Abstract
Background
In many developing countries, shigellosis is endemic and also occurs in epidemics and treatment of multidrug-resistant (MDR) isolates are important. The aims of this study were to determine the antimicrobial susceptibility, prevalence of class 1 and 2 integrons and the clonal relatedness of isolates.
Materials and Methods
Antimicrobial susceptibility tests were performed by disc diffusion method. Polymerase chain reaction (PCR)-sequencing technique was employed for detection and characterization of integrons. The genetic relatedness was evaluated by using enterobacterial repetitive intergenic consensus (ERIC) PCR.
Results
There was a high percentage of resistance to trimethoprim-sulfamethoxazole (TMP/SMX) (93.7%), ampicillin (AMP) (87.3%), streptomycin (STR) (84.5%) and tetracycline (TET) (78.9%). Multidrug resistant phenotype was seen in 95.1% of total isolates. Most common MDR profile was TMP/SMX/STR/AMP resistant pattern. Among the 142 Shigella spp. analyzed in this study, 28 isolates were positive for class 1 integron with two types of gene cassette arrays (dfrA17/aadA5 = 31.7% and dfrA7 = 3.8%). The class 2 integron was more frequently detected among the isolates (94.7%) with dfrA1/sat1/aadA1 (69.4%) and dfrA1/sat1 (30.6%) gene cassettes. ERIC-PCR results showed 6, 5, 4 and 3 main genotypes among S. flexneri, S. sonnei, S. boydii and S. dysenteriae isolates, respectively.
Conclusions
Our findings revealed that multidrug resistant Shigella species with high prevalence of class 2 integron were very common in Iran. In addition, ERIC-PCR patterns showed limited variety of clones are responsible for shigellosis in the region of the study.
Keywords: Shigella, Integron, Multidrug resistant, Enterobacterial repetitive intergenic consensus, Polymerase chain reaction
Introduction
Shigellosis, caused by Shigella species, is an acute enteric infection that is characterized by a watery and mucoid bloody diarrhea, also known as bacillary dysentery [1,2]. Antibiotic therapy can reduce the duration and severity of the disease and has been effective in improving the dysenteric syndrome of shigellosis and reduce the risk of transmission [3]. However, resistance to commonly used antibiotics has been increasing among Shigella spp. worldwide and multidrug-resistant (MDR) Shigella infections are widespread [4,5]. In Shigella species, resistance to ampicillin, trimethoprim, streptothricin and streptomycin is often associated with the presence of class 1 and class 2 integrons that contain resistance gene cassettes [3]. However, there are few data available to describe the prevalence of class 1 and class 2 integrons of Shigella species in Iran and there is no report on this subject in Northwest of Iran. Additionally, typing of bacterial strains helps us to understand transmission dynamics and control the spread of disease and accurately interpret epidemiological evolution of infectious diseases in the societies [6,7].
The objective of this work was to determine the antimicrobial susceptibility pattern in Shigella species isolated from Iran. Also, we have investigated the prevalence of class 1 and 2 integrons and their cassette arrays among the isolates. Moreover, the clonal relatedness of all Shigella species was evaluated using enterobacterial repetitive intergenic consensus (ERIC) PCR genotyping method for the first time in Iran.
Materials and Methods
1. Bacteria isolates
Between May 2014 and May 2015, a total of 142 non-duplicate Shigella isolates were obtained from the feces of patients suffered from acute diarrhea and gastroenteritis in Iran (Table 1). Moreover, fresh stool samples were cultured on selective media including Xylose lysine desoxycholate (XLD) (Merck, Hamburg, Germany) agar and Hektoen Enteric agar plates (Merck, Germany) which were incubated at 37˚C for 18–24 h. All presumptive colonies (non-lactose-fermenting colonies) were more identified as Shigella spp. by standard Enterobacteriaceae differentiation biochemical tests [6]. Furthermore, specific polyvalent antisera (SIFIN, GmbH Berlin, Germany) were used for serogrouping of Shigella isolates by a slide agglutination test [7].
Table 1. Shigella species isolated from different parts of Iran used in this study.
| City | No. (%) isolates | ||||
|---|---|---|---|---|---|
| Shigella dysenteriae | Shigella flexneri | Shigella boydii | Shigella sonnei | Total | |
| Ardabil | 0 (0) | 10 (55.6) | 4 (22.2) | 4 (22.2) | 18 |
| Kerman | 2 (4) | 31 (62) | 4 (8) | 13 (26) | 50 |
| Urmia | 1 (2.5) | 10 (25) | 5 (12.5) | 24 (60) | 40 |
| Tabriz | 3 (8.8) | 22 (64.7) | 3 (8.8) | 6 (16.7) | 34 |
| Total | 6 (4.2) | 73 (51.4) | 16 (11.3) | 47 (33.1) | 142 |
2. Antimicrobial susceptibility
Antibiotic susceptibility testing was performed by using Kirby–Bauer disc diffusion method according to Clinical and Laboratory Standards Institute recommendations (CLSI) [8]. The antimicrobial disks tested were ampicillin (AMP), cefoxitin (FOX), cefazolin (CFZ), ceftriaxone (CRO), cefotaxime (CTX), ceftazidime (CAZ), cefepime (FEP), amoxicillin-clavulanate (AMC), aztreonam (ATM), gentamicin (GEN), streptomycin (STR), amikacin (AMK), nalidixic acid (NAL), ofloxacin (OFX), ciprofloxacin (CIP), levofloxacin (LVX), chloramphenicol (CHL), trimethoprim-sulfamethoxazole (TMP/SMX), tetracycline (TET), azithromycin (AZM), imipenem (IPM) and meropenem (MEM) (Mast Co., Bootle, Merseyside, UK). Escherichia coli ATCC 25922 was used as the quality control strain. The MDR was defined as resistance to 3 or more unrelated antibiotics [9].
3. Detection of class 1 and 2 integrons
Total DNA of the isolates was prepared using the boiling method, as previously described [10]. The presence of class 1 and 2 integrons were investigated by PCR method of the conserved integrase gene with specific primers set of int 1 and int 2. Also, to detect the gene cassettes of class 1 integron, PCR was performed with the primer pair CS-F/CS-R, which spans the entire cassette integrating site (3'-CS and 5'-CS) of integron (Table 2). Cassettes of class 2 integron was investigated by another PCR with the primer pair hep74 and hep51, specific to the conserved regions of class 2 integron, following previously described conditions and procedures [10,11,12]. Eventually, to determine the content of gene cassettes both DNA strands of the PCR products were sequenced by Macrogen Inc. (Seoul, South Korea).
Table 2. Oligonucleotides used in this study.
| Primer | Oligonucleotide (5′-3′) | Produced size (bp) | References |
|---|---|---|---|
| int1-F | CAGTGGACATAAGCCTGTTC | 160 | [10, 11, 12] |
| int1-R | CCCGAGGCATAGACTGTA | ||
| int2-F | CACGGATATGCGACAAAAAGGT | 789 | [10, 11, 12] |
| int2-R | GTAGCAAACGAGTGACGAAATG | ||
| CS-F | GGCATCCAAGCAGCAAG | Variable | [10, 11, 12] |
| CS-R | AAGCAGACTTGACCTGA | ||
| hep74 | CGGGATCCCGGACGGCATGCACGATTTGTA | Variable | [10, 11, 12] |
| hep51 | GATGCCATCGCAAGTACGAG | ||
| ERIC-1 | ATGTAAGCTCCTGGGGATTCAC | Variable | [13] |
| ERIC-2 | AAGTAAGTGACTGGGGTGAGCG |
4. ERIC-PCR
Genomic DNA was extracted from all isolates by DNA extraction kit (Bioneer, Daejeon, South Korea) following the manufacturer's instructions. Genotyping of the Shigella isolates was performed using primers (Table 2) and protocol of Dalla-Costa et al. [13]. ERIC-PCR patterns were evaluated with GelClust software [14]. Similarity for a potential clonal relatedness was determined with Dice Unweighted Pair Group Method with Arithmetic Mean (UPGENA) method [13].
Results
1. Patient data
The age distribution data revealed that Shigella species were isolated from 59 cases (41.5%) in the <5-years, 44 (31%) in the 6–12-years and 39 (27.5%) in >13-years old age groups. Considering the gender of patients, 81 (57%) were male and 61 (43%) were female patients.
2. Antimicrobial profiles
There was a high percentage of resistance to TMP/SMX, AMP, STR and TET. A lower percentage of resistance was also against AZM, FOX, OFX, CIP and LVX while no resistance was found to AMK and carbapenems including IPM and MEM (Table 3). MDR phenotype was seen in 95.1% of the total isolates.
Table 3. Antimicrobial resistance among Shigella isolates collected from Iran.
| Antibiotics | No. (%) isolates | ||||
|---|---|---|---|---|---|
| Shigella flexneri (N = 73) | Shigella sonnei (N = 47) | Shigella boydii (N = 16) | Shigella dysenteriae (N = 6) | Total (N = 142) | |
| Trimethoprim-sulfamethoxazole | 66 (90.4) | 47 (100) | 14 (87.5) | 6 (100) | 133 (93.7) |
| Streptomycin | 67 (91.8) | 38 (80.9) | 10 (62.5) | 5 (83.3) | 120 (84.5) |
| Ampicillin | 68 (93.2) | 36 (76.6) | 14 (87.5) | 6 (100) | 124 (87.3) |
| Tetracycline | 62 (84.9) | 34 (72.3) | 11 (68.7) | 5 (83.3) | 112 (78.9) |
| Cefazolin | 46 (63) | 34 (72.3) | 10 (62.5) | 3 (50.0) | 93 (65.5) |
| Amoxicillin-clavulanate | 52 (71.2) | 22 (48.6) | 7 (43.8) | 2 (33.3) | 83 (58.5) |
| Ceftriaxone | 38 (52.0) | 30 (63.8) | 7 (43.8) | 3 (50.0) | 78 (54.9) |
| Cefotaxime | 39 (53.4) | 30 (63.8) | 6 (37.5) | 3 (50.0) | 78 (54.9) |
| Aztreonam | 25 (34.2) | 16 (34.0) | 5 (31.2) | 3 (50.0) | 49 (34.5) |
| Nalidixic acid | 19 (26.0) | 17 (36.2) | 8 (50.0) | 0 (0) | 44 (31.0) |
| Ceftazidime | 25 (34.2) | 12 (25.5) | 5 (31.2) | 2 (33.3) | 44 (31.0) |
| Cefepime | 21 (28.8) | 12 (25.5) | 5 (31.2) | 3 (50.0) | 41 (28.9) |
| Chloramphenicol | 28 (38.4) | 1 (2.1) | 4 (25.0) | 0 (0) | 33 (23.2) |
| Gentamicin | 6 (8.2) | 16 (34.0) | 3 (18.8) | 1 (16.7) | 26 (18.3) |
| Azithromycin | 6 (8.2) | 6 (12.8) | 4 (25.0) | 0 (0) | 16 (11.3) |
| Cefoxitin | 7 (9.5) | 3 (6.4) | 0 (0) | 0 (0) | 10 (7.0) |
| Ciprofloxacin | 1 (1.4) | 0 (0) | 5 (31.2) | 0 (0) | 6 (4.2) |
| Ofloxacin | 1 (1.4) | 0 (0) | 5 (31.2) | 0 (0) | 6 (4.2) |
| Levofloxacin | 0 (0) | 0 (0) | 4 (25.0) | 0 (0) | 4 (2.8) |
| Amikacin | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Meropenem | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Imipenem | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
3. Distribution of class 1 and 2 integrons
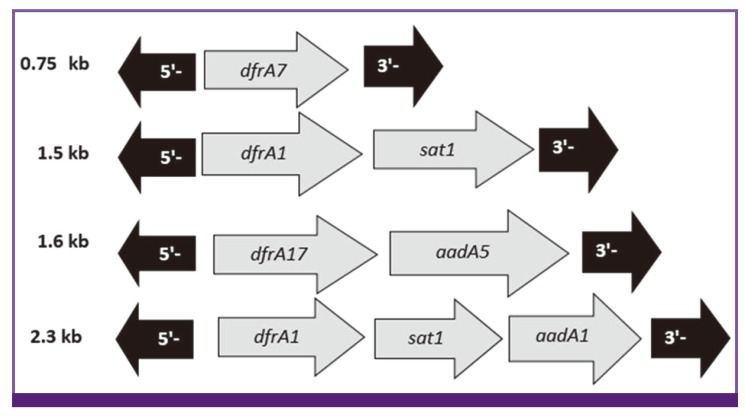
Among the total of 142 isolates, 79 (55.6%) carried class 1 integron (int+) and 114 (80.3%) carried class 2 integron (int+). PCR amplification of internal variable region of 79 class 1 integron positive strains using CS-F/CS-R primers produced two different products of approximately 750 and 1600 bp in 3 (3.8%) and 25 (31.7%) isolates, respectively. Fifty-one (64.5%) isolates produced no products, which is indicative of an empty integron with no resistance gene cassette. Sequence analysis of variable region indicated the presence of dfrA7, and dfrA17-aadA5 resistance gene cassettes among the isolates, which correspond to 750 and 1600 bp PCR products, respectively (Fig.1). Amplification of internal variable region for 114 class 2 integron positive isolates using hep74 and hep51 primers showed, 108 (94.7%) isolates harbored resistance gene cassettes; however, no gene cassette was obtained for another six (5.3%) integron-positive isolates. In addition, two types gene cassette were identified in class 2 integrons. Seventy-five (69.4%) strains had a 2300 bp fragment and 33 (30.6%) strains had a 1500 bp fragment. Sequence analysis of the gene cassette indicated the presence dfrA1-sat1-aadA1 and dfrA1-sat1 resistance gene cassettes that are related to 2,300 and 1,500 bp fragments, respectively (Fig.1).
Figure 1.
Structures of class 1, 2 integrons variable regions found in this study.
4. Genotyping
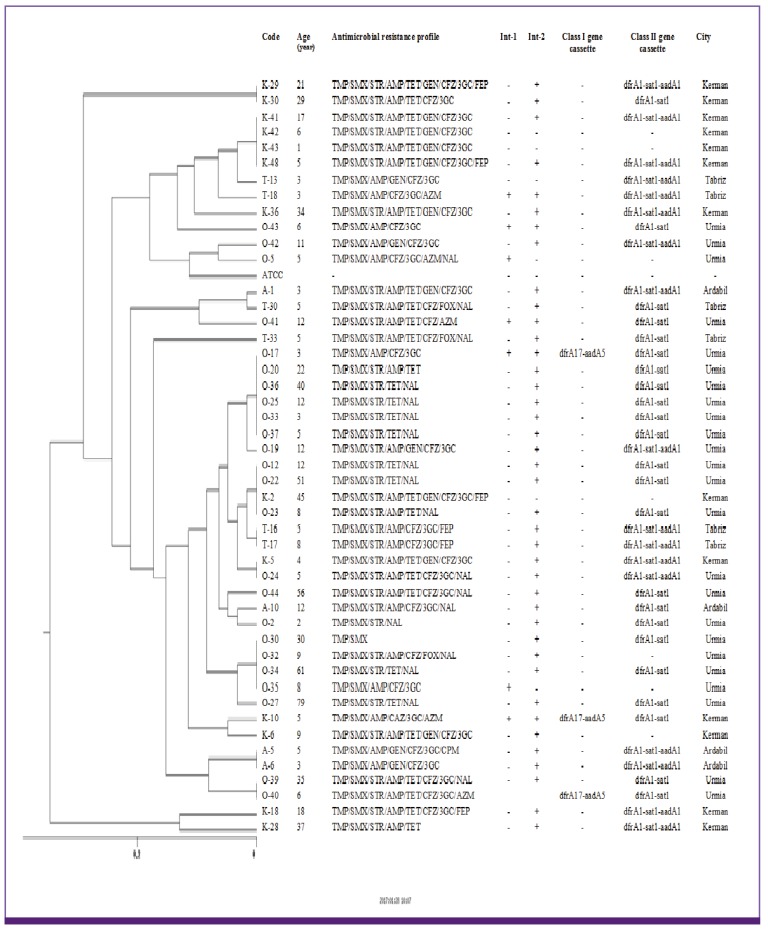
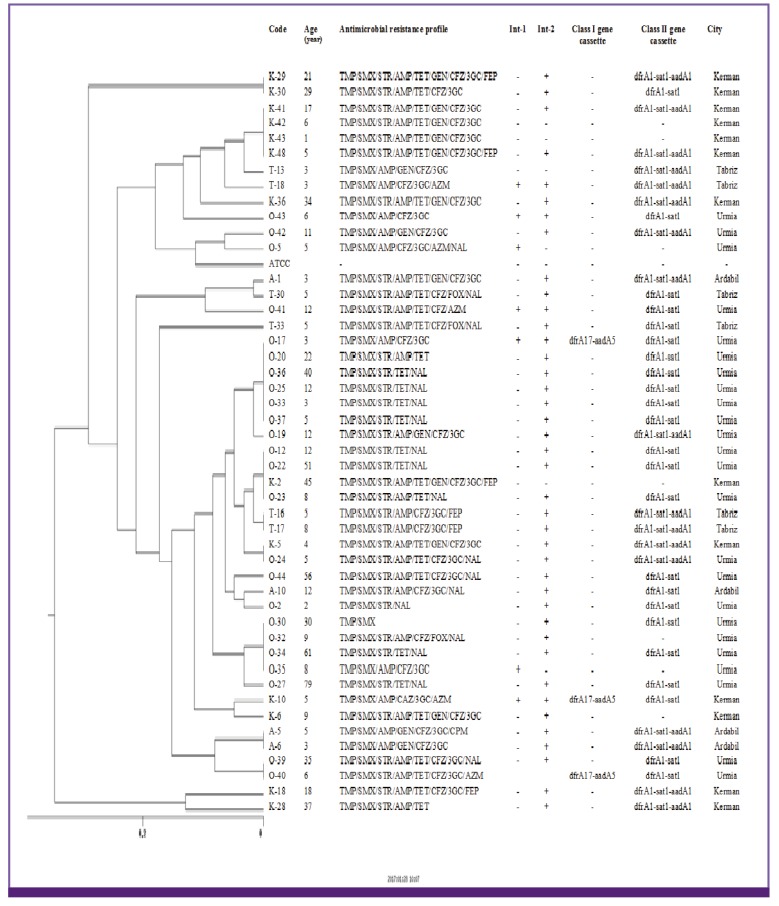
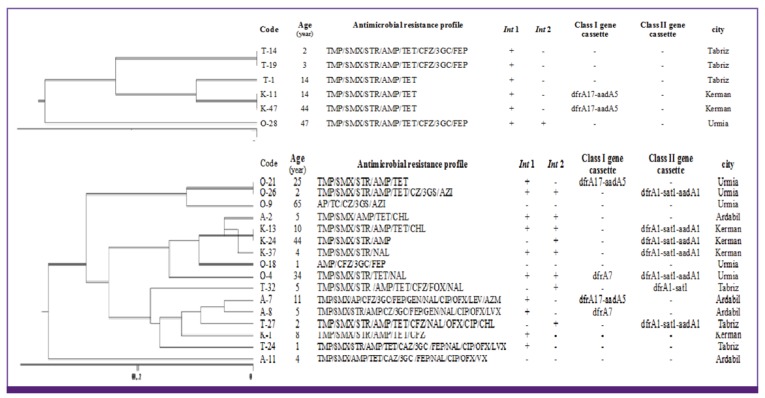
The 73 S. flexneri isolates were classified into 6 major clusters (A to F) and 41 subcluster. Moreover, five different genotypes (A to E) and 28 subtypes were found among the 47 S. sonnei isolates. All of 16 S. boydii and 6 S. dysenteriae isolates were also classified into 4 major clusters (A to D) and 13 subcluster as well as 3 cluster (A to C) and 4 sub cluster, respectively (Fig. 2, 3, 4).
Figure 2.
Dendrogram based on ERIC-PCR of 73 Shigella flexneri in relation to the resistance profile and integron resistance gene content.
ERIC, enterobacterial repetitive intergenic consensus; PCR, polymerase chain reaction; AMP, ampicillin; FOX, cefoxitin; CFZ, cefazolin; 3GC, 3-th generation of cephalosporins; FEP, cefepime; GEN, gentamicin; STR, streptomycin; NAL, nalidixic acid, OFX, ofloxacin; CIP; ciprofloxacin; LVX, levofloxacin; CHL, chloramphenicol; TMP/SMX, trimethoprim-sulfamethoxazole; TET, tetracycline; AZM, azithromycin; Int 1, integrase 1; Int 2, integrase 2.
Figure 3.
Dendrogram based on ERIC-PCR of 47 Shigella sonnei in relation to the resistance profile and integron resistance gene content.
ERIC, enterobacterial repetitive intergenic consensus; PCR, polymerase chain reaction; AMP, ampicillin; FOX, cefoxitin; CFZ, cefazolin; 3GC, 3rd generation of cephalosporins; FEP, cefepime; GEN, gentamicin; STR, streptomycin; NAL, nalidixic acid; OFX, ofloxacin; CIP, ciprofloxacin; LVX, levofloxacin; CHL, chloramphenicol; TMP/SMX, trimethoprim-sulfamethoxazole; TET, tetracycline; AZM, azithromycin; Int 1, integrase 1; Int 2, integrase 2.
Figure 4.
Dendrogram based on ERIC-PCR of 16 Shigella boydii and 6 Shigella dysenteriae in relation to the resistance profile and integron resistance gene content.
ERIC, enterobacterial repetitive intergenic consensus; PCR, polymerase chain reaction; AMP, ampicillin; FOX; cefoxitin; CFZ, cefazolin; 3GC, 3rd generation of cephalosporins; FEP, cefepime; GEN, gentamicin; STR, streptomycin; NAL, nalidixic acid; OFX, ofloxacin; CIP, ciprofloxacin; LVX, levofloxacin; CHL, chloramphenicol; TMP/SMX, trimethoprim-sulfamethoxazole; TET, tetracycline; AZM, azithromycin; Int 1, integrase 1; Int 2, integrase 2.
Discussion
The emergence and dissemination of MDR Shigella strains have become an important concern worldwide which can complicate the selection of empirical agents for the treatment of shigellosis [15]. Our findings revealed that 95.1% of Shigella spp. isolated from patients showed a MDR phenotype. Most common MDR profiles observed in this study were TMP/SMX/STR/AMP (76.1%), TMP/SMX/STR/TET (73.2%) and TMP/SMX/STR /AMP/TET (62.7%). Previous studies have also shown the dominance of resistance to TMP/SMX and STR and foremost resistance phenotype were TMP/SMX/TET/TMP among Shigella spp. in Iran and other countries [5,15,16,17]. Therefore, these antibiotics should no longer be considered as appropriate empirical therapy. Additionally, cephalosporins are considered as alternative drugs for shigellosis treatment. Unfortunately, this study has demonstrated emerging resistance to these drugs with 65.5%, 54.9%, 54.9% and 31% resistance rate to cefazolin, cefotaxime, ceftriaxone and ceftazidime, respectively. A previous study in China had shown low cephalosporin resistance among Shigella spp [15]. It is also noteworthy that the result of present study showed a high dissemination of cephalosporins resistance among Shigella spp. in Iran compared with two previous studies [2,18]. Importantly, 28.9% of our collected Shigella spp. were also resistant to cefepime. The emergence of co-resistance to older antibiotics and 3rd- or 4th-generation cephalosporin's in Shigella is an excessive challenge to the public health for the effective treatment of shigellosis. The main mechanism of resistance to cephalosporin's in our isolates was production of extended spectrum beta-lactamase enzymes (unpublished data). However, in the present study, we observed high in vitro activity of azithromycin (88.7%), fluoroquinolone (95.8-97.2%), amikacin and carbapenems (100%), making them the recommended drugs of choice for treatment of shigellosis.
Noteworthy this is the first comprehensive report about the integrons cassettes in all four Shigella species from different parts of Iran. Previous studies have also shown the similar array of gene cassettes of class 1 integrons among enteric bacteria including Proteus, E. coli, Klebsiella pneumoniae and Shigella spp. in Iran and other countries, which provide evidence for transfer of resistance genes through integrons between different species [16,17,19,20]. The class 2 integron was more frequently detected among the isolates (94.7%). Similar observations in other countries have shown the high prevalence of class 2 integron among Shigella spp. [3,21,22].
Furthermore, two different gene cassettes detected in class 2 integrons among our isolates (1,500 and 2,300 bp in size). These two arrays were defined as dfrA1/sat1 (30.6%) and dfrA1/sat1/aadA1 (69.4%). Interestingly, this high prevalence of class 2 integron with dominant of dfrA1/sat1/aadA1 arrays found in Shigella spp. in the present study is in conformity with previous studies conducted [23,24]. The occurrence of gene cassettes dfrA1/sat1/aadA1 in Shigella flexneri (73.9%) and gene cassettes dfrA1/sat1 in Shigella sonnei (51%) were more than the other Shigella species.
Uncommonly, nineteen isolates with empty class 1 and class 2 integron were resistance to TMP/SMX This can be attributed to sulfanilamide resistance gene (sul1) offered as part of the 3 conserved segment of all class 1 integrons [25]. Furthermore, taking into account 16 (11.2%) isolates resistant to STR that did not carry a class 1 or 2 integron, indicated that mechanisms other than integrons mediate STR resistance in this isolates of Shigella.
All dfrA carrying isolates were resistant to TMP/SMX, except three S. flexneri (T2, K32, K45) that this isolates harboring dfrA1/sat1/aadA1 gene cassette of class 2 integron and were sensitive to TMP/SMX. Also all aadA carrying isolates were resistant to streptomycin, except four Shigella isolates (T8, T18, A7, K10) harboring resistance gene cassette with sensitive phenotype, maybe due to the different promoter strength or even inactive promoters. Some previous studies have reported similar results in Shigella species [17,24]. Except two, all isolates that carried dfrA1/sat1/aadA1 gene cassette array, were resistant to TMP/SMX and STR. The results indicate the importance of this gene cassette array in high resistance to TMP/SMX and STR.
A little information on the integron content of S. boydii and S. dysenteriae strains is available. In our study among 16 isolates of S. boydii, five isolates carried class 1 integron with two different gene arrays (dfrA17-aadA1 in 2, dfrA7 in one isolate) and six isolates contained class 2 integron with only dfrA1/sat1/aadA1 gene cassette array. All of the six S. dysenteriae in the present study were resistance to streptomycin and trimethoprim-sulfamethoxazole. However, only two isolates (K11, K47) harbored dfrA17-aadA5 gene cassette that related to a 1,600 bp product of class 1 integron and 4 other isolates did not carry any gene cassette of class 1 or 2 integron. So the cause of resistance to these antibiotics in these isolates could be due to other mechanisms of resistance that need to further researches.
Notably, our results showed that 21 Shigella isolates (14.7%) harbored both class 1 and 2 integrons simultaneously: 13 S. flexneri (with dfrA1/sat1/aadA1 + dfrA17/aadA5 gene arrays), six S. sonnei (with dfrA1/sat1 + dfrA17-aadA5 gene arrays) and two S. boydii (one with dfrA1/sat1/aadA1 + dfrA17-aadA5 and one with dfrA1/sat1 + dfrA7 gene cassettes arrays). This dual occurrence has been reported earlier only in 1, 7 and 10 isolates from Brazil [26] and Korea [27].
The results of ERIC-PCR analysis of 73 S. flexneri showed a major cluster (cluster E) with 22 isolate constituting 30.13% of total isolates specially distributes in Kerman and Tabriz province, all of which uniformly showed identical dfrA1/sat1/aadA1 resistance gene cassette array of class 2 integron content. ERIC-PCR patterns of S. sonnei in this study has shown the main cluster with 30 isolates (cluster D) constituted 63.8% of the total S. sonnei, all of which (except seven isolates) uniformly showed dfrA1/sat1 resistance gene cassette of class 2 integron and absence of class 1 integron in these isolates (except three isolates). Considering the ERIC-PCR results an impressive feature is the high genetic homogeneity of the studied isolates. This result strongly suggests the distribution of a limited S. sonnei clone among Iranian population. Similar limited diversity in strains of Shigella spp. has been reported previously in India [28] and Japan [29]. Of the 16 S. boydii isolates, 4 different genotypes were obtained: 3 isolates in type A, 6 isolates each in type B and C and one isolate in type D. From six fluoroquinolone resistance isolates in this study, four isolates belonging to major cluster C. In S. boydii, strains belonging to the same ERIC cluster but having different antimicrobial resistance profile were observed, this is probably due to an integron or other mobile elements carrying the resistance gene integrated in the chromosome [30]. Eventually, in S. dysenteriae, isolates with the similar antimicrobial resistance profile and integron content placed in unique cluster and were found to be closely related.
In conclusion, we have identified prevalence of high resistance to widely used antimicrobial agents for shigellosis and demonstrated that MDR phenotype is common among Shigella species in Iran. To the best of our knowledge, this study was the first comprehensive and largest analysis of the genetic characteristics of integrons, their association with antibiotic resistance and ERIC-PCR typing of the clinical isolates of Shigella species from Iran. Significantly, the ERIC patterns in this study were associated with similar resistance phenotypes and the predominance of class 2 integrons in most strains of Shigella spp. Finally, the results suggest a few specific clones are responsible of shigellosis in these regions of Iran.
Acknowledgements
This work was supported financially by the Immunology Research Center, Tabriz University of Medical Sciences (grant No. 94-08). It is also a report originating from a database developed for the Ph.D. thesis of the first author. We would appreciate the cooperation of microbiology laboratory personnel from hospitals of Urmia, Tabriz, Ardabil and Kerman in the collection of clinical isolates. We also thank Dr. Kamali, the chief of Aryan laboratory to his worthy assistance in our work.
Footnotes
Conflicts of Interest: No conflicts of interest.
References
- 1.Ahmed AM, Shimamoto T. Molecular characterization of multidrug-resistant Shigella spp. of food origin. Int J Food Microbiol. 2015;194:78–82. doi: 10.1016/j.ijfoodmicro.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Tajbakhsh M, García Migura L, Rahbar M, Svendsen CA, Mohammadzadeh M, Zali MR, Aarestrup FM, Hendriksen RS. Antimicrobial-resistant Shigella infections from Iran: an overlooked problem? J Antimicrob Chemother. 2012;67:1128–1133. doi: 10.1093/jac/dks023. [DOI] [PubMed] [Google Scholar]
- 3.Pan JC, Ye R, Meng DM, Zhang W, Wang HQ, Liu KZ. Molecular characteristics of class 1 and class 2 integrons and their relationships to antibiotic resistance in clinical isolates of Shigella sonnei and Shigella flexneri . J Antimicrob Chemother. 2006;58:288–296. doi: 10.1093/jac/dkl228. [DOI] [PubMed] [Google Scholar]
- 4.Ranjbar R, Mirsaeed Ghazi F. Antibiotic sensitivity patterns and molecular typing of Shigella sonnei strains using ERIC-PCR. Iran J Public Health. 2013;42:1151–1157. [PMC free article] [PubMed] [Google Scholar]
- 5.Memish ZA, Venkatesh S, Shibl AM. Impact of travel on international spread of antimicrobial resistance. Int J Antimicrob Agents. 2003;21:135–142. doi: 10.1016/s0924-8579(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 6.Perilla MJ, Ajello G, Bopp C, Elliott J, Facklam R, Knapp JS, Popovic T, Wells J, Dowell SF. Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world. Atlanta, GA: CDC; 2003. [Google Scholar]
- 7.Guardabassi L, Dijkshoorn L, Collard JM, Olsen JE, Dalsgaard A. Distribution and in-vitro transfer of tetracycline resistance determinants in clinical and aquatic Acinetobacter strains. J Med Microbiol. 2000;49:929–936. doi: 10.1099/0022-1317-49-10-929. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI) 25th informational supplement. Document M100-S25. Wayne, PA: CLSI; 2015. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 9.Rezaee MA, Abdinia B, Abri R, Kafil HS. Comparison of the antibiotic resistance patterns among Shigella species isolated from pediatric hospital between 1995-1999 and 2009-2013 in North-West of Iran. J Anal Res Clin Med. 2014;2:118–122. [Google Scholar]
- 10.Ahmed AM, Shimamoto T, Shimamoto T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int J Med Microbiol. 2013;303:475–483. doi: 10.1016/j.ijmm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Tamang MD, Oh JY, Seol SY, Kang HY, Lee JC, Lee YC, Cho DT, Kim J. Emergence of multidrug-resistant Salmonella enterica serovar Typhi associated with a class 1 integron carrying the dfrA7 gene cassette in Nepal. Int J Antimicrob Agents. 2007;30:330–335. doi: 10.1016/j.ijantimicag.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Mazel D, Dychinco B, Webb VA, Davies J. Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrob Agents Chemother. 2000;44:1568–1574. doi: 10.1128/aac.44.6.1568-1574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalla-Costa LM, Irino K, Rodrigues J, Rivera IN, Trabulsi LR. Characterisation of diarrhoeagenic Escherichia coli clones by ribotyping and ERIC-PCR. J Med Microbiol. 1998;47:227–234. doi: 10.1099/00222615-47-3-227. [DOI] [PubMed] [Google Scholar]
- 14.Khakabimamaghani S, Najafi A, Ranjbar R, Raam M. GelClust: a software tool for gel electrophoresis images analysis and dendrogram generation. Comput Methods Programs Biomed. 2013;111:512–518. doi: 10.1016/j.cmpb.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Jin H, Hu J, Yuan Z, Shi W, Yang X, Xu X, Meng J. Antimicrobial resistance of Shigella spp. from humans in Shanghai, China, 2004–2011. Diagn Microbiol Infect Dis. 2014;78:282–286. doi: 10.1016/j.diagmicrobio.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Eftekhari N, Bakhshi B, Pourshafie MR, Zarbakhsh B, Rahbar M, Hajia M, Ghazvini K. Genetic diversity of Shigella spp. and their integron content. Foodborne Pathog Dis. 2013;10:237–242. doi: 10.1089/fpd.2012.1250. [DOI] [PubMed] [Google Scholar]
- 17.Alizadeh-Hesar M, Bakhshi B, Najar-Peerayeh S. Clonal dissemination of a single Shigella sonnei strain among Iranian children during Fall 2012 in Tehran, IR Iran. Infect Genet Evol. 2015;34:260–266. doi: 10.1016/j.meegid.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Mardaneh J, Poor SA, Afrugh P. Prevalence of Shigella species and antimicrobial resistance patterns of isolated strains from infected pediatrics in Tehran. Int J Enteric Pathog. 2013;1:e10705. [Google Scholar]
- 19.Young HK, Amyes S. Plasmid trimethoprim resistance in Vibrio cholerae: migration of the type I dihydrofolate reductase gene out of the Enterobacteriaceae. J Antimicrob Chemother. 1986;17:697–703. doi: 10.1093/jac/17.6.697. [DOI] [PubMed] [Google Scholar]
- 20.Najibi S, Bakhshi B, Fallahzad S, Pourshafie MR, Katouli M, Sattari M, Alebouyeh M, Tajbakhsh M. Distribution of class 1 integrons among enteropathogenic Escherichia coli . Can J Microbiol. 2012;58:637–643. doi: 10.1139/w2012-035. [DOI] [PubMed] [Google Scholar]
- 21.Cui X, Yang C, Wang J, Liang B, Yi S, Li H, Liu H, Li P, Wu Z, Xie J, Jia L, Hao R, Wang L, Hua Y, Qiu S, Song H. Antimicrobial resistance of Shigella flexneri serotype 1b isolates in China. PLoS One. 2015;10:e0129009. doi: 10.1371/journal.pone.0129009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tariq A, Haque A, Ali A, Bashir S, Habeeb MA, Salman M, Sarwar Y. Molecular profiling of antimicrobial resistance and integron association of multidrug-resistant clinical isolates of Shigella species from Faisalabad, Pakistan. Can J Microbiol. 2012;58:1047–1054. doi: 10.1139/w2012-085. [DOI] [PubMed] [Google Scholar]
- 23.McIver CJ, White PA, Jones LA, Karagiannis T, Harkness J, Marriott D, Rawlinson WD. Epidemic strains of Shigella sonnei biotype g carrying integrons. J Clin Microbiol. 2002;40:1538–1540. doi: 10.1128/JCM.40.4.1538-1540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jové T, Da Re S, Denis F, Mazel D, Ploy MC. Inverse correlation between promoter strength and excision activity in class 1 integrons. PLoS Genet. 2010;6:e1000793. doi: 10.1371/journal.pgen.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulsen IT, Littlejohn TG, Rådström P, Sundström L, Sköld O, Swedberg G, Skurray RA. The 3'conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother. 1993;37:761–768. doi: 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peirano G, Agersø Y, Aarestrup FM, dos Prazeres Rodrigues D. Occurrence of integrons and resistance genes among sulphonamide-resistant Shigella spp. from Brazil. J Antimicrob Chemother. 2005;55:301–305. doi: 10.1093/jac/dki012. [DOI] [PubMed] [Google Scholar]
- 27.Jin YH, Oh YH, Jung JH, Kim SJ, Kim JA, Han KY, Kim MY, Park SG, Lee YK. Antimicrobial resistance patterns and characterization of integrons of Shigella sonnei isolates in Seoul, 1999-2008. J Microbiol. 2010;48:236–242. doi: 10.1007/s12275-010-9220-z. [DOI] [PubMed] [Google Scholar]
- 28.Das A, Natarajan M, Mandal J. The emergence of quinolone resistant Shigella sonnei, Pondicherry, India. PLoS One. 2016;11:e0160290. doi: 10.1371/journal.pone.0160290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terajima J, Tamura K, Hirose K, Izumiya H, Miyahara M, Konuma H, Watanabe H. A multi-prefectural outbreak of Shigella sonnei infections associated with eating oysters in Japan. Microbiol Immunol. 2004;48:49–52. doi: 10.1111/j.1348-0421.2004.tb03486.x. [DOI] [PubMed] [Google Scholar]
- 30.Navia MM, Capitano L, Ruiz J, Vargas M, Urassa H, Schellemberg D, Gascon J, Vila J. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J Clin Microbiol. 1999;37:3113–3117. doi: 10.1128/jcm.37.10.3113-3117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]