utilization in castor bean. Cytosolic ACL, however, could supply the acetyl-CoA needed for the mevalonic acid pathway leading to synthesis of sterols and other isoprenoid metabolites. In addition, the findings in sweet potato (Takeuchi et al., 1981) of an increase in ACL activity along with an accumulation of sesquiterpenoid phytoalexins on infection with a pathogen suggest that cytosolic ACL may be involved in the protection of some cells against pathogens and in providing acetyl-CoA for isoprenoid biosynthesis. The presence of one of the two forms of acetyl-CoA carboxylase in the cytosol of higher plants—the second form being in the plastids (see Sasaki et al., 1995)—is also evidence that cytosolic ACL might provide acetyl-CoA for chain elongation of fatty acids up to C20 to C30 because of the absence of acetyl-CoA synthetase in the cytosol (Kuhn et al., 1981) and the confinement of pyruvate dehydrogenase activity to the plastids (Camp and Randall, 1985; Lernmark and Gardeström, 1994). It is thus reasonable to assume that cytosolic ACL might be involved in the supply of acetyl-CoA for several metabolic pathways. However, the regulatory role of ACL in carbon metabolism and differences in the amount of ACL present in different plant species have yet to be explored.
ACKNOWLEDGMENTS
The authors thank Dr. Nebil Elshourbagy (SmithKline Beecham, Philadelphia) for the rat ACL antibody. We are grateful to Dr. D.R. Threlfall, Professor of Plant Biochemistry in the authors' department, for his advice and critical appraisal of the manuscript.
Footnotes
D.R. received financial support from the Commonwealth Scholarship Committee, UK.
LITERATURE CITED
- Alexander MC, Kowaloff EM, Witters LA, Denniby DT, Avruch J. Purification of a hepatic 123,000 dalton hormones-stimulated 32P-peptide and its identification as ATP:citrate lyase. J Biol Chem. 1979;252:8052–8056. [PubMed] [Google Scholar]
- Anderson LE. Ribulose-1,5-bisphosphate carboxylase from Rhodospirillum rubrum. Methods Enzymol. 1975;42:457–461. doi: 10.1016/0076-6879(75)42151-6. [DOI] [PubMed] [Google Scholar]
- Boulton CA, Ratledge C. ATP:citrate lyase: the regulatory enzyme for lipids biosynthesis in Lipomyces starkeyi? J Gen Microbiol. 1983;127:423–426. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camp PJ, Randall DD. Purification and characterization of pea chloroplasts pyruvate dehydrogenase complex. Plant Physiol. 1985;77:571–577. doi: 10.1104/pp.77.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy NA, Near JC, Kmetz PJ, Sathe GM, Southern C, Stickler JE, Gross M, Young FJ, Well TN, Groot HE. Rat ATP:citrate lyase: molecular cloning and sequence analysis of a full length cDNA and mRNA abundance as a function of diet, organ and age. J Biol Chem. 1990;204:491–499. [PubMed] [Google Scholar]
- Entwistle G, ap Rees T. Enzymic capacities of amyloplasts from wheat endosperm. Biochem J. 1988;255:391–396. doi: 10.1042/bj2550391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CT, Ratledge C. Possible regulatory roles of ATP:citrate lyase, malic enzyme and AMP deaminase in lipid accumulation by Rhodosporidium toruloides CBS14. Can J Microbiol. 1985a;31:1000–1005. [Google Scholar]
- Evans CT, Ratledge C. The physiological significance of citric acid in the control of metabolism in lipid-accumulating yeasts. In: Russell GE, editor. Biotechnology and Genetic Engineering Reviews. Vol. 3. Newcastle upon Tyne, UK: Intercept; 1985b. pp. 349–375. [Google Scholar]
- Fritsch H, Beevers H. ATP:citrate lyase from germinating castor bean endosperm. Plant Physiol. 1979;63:687–691. doi: 10.1104/pp.63.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. A simple spectrophotometric assay for fumarate hydratase in crude tissue extracts. Anal Biochem. 1978;85:271–275. doi: 10.1016/0003-2697(78)90299-3. [DOI] [PubMed] [Google Scholar]
- Kaethner TM, ap Rees T. Intracellular location of ATP:citrate lyase in leaves of Pisum sativum. Planta. 1985;163:290–294. doi: 10.1007/BF00393520. [DOI] [PubMed] [Google Scholar]
- Kang F, Rawsthorne S. Starch and fatty acid synthesis in plastids from developing embryos of oil seed rape. Plant J. 1994;6:795–805. [Google Scholar]
- Kohlhaw GB, Tan-Wilson A. Carnitine acetyltransferase: candidate for the transfer of acetyl groups through the mitochondrial membrane of yeast. J Bacteriol. 1977;129:1159–1161. doi: 10.1128/jb.129.2.1159-1161.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DN, Knauf MJ, Stumpf PK. Subcellular locations of acetyl-CoA synthetase in leaf protoplasts of Spinach oleracea. Arch Biochem Biophys. 1981;209:441–450. doi: 10.1016/0003-9861(81)90301-5. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lernmark U, Gardeström P. Distribution of pyruvate dehydrogenase complex activities between chloroplasts and mitochondria from leaves of different species. Plant Physiol. 1994;106:1633–1638. doi: 10.1104/pp.106.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Rinne RW. The role of citrate in lipid biosynthesis in developing soybean cotyledons. Plant Physiol. 1977;55:69–72. doi: 10.1104/pp.55.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Jaworski JG, Post-Beittenmiller D. De novo fatty acid biosynthesis. In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 3–32. [Google Scholar]
- Patel TB, Clark JB. Lipogenesis in the brain of suckling rats: studies on the mechanism of mitochondrial-cytosolic carbon transfer. Biochem J. 1980;188:163–168. doi: 10.1042/bj1880163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller MH, Roughan G, Ohlrogge JB. Regulation of plant fatty acid synthesis: analysis of acetyl-CoA and acyl-ACP substrate pools in chloroplasts from spinach and pea. Plant Physiol. 1992;100:923–930. doi: 10.1104/pp.100.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick WP, Scheibe R, Neuhaus HE. Induction of hexose-phosphate translocater activity in spinach chloroplasts. Plant Physiol. 1995;109:113–121. doi: 10.1104/pp.109.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy D, Ratledge C. Genetic enhancement of fatty acid synthesis by targeting rat liver ATP:citrate lyase into plastids of tobacco. Plant Physiol. 2000;122:1231–1238. doi: 10.1104/pp.122.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C. Microbial lipids. In: Rehm HJ, Reid G, Pühler A, Stadler P, editors. Biotechnology. Ed 2. Vol. 7. Weinheim, Germany: VCH; 1997. pp. 133–197. [Google Scholar]
- Ratledge C, Bowater MDV, Taylor PN. Correlation of ATP:citrate lyase activity with lipid accumulation in developing seeds of Brassica napus L. Lipids. 1997;32:7–12. doi: 10.1007/s11745-997-0002-7. [DOI] [PubMed] [Google Scholar]
- Ratledge C, Evans CT. Lipids and their metabolism. In: Rose AH, Harrison JS, editors. The Yeasts. Ed 2. Vol. 3. London: Academic Press; 1989. pp. 368–446. [Google Scholar]
- Robinson C, Barnett LK. Isolation and analysis of chloroplasts. In: Shaw CH, editor. Plant Molecular Biology. Oxford: IRL Press; 1988. pp. 67–78. [Google Scholar]
- Sasaki BM, Pascoe VM, Nagarno Y. The compartmentation of acetyl-CoA carboxylase in plants. Plant Physiol. 1995;108:445–448. doi: 10.1104/pp.108.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi K, Bechwat AK, Joseph R. ATP:citrate lyase of Rhodotorula gracilis: purification and properties. Biochim Biophys Acta. 1990;1033:23–30. doi: 10.1016/0304-4165(90)90189-4. [DOI] [PubMed] [Google Scholar]
- Singh M, Richard EG, Mukherjee A, Srere PA. Structure of ATP:citrate lyase from rat liver. J Biol Chem. 1976;251:5242–5240. [PubMed] [Google Scholar]
- Smith RG, Gauthier DA, Dennis DT, Turpin DH. Malate and pyruvate dehydrogenase-dependent fatty acid synthesis in leucoplasts from developing castor endosperm. Plant Physiol. 1992;98:1233–1238. doi: 10.1104/pp.98.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf PK. Fatty acid biosynthesis in higher plants. In: Numa S, editor. Fatty Acid Metabolism and Its Regulation. New York: Elsevier; 1984. pp. 155–179. [Google Scholar]
- Sullivan AC, Triscari J, Hamilton JG, Muller O, Wheatley VR. Effect of (−) hydroxycitrate from the accumulation of lipid in the rat lipogenesis. Lipids. 1973;9:121–128. doi: 10.1007/BF02532136. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Yamaguchi M, Uritani I. ATP:citrate lyase from opomea potatoes root tissue infected with Ceratocystis fimbriata. Phytochemistry. 1981;20:1235–1239. [Google Scholar]
- Wedding TR, Kline K. Comparative studies of coupled assays for phosphoenolpyruvate carboxylase. Physiol Plant. 1994;92:197–200. [Google Scholar]