Abstract
Urinary tract infections (UTIs) are infectious diseases that commonly occur in communities. Although several international guidelines for the management of UTIs have been available, clinical characteristics, etiology and antimicrobial susceptibility patterns may differ from country to country. This work represents an update of the 2011 Korean guideline for UTIs. The current guideline was developed by the update and adaptation method. This clinical practice guideline provides recommendations for the diagnosis and management of UTIs, including asymptomatic bacteriuria, acute uncomplicated cystitis, acute uncomplicated pyelonephritis, complicated pyelonephritis related to urinary tract obstruction, and acute bacterial prostatitis. This guideline targets community-acquired UTIs occurring among adult patients. Healthcare-associated UTIs, catheter-associated UTIs, and infections in immunocompromised patients were not included in this guideline.
Keywords: Urinary Tract Infection, Guideline, Cystitis, Pyelonephritis, Prostatitis
I. Introduction
1. Background of guidelines
Urinary tract infections (UTIs) are infectious diseases that commonly occur in communities. They are classified as upper UTIs (pyelonephritis) and lower UTIs (cystitis, prostatitis) depending on the site of infection and as uncomplicated or complicated according to underlying diseases and anatomical or functional abnormalities of the urinary tract. Clinical manifestations of UTIs vary from asymptomatic bacteriuria to septic shock. Having a sufficient understanding of these symptoms and using appropriate antibiotics are crucial for preventing serious complications and antibiotic misuse and for inhibiting the expression of resistant bacteria.
The global monitoring report on antibiotic resistance released by the World Health Organization in 2014 reported the increased resistance of major bacteria to cephalosporins and fluoroquinolones to be a serious worldwide health issue, and Korea should also take measures against this trend. Efforts to raise awareness about this issue and resolve it are necessary. Policies to establish and distribute standard guidelines for antibiotic selection for appropriate treatment of UTIs and inhibition of the emergence of antibiotic-resistant bacteria have been in demand.
The causative bacteria of UTIs in Korea have higher resistance to trimethoprim/sulfamethoxazole (TMP/SMX) than those in the United States and Europe where TMP/SMX is recommended as a primary antibiotic for UTIs. In Korea, fluoroquinolones are mainly recommended as a primary antibiotic for UTIs. However, since the causative bacteria of UTIs in Korea are more resistant to fluoroquinolones than those in the United States and Europe, the failure rate of fluoroquinolone treatments is high. Although nitrofurantoin and fosfomycin have been recommended in foreign countries as substitutes for fluoroquinolones, they can be used for lower UTIs only, and they have not been widely used in Korea owing to lack of advertisement and constraints on the introduction of new drugs. Intravenous cephalosporins are commonly administered for upper UTIs accompanied by fever. However, as the population of resistant bacteria that produce extended-spectrum β-lactamases (ESBLs) has increased, the failure rate of cephalosporin treatment has also increased.
Various treatment guidelines providing the clinicians treating UTIs with scientific evidence have been developed in foreign countries. In Korea, the Korean Society of Infectious Diseases, the Korean Society for Chemotherapy, the Korean Association of Urogenital Tract Infection and Inflammation, and the Korean Society of Clinical Microbiology developed and published guidelines for UTIs in 2011. Recently, the Korean Association of Urogenital Tract Infection and Inflammation published new guidelines for UTIs in 2016. Owing to the changes in the antibiotic resistance of causative bacteria and the recent publication of studies on them, an update on the domestic guidelines has been demanded. In compliance with the policies of the related academic societies, and with the support of the Korea Centers for Disease Control and Prevention, the present guideline has been developed.
2. Patients for whom the guideline is applicable
This guideline is on the use of antibiotics for community-acquired UTIs affecting patients aged 18 years or older. The guideline targets asymptomatic bacteriuria, acute uncomplicated cystitis, acute uncomplicated pyelonephritis, complicated pyelonephritis related to urinary tract obstruction, and acute bacterial prostatitis. Hospital-acquired infections that occurred 48 hours after hospital admission or catheter-associated UTIs were excluded. Patients with UTIs who were diabetic, were immunosuppressed, or had other underlying chronic illnesses, and were thus associated with complex factors aside from urinary tract obstruction, were also excluded from the target patient group of this guideline.
3. Professionals for whom guideline use is recommended
This guideline has been developed for easy reference by health professionals, experts, and general physicians from various departments who treat UTIs in large-scale hospitals or clinics.
4. Principles and method of guideline update
This guideline was developed based on domestic and foreign clinical guidelines that could be referred to during the development period as well as recently published literature. This guideline may be subject to revisions if new antibiotic treatments for UTIs are developed or if important changes in the antibiotic resistance of causative bacteria of UTIs occur.
Summary of recommendations
II. The Process of Guideline Development
1. Forming a treatment guideline committee
The development committee consisted of a chairman (Seong-Heon Wie, the Catholic University College of Medicine) and five committee members recommended by the Korean Society for Chemotherapy and the Korean Society of Infectious Diseases, one committee member recommended by the Korean Association of Urogenital Tract Infection and Inflammation, two committee members recommended by the Korean Urological Association, and two committee members recommended by the Korean Society of Nephrology.
2. The process of treatment guideline development
The committee determined the method of treatment guideline development. The basic objective was to update the Korean guideline developed in 2011. For the existing key questions from the guideline, study findings that were published after the guideline was developed in 2011 were searched and added to the guideline, and the level of recommendation was determined. In addition, important questions that required additional findings were answered using the adaptation method. For questions regarding uncomplicated cystitis, the adaptation method was used. For the remaining four diseases, key questions were summarized and partially revised in the guideline.
1) Guideline search
Guidelines for the five diseases that were developed after 2011 were searched in the National Guideline Clearinghouse (NGC) and OVID MEDLINE. They were searched using disease names on the NGC, and using Mesh terms related to the diseases and guideline search filters on MEDLINE [1]. Search results are shown in Table 1. Two committee members selected seven guidelines for the five diseases from the search results.
Table 1. Guideline search results by disease.
| Disease | Number of guidelines found | Number after duplicate removal | |
|---|---|---|---|
| National Guideline Clearinghouse | OVID MEDLINE (search equation + guideline search filter) | ||
| Bacterial prostatitis | Search word: prostatitis | Search equation: prostatitis.mp. or exp Prostatitis/ | 3 |
| Result: 86 | Result: 17 | ||
| Selected: 1 | Selected: 2 | ||
| Cystitis | Search word: cystitis | Search equation: exp cystitis, interstitial/ or exp cystitis/ or cystitis.mp. | 6 |
| Result: 19 | Result: 89 | ||
| Selected: 1 | Selected: 5 | ||
| Bacteriuria | Search word: bacteriuria | Search equation: bacteriuria.mp. or exp bacteriuria/ | 3 |
| Result: 14 | Result: 44 | ||
| Selected: 2 | Selected: 3 | ||
| Complicated UTI | Search word: urinary tract infection | Search equation: urinary tract infections.mp. or exp urinary tract infections/ | 2 |
| Result: 113 | Result: 333 | ||
| Selected: 2 | Selected: 1 | ||
| Acute pyelonephritis | Search word: pyelonephritis | Search equation: exp pyelonephritis, xanthogranulomatous/ or exp pyelonephritis/ or pyelonephritis.mp. | 6 |
| Result: 12 | Result: 36 | ||
| Selected: 2 | Selected: 6 | ||
UTI, urinary tract infection.
2) Formulating key clinical questions
Referring to the seven selected guidelines, the key questions of the 2011 guideline were reorganized into the PICO format, and questions about uncomplicated cystitis were formulated, resulting in a total of 23 key questions.
3) Guideline assessment and selection
Three experts assessed the quality of the seven guidelines using the AGREE II tool. The quality assessment results are shown in Table 2. Through a discussion among the committee members, five guidelines to be included in the adaptation of the uncomplicated cystitis guideline were finally selected. The selected guidelines are listed in Table 2. The 2016 guideline by the Korean Association of Urogenital Tract Infection and Inflammation and the 2011 guideline by the Korean Society of Infectious Diseases had relatively low AGREE scores. They were nonetheless selected because they were domestically developed guidelines and thus have high applicability.
Table 2. Characteristics of the selected guidelines.
| AGREE Area of assessment | AGREE score | ||||||
|---|---|---|---|---|---|---|---|
| IDSA, 2010 | DGU, 2011 | SIGN, 2012 | EAU, 2015 | ACOG, 2016 | KAUTII, 2016 | KSID, 2011 | |
| Scope and purpose | 81.5% | 66.7% | 92.6% | 59.3% | 77.8% | 33.3% | 31.5% |
| Stakeholder participation | 42.6% | 57.4% | 81.5% | 42.6% | 18.5% | 18.5% | 25.9% |
| Strictness in the developmental process | 77.8% | 47.2% | 69.4% | 37.5% | 29.2% | 31.9% | 18.1% |
| Expression clarity | 88.9% | 66.7% | 88.9% | 74.1% | 70.4% | 64.8% | 44.4% |
| Applicability | 36.1% | 29.2% | 62.5% | 22.2% | 19.4% | 13.9% | 16.7% |
| Independence of editing | 72.2% | 22.2% | 50.0% | 83.3% | 5.6% | 5.6% | 27.8% |
| Overall result: recommended to use or not | Recommended | Not recommended | Recommended | Recommended | Not recommended | Recommended (revision needed) | Recommended (revision needed) |
| Selected or excluded? | Selected | Excluded | Selected | Selected | Excluded | Selected | Selected |
IDSA, Infectious Diseases Society of America; DGU, German Society of Urology; SIGN, Scottish Intercollegiate Guidelines Network; EAU, European Association of Urology; ACOG, American Congress of Obstetricians and Gynecologists; KAUTII, Korean Association of Urogenital Tract Infection and Inflammation; KSID, Korean Society of Infectious Diseases.
4) Adaptation
For the key questions on uncomplicated cystitis from the five selected guidelines, the recommendation status and the corresponding recommendation level were determined. The guideline committee assessed the acceptability and applicability of the guidelines based on these results.
5) Additional evidence search
After the guidelines selected for the revision and adaptation processes were confirmed to be up-to-date, additional evidence was searched for certain time periods. Two committee members selected the search results and included them in the revision contents.
6) Writing the guideline and determining the recommendation level
Recommendations were rewritten to be more relevant to the revised key questions in the revision process. In the adaptation process, the five selected guidelines were referred to create a new guideline.
The recommendation levels for asymptomatic bacteriuria, uncomplicated pyelonephritis, and acute bacterial prostatitis outlined in the 2011 guideline were revised as shown in Table 3. As part of the adaptation process, the recommendation levels were converted to criteria of GRADE methods.
Table 3. Revised recommendation levels.
| Levels of evidence of the 2011 guideline | Revised evidence level | |
|---|---|---|
| 1 | Evidence from at least one randomized controlled trial | High |
| 2 | Evidence from a well-designed clinical study, albeit not a randomized trial | Moderate |
| 3 | Expert opinions based on clinical experiences or committee reports | Very Low |
7) Consensus methodology on guideline adoption
The guideline committee held a meeting to discuss and to reach a consensus about the contents of the guidelines and their recommendation grades.
8) Assessment and review by external experts
A guideline established through a discussion among the guideline committee members was presented at the annual meeting hosted by the Korean Society for Chemotherapy and the Korean Society of Infectious Diseases, and at the primary physician educational program held by the Korean Society for Chemotherapy. The contents discussed at the conference were revised and improved through additional discussion among the committee members. The final, revised guideline was reviewed and approved by the Korean Society for Chemotherapy, the Korean Society of Infectious Diseases, the Korean Urological Association, the Korean Association of Urogenital Tract Infection and Inflammation, and the Korean Society of Nephrology.
9) Support
This guideline was developed with the support of the Policy Research Project of the Korea Centers for Disease Control and Prevention in 2017. The committee members who participated in the development of this guideline were not influenced by any government authorities, academic societies, pharmaceutical companies, or interest groups.
III. Clinical practice guidelines by specific diseases
1. Asymptomatic bacteriuria
1) Background and epidemiology
Asymptomatic bacteriuria is a common infection. It frequently affects people with anatomical or functional anomalies of the urinary system, and can also be observed in those with a normal urinary system [2,3]. In general, asymptomatic bacteriuria does not have secondary effects [4]. Unnecessary antibiotic administration for asymptomatic bacteriuria can induce antibiotic resistance and cause drug-related adverse events [5]. Therefore, treatment with antibiotics should be limited to those for whom the benefits of these antibiotics have been proven [5].
In women, the incidence of asymptomatic bacteriuria increases with age. Asymptomatic bacteriuria is detected in 20% of women aged 80 years or older. Asymptomatic bacteriuria is rare in men younger than 70 years of age. Approximately 5–10% of asymptomatic bacteriuria in men occurs in those aged 80 years or older. Asymptomatic bacteriuria does not increase the incidence of renal failure, hypertension, or mortality [6].
Escherichia coli is the most common causative microorganism of asymptomatic bacteriuria affecting women residing in local communities. Other common causative microorganisms include Enterobacteriaceae such as Klebsiella pneumoniae and gram-positive bacteria such as enterococci [7,8]. Although E. coli is the most common cause of asymptomatic bacteriuria among Korean inpatients, the incidence of asymptomatic bacteriuria caused by E. coli is lower among these domestic inpatients than among residents of local communities. In intensive care units, antibiotic-resistant enterococci, K. pneumoniae, Pseudomonas aeruginosa, and Candida are also frequently isolated [9,10].
2) Diagnosis
Asymptomatic bacteriuria is defined as a significant level of bacteria in the urine in the absence of symptoms or signs of UTIs. A significant level of bacteria is defined as a bacterial count of 105 of identical bacteria species per 1 mL of clean-catch midstream urine in two cultures [2,3]. For men, a bacterial count of 105 per 1 mL of clean-catch midstream urine in only one culture may be significant. For catheter urine samples collected from men and women, a bacterial count of 102 per 1 mL of urine may be significant [2,3].
3) Guideline by key questions
① Can the treatment of asymptomatic bacteriuria prevent the symptomatic UTIs and perinatal complications in pregnant women?
<Recommendation>
Pregnant women in early gestational stages should be screened for bacteriuria and bacteriuria in pregnant women should be treated (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
According to a guideline published by the Infectious Diseases Society of America (IDSA) in 2005, women in early gestational stages should be screened and treated for bacteriuria [2]. However, recent study results raise a question as to whether bacteriuria screening is necessary for pregnant women. In a meta-analysis/systematic review of 14 randomized controlled trials (RCTs) on 2,302 pregnant women with asymptomatic bacteriuria, although the incidence of pyelonephritis, low birth weight, and premature birth were significantly reduced in antibiotic-treated groups relative to the control groups, the authors emphasized that their meta-analysis was based on low-quality studies [11]. Although asymptomatic bacteriuria was associated with the incidence of pyelonephritis in pregnant women in an RCT conducted in the Netherlands, the risk of asymptomatic bacteriuria was low [12]. This study also reported no association between asymptomatic bacteriuria and premature birth. A recent systematic review reported that asymptomatic bacteriuria screening has no benefits for pregnant women [13]. Based on these study results, the level of evidence for bacteriuria screening and treatment in the early gestational period was lowered. However, considering the importance of the health of both pregnant mother and infants, the recommendation grade was maintained at strong until similar study results were added.
② Can the treatment of asymptomatic bacteriuria prevent symptomatic UTIs in non-pregnant women?
<Recommendation>
The screening and treatment of asymptomatic bacteriuria are not recommended for non-pregnant women (level of evidence: high; recommendation grade: strong).
<Summary of evidence>
In an RCT involving 673 young women with asymptomatic bacteriuria, no difference in the incidence of bacteriuria was found between the treatment and control groups at 3 months after treatment; however, the incidence of recurrent bacteriuria was higher in the treatment group at 6 months (29.7% vs. 7.6%, P <0.0001) [14]. In another RCT, young women who received antibiotic treatment for asymptomatic bacteriuria had higher antibiotic resistance to the bacteria that were later isolated [15]. Asymptomatic bacteriuria did not affect long-term prognoses (hypertension, chronic kidney disorders, cancer of the urogenital system, or reduced survival time) [2]. Treatment of asymptomatic bacteriuria did not decrease the frequency of symptomatic UTIs or the incidence of asymptomatic bacteriuria [2]. According to the guidelines from the IDSA and the European Association of Urology (EAU), there is no need to screen or treat premenopausal, non-pregnant women for asymptomatic bacteriuria [2,3]. In an RCT involving elderly women with asymptomatic bacteriuria and without mobility difficulties, the incidence of bacteriuria decreased in the antibiotic-treated groups (one-time administration of TMP/SMX or 3-day administration of cefaclor) relative to the control group at 6 months after treatment (36.4% vs. 65.5%, P = 0.004). However, the incidence of symptomatic UTIs was similar among the groups during the 6 months (7.9% vs. 16.4%, P = 0.18). Therefore, there was no difference in the incidence of symptomatic UTIs even after antibiotic treatment [16]. The IDSA and the EAU also do not recommend asymptomatic bacteriuria screening and treatment for elderly women residing in local communities [2,3].
③ Can the treatment of asymptomatic bacteriuria prevent symptomatic UTIs in female residents of nursing homes?
<Recommendation>
The screening and treatment of asymptomatic bacteriuria are not recommended for female residents of nursing homes (level of evidence: high; recommendation grade: strong).
<Summary of evidence>
A prospective controlled study in which elderly residents of nursing homes were treated with antibiotics for asymptomatic bacteriuria reported no benefits of screening and treatment of asymptomatic bacteriuria [17,18,19]. Antibiotic treatment did not decrease the incidence of symptomatic UTIs, prolong survival, or alleviate chronic urogenital symptoms [17,18,19]. Instead, the incidence of adverse events from the antibiotics increased, and subjects were later infected by antibiotic-resistant bacteria [18]. The IDSA and the EAU also do not recommend screening and treating women residing in nursing homes for asymptomatic bacteriuria [2,3].
④ Can the treatment of asymptomatic bacteriuria prevent symptomatic UTIs in diabetic women?
<Recommendation>
The screening and treatment of asymptomatic bacteriuria are not recommended for diabetic women (level of evidence: high; recommendation grade: strong).
<Summary of evidence>
In a prospective RCT in which diabetic women with asymptomatic bacteriuria were followed up every 3 months for up to 3 years, antibiotic treatment showed no benefits [20]. Antibiotic treatment did not delay or inhibit the development of symptomatic UTIs and did not reduce the number of hospital admissions for reasons not exclusive to UTIs. The number of days of antibiotic administration was five times higher and the incidence of adverse drug reactions caused by the antibiotic agents was significantly higher in the antibiotic-treated group than in the control group. In another RCT involving diabetic women, antibiotic treatment against asymptomatic bacteriuria reduced the duration of bacteriuria and significantly shortened the period during which subjects were carriers of certain bacterial strains; however, the bacteria formed new colonies following treatment in most subjects [21] The IDSA and EAU do not recommend screening and treatment of asymptomatic bacteriuria for diabetic women [2,3].
⑤ Can the treatment of asymptomatic bacteriuria prevent symptomatic UTIs in patients with spinal cord injuries?
<Recommendation>
The screening and treatment of asymptomatic bacteriuria are not recommended for patients with spinal cord injuries (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
In a small controlled experiment and an RCT involving patients with spinal cord injuries and asymptomatic bacteriuria who were on intermittent catheterization, there was no difference in the incidence of symptomatic UTIs or recurrent bacteriuria between the antibiotic-treated group and the control group [22,23]. The IDSA and EAU do not recommend the screening and treatment of asymptomatic bacteriuria for patients with spinal cord injuries [2,3].
⑥ Can the treatment of asymptomatic bacteriuria prevent symptomatic UTIs in patients with indwelling urinary catheters?
<Recommendation>
The screening and treatment of asymptomatic bacteriuria are not recommended for patients with indwelling urinary catheters (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
Treatment of asymptomatic bacteriuria in patients with indwelling urinary catheters in intensive care units does not decrease the incidence of symptomatic UTIs [24,25,26]. The IDSA and EAU do not recommend the screening and treatment of asymptomatic bacteriuria for patients with indwelling urinary catheters [2,3,27].
⑦ Can the treatment of asymptomatic bacteriuria prevent infectious complications in patients undergoing urological procedures such as transurethral resection of the prostate (TUR-P) in which mucosal bleeding is predicted?
<Recommendation>
Patients undergoing urological procedures in which mucosal bleeding is expected are recommended to be screened and treated for asymptomatic bacteriuria before undergoing the procedures (level of evidence: high; recommendation grade: strong).
<Summary of evidence>
In four RCTs involving men with bacteriuria scheduled for TUR-P, antibiotic administration effectively prevented infectious complications [28,29,30,31]. The IDSA and EAU recommend patients scheduled for TUR-P to be screened and treated for asymptomatic bacteriuria [2,3]. Other urological procedures that are highly likely to cause mucosal bleeding are also associated with the risk of infectious complications [32]. It is therefore recommended that patients be screened and treated for asymptomatic bacteriuria before they undergo urological procedures that are likely to cause mucosal bleeding. Antibiotic administration should begin the night before the day of the procedure or immediately before the procedure [32,33] and end immediately upon completion of the procedure [30,31,33].
2. Acute uncomplicated cystitis
1) Background and epidemiology
Acute uncomplicated UTIs are common infectious diseases that are experienced by approximately 10% of women at least every year and by 60% of women at least once in their lifetime [34]. The diseases recur in approximately 5% of women, and approximately 44% of them recur within 1 year. For this reason, acute uncomplicated UTIs are the diseases for which antibiotic treatment is frequently prescribed [35]. In the United States and Europe, approximately 15% of all antibiotics used in outpatient clinics are prescribed for UTIs [36].
UTIs are classified as upper UTIs (pyelonephritis) and lower UTIs (cystitis) depending on the infected site. UTIs are also classified as uncomplicated in the absences of structural or neurological abnormalities of the urological system and as complicated otherwise. This section mainly discusses uncomplicated cystitis in women.
Uropathogenic E. coli accounts for 70–83% of all major causative bacteria of uncomplicated cystitis [37,38,39]. Therefore, empirical treatment of uncomplicated cystitis should target E. coli, and appropriate antibiotic selections should be made according to the patterns of the bacteria's antibiotic resistance. Results regarding antibiotic susceptibility from recent domestic studies on E. coli isolated from uncomplicated cystitis are shown in Table 4.
Table 4. Antibiotic susceptibility of Escherichia coli isolated from Korean patients with uncomplicated cystitis.
| Antimicrobial agent | Year of study | ||||
|---|---|---|---|---|---|
| 2006/2002 [40] | 2008 [38] | 2009 [37] | 2010–2014 [41] | 2013–2015 [42] | |
| Ampicillin | 35.2/37.2 | 46.7 | 38.5 | 35.3 | 30.4 |
| Ampicillin/sulbactam | 52.4/44.5 | 83.5 | - | - | - |
| Amoxicillin/clavulanate | - | - | 80.7 | 84.5 | 64.6 |
| Piperacillin/tazobactam | 98.6/97.4 | 98.8 | - | 96 | 94.8 |
| Ciprofloxacin | 76.6/84.8 | 79.6 | 74.6 | 58.3 | 73.6 |
| Gatifloxacin | 78.2/NA | - | - | - | - |
| Cefazolin | 92.4/92.2 | - | 86 | 83.8 | 72.1 |
| Amikacin | 99.5/99.0 | 99.1 | 99.5 | 100 | 99.5 |
| Gentamicin | 77.6/81.7 | 79.8 | 76.6 | 69.1 | 72.3 |
| Tobramycin | 78.2/85.9 | 82.9 | 80.9 | 74.8 | - |
| TMP/SMX | 70.6/61.3 | 67 | 67.3 | 66 | 61.6 |
| Cefuroxime | - | - | 86.1 | - | - |
| Ceftriaxone | - | 95.3 | 94.7 | - | - |
| Ertapenem | - | - | - | 100 | 99.8 |
| Imipenem | - | - | - | 100 | 99.5 |
| Cefoxitin | - | - | - | 92.9 | 89.8 |
| Cefepime | - | 95.3 | - | 92.3 | 77.6 |
| Ceftazidime | - | - | - | 93.1 | 76.1 |
| Cefotaxime | - | 95.1 | - | 87.3 | 75.8 |
| Aztreonam | - | 97.1 | - | 90.7 | - |
TMP, trimethoprim; SMX, sulfamethoxazole.
Results are shown as percentages.
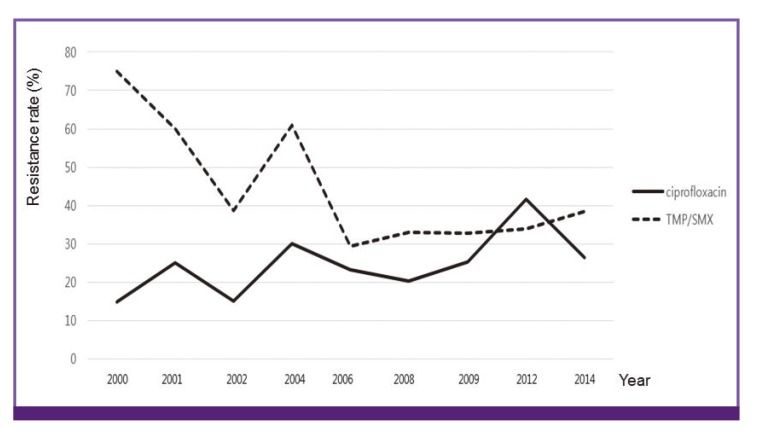
Although the antibiotic resistance to ciprofloxacin, which was increasingly used as an empirical antibiotic for acute cystitis, increased, that to TMP/SMX, which has been used less and less over time, decreased [40,41] (Fig. 1).
Figure 1.
Changes in the antibiotic resistance of Escherichia coli isolated from cystitis to ciprofloxacin and trimethoprim/sulfamethoxazole (TMP/SMX)
Resistance patterns also differ across regions [42]. In 2006, the antibiotic resistance of E. coli isolated from cystitis to ciprofloxacin was 24.6% in Seoul, 40.0% in Gyeongsang Province, 14.7% in Gyeonggi Province, and 32.1% in Chungcheong Jeolla Province [40]. A study conducted during a similar period reported antibiotic resistance of 24.6% in South Jeolla Province [43]. Therefore, selection of empirical antibiotics should be based on data from different time periods and regions.
2) Diagnosis
Acute cystitis can be diagnosed by the presence of clinical symptoms and pyuria [44].
(1) Clinical diagnosis
A patient's medical history is crucial in the diagnosis of uncomplicated cystitis. In the absence of vaginal secretions or pains, the positive predictive value of acute cystitis screening is 90% in the presence of newly developed polyuria, dysuria, and urinary urgency [45]. Acute cystitis may be accompanied by hematuria and urinary incontinence. Clinical symptoms of acute cystitis often go unreported by older adults with cognitive impairment or may present as systemic symptoms such as lower abdominal pain; therefore, acute cystitis screening should be performed with care [46].
(2) Laboratory diagnosis
Pyuria is defined as a white blood cell count of 10 or more in a high-magnification analysis of uncentrifuged urine [3,47]. The urine dipstick test or microscopy analysis may be used to assess pyuria and bacteriuria. A urine culture is performed to screen for bacteriuria, and an antibiotic susceptibility test is performed using the cultured pathogens. For women showing symptoms of pyuria, a colony count of 103 colony forming units/mL in midstream urine holds diagnostic significance [48]. However, women showing typical symptoms can immediately be diagnosed and treated for cystitis, and laboratory diagnosis is not required in such cases.
3) Recommendation by key question
① Does the clinical course of acute cystitis change according to the types of antibiotics used and the duration of antibiotic treatment for patients with acute uncomplicated cystitis?
<Recommendation>
The following antibiotics can be recommended for the treatment of acute uncomplicated cystitis in outpatient clinics.
ⓐ Nitrofurantoin monohydrate/macrocrystals – Recommended dosage: 100 mg per administration, twice daily, for more than 5 days (level of evidence: high; recommendation grade: strong).
ⓑ Fosfomycin trometamol – Recommended dosage: one-time administration of 3 g. (Level of evidence: high; recommendation grade: strong).
ⓒ Pivmecillinam - Recommended dosage: 400 mg per administration, three times daily, for more than three days (level of evidence: high; recommendation grade: strong).
ⓓ Oral fluoroquinolones for more than three days. (Level of evidence: low; recommendation grade: strong).
ⓔ β-Lactams, oral cephalosporins may be used. Administration of cefpodoxime, cefdinir, cefcapene, cefditoren, and cefixime for 5 or more days is recommended (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
A. Empirical antibiotics for outpatients
When selecting empirical antibiotics, the individual patient's situation, antibiotic resistance within each local community, treatment costs, and treatment failure rates should be considered. The antibiotics recommended by this guideline are indicated for women diagnosed with community-acquired acute cystitis who are at low risk of getting infected by resistant bacteria. If patients do not show a treatment response, the possibility of antibiotic resistance is reevaluated, and appropriate antibiotics should be used (Table 5).
Table 5. Empirical antibiotics that may be used for domestic cases of acute uncomplicated cystitis.
| Empirical antibiotics | Dosage | Minimum duration, days | |
|---|---|---|---|
| Fosfomycin | One-time administration of 3 g | 1 | |
| Ciprofloxacin | 500 mg, twice daily | 3 | |
| 250 mg, twice daily | |||
| β-Lactams | |||
| Cefpodoxime proxetil | 100 mg, twice daily | 5 | |
| Cefdinir | 100 mg, three times daily | 5 | |
| Cefcapene pivoxil | 100 mg, three times daily | 5 | |
| Cefditoren pivoxil | 100 mg, three times daily | 3 | |
| Cefixime | 400 mg, once daily | 3 | |
| 200 mg, twice daily | |||
| After introduction in Korea | |||
| Nitrofurantoin | 100 mg, twice daily | 5 | |
| Pivmecillinam | 400 mg, three times daily | 3 | |
| After antibiotic susceptibility test | |||
| Amoxicillin/clavulanate | 500/125 mg, twice daily | 7 | |
| TMP/SMX | 160/800 mg, twice daily | 3 | |
Abbreviations: TMP, trimethoprim; SMX, sulfamethoxazole.
Based on foreign guidelines, fosfomycin is an appropriate choice for uncomplicated cystitis in Korea. With the current situation in Korea taken into account, however, the use of fluoroquinolone, oral β-lactam, and more importantly, cephalosporin is inevitable until primary antibiotics such as nitrofurantoin and pivmecillinam are introduced.
(a) Fluoroquinolones
Fluoroquinolones have been used as primary medications against uncomplicated cystitis in regions with high resistance to other antibiotics. Fluoroquinolones are highly preferred as empirical antibiotics in Korea where E. coli is highly resistant to TMP/SMX.
In a comparison between fluoroquinolones and TMP/SMX, both antibiotics improved short-term and long-term symptoms to a similar extent, and in a comparison between fluoroquinolones and β -lactams, fluoroquinolones more effectively improved short-term symptoms and produced better bacterial treatment outcomes [49]. There is no difference in the clinical or microbiological effects among fluoroquinolones in the treatment of acute uncomplicated cystitis, and a 3-day therapy using ciprofloxacin, levofloxacin, and ofloxacin may be used [50].
Fluoroquinolone antibiotics differ in their stability in terms of the frequency and patterns of adverse drug reactions. Adverse reactions in ligaments, muscles, joints, nerves, and the central nervous system have been reported following fluoroquinolone antibiotics use. Accounting for these severe side effects, the U.S. Food and Drug Administration recommended not using fluoroquinolones against acute sinusitis, acute bronchitis, and uncomplicated UTIs when other antibiotics can be used [51]. The Ministry of Health and Welfare of Korea has announced a revision that does not acknowledge medical care expenses arising from the oral administration of ciprofloxacin as a primary medication for uncomplicated UTIs. Considering the resistance to antibiotics used for complicated UTIs in Korea, antibiotics such as nitrofurantoin and pivmecillinam, which can be used as primary empirical antibiotics, are currently in poor supply. Until antibiotics such as nitrofurantoin and pivmecillinam are introduced and widely used in Korea, the use of fluoroquinolones as primary antibiotics in clinical settings is inevitable.
(b) Fosfomycin trometamol
In a meta-analysis comparing the therapeutic effects of fosfomycin against cystitis with those of other antibiotics, fosfomycin produced similar effects to fluoroquinolones, TMP/SMX, β-lactams, and nitrofurantoin and caused fewer adverse events [52].
In an epidemiological study on the antibiotic resistance of the causative bacteria of cystitis conducted in Europe and Brazil, E. coli was the most common causative bacterium (76.7%), which showed high susceptibility to fosfomycin (98.1%) [53]. ESBL-producing bacteria, AmpC β-lactamase-producing bacteria, and carbapenem-nonsusceptible, multidrug-resistant E. coli are also highly susceptible to fosfomycin [54]. In a domestic study, E. coli that showed 22% resistance rate and 29.2% resistance rate to TMP/SMX showed 0% resistance rate to fosfomycin [55]. Therefore, it is appropriate to use fosfomycin as a primary empirical antibiotic for acute uncomplicated cystitis in Korea.
(c) β-Lactam antibiotics
In a study comparing symptom improvement and therapeutic effects between groups treated with β-lactam and nitrofurantoin, and another study of groups treated with β-lactam and TMP/SMX, no significant differences were found between either of the two groups. In an intention-to-treat analysis of β-lactams, fluoroquinolones were more effective than β-lactams [49].
Considering the antibiotic resistance of bacteria within Korea, cephalosporins, which are β-lactam antibiotics, may be used empirically. Of these, second- and third-generation cephalosporins may be considered. These antibiotics have indication for the treatment of UTIs. In a study comparing the effects of oral cephalosporins, cefpodoxime was inferior to ciprofloxacin in terms of therapeutic effects [56]. However, no difference in therapeutic effects was found between cefpodoxime and TMP/SMX [57]. In a comparison between cefixime, ofloxacin, and ciprofloxacin, there was no difference in therapeutic effects among these antibiotics [58,59]. Regarding antibiotic resistance to oral antibiotics, antibiotic susceptibility to cefuroxime, cefpodoxime, and amoxicillin/clavulanate was 86.1%, 93.6%, and 80.7%, respectively, based on 2008–2009 data [37]. In 2007–2008 data, antibiotic susceptibility to cefcapene pivoxil was 88.9%, and administration of 100 mg of cefcapene pivoxil, three times daily for 5 days led to significant symptom improvement and bacterial treatment efficacy [60]. In 2004–2005 data, antibiotic susceptibility to cefixime was reported at 87.3% [61]. Although there is no susceptibility data for the causative bacteria of uncomplicated cystitis, the susceptibility of E. coli isolated from community-acquired UTIs to cefaclor and cefuroxime was reported to be 90% and 90%, respectively, in 2003. Antibiotic susceptibility may be estimated based on these data [62]. On the other hand, the antibiotic susceptibility to cefaclor in E. coli isolates from pediatric UTIs was very low at 6.4% in 2004–2005 [63]. Additional research data are needed before cefaclor can be used as an empirical antibiotic.
The antibiotic resistance of E. coli isolated in Korea to amoxicillin/clavulanate is reported at 20–35%, and it is not yet possible to recommend amoxicillin/clavulanate as a primary empirical antibiotic until antibiotic susceptibility to this antibiotic is assessed in urine culture tests.
(d) Trimethoprim/sulfamethoxazole
In Korea, TMP/SMX can be used to treat patients with cystitis only after the antibiotic susceptibility of the causative bacteria is tested. In the past, TMP/SMX had been a standard medication that was recommended as a primary antibiotic in place of fluoroquinolones, which are expensive, when the antibiotic resistance to TMP/SMX was less than 20% [64]. In cases where the causative bacteria show susceptibility to TMP/SMX, the antibiotic is on a par with fluoroquinolones in terms of short-term symptom improvement and therapeutic effects from the microbiological aspect [49]. TMP/SMX produced similar symptom improvement and therapeutic effects to those of nitrofurantoin.
When antibiotic resistance within the community is less than 20%, the use of TMP/SMX as an empirical antibiotic is recommended. However, domestic antibiotic susceptibility data show that the antibiotic resistance to TMP/SMX has consistently been over 35%, making it difficult to recommend TMP/SMX as an empirical antibiotic [65]. Once antibiotic susceptibility to TMX/SMX is verified through a urine culture test, it may be used as an empirical antibiotic.
B. Antibiotics that should be domestically introduced
(a) Nitrofurantoin
The antibiotic resistance of E. coli to nitrofurantoin is very low at 0.6% in Korea [55]. It is not yet possible to use the antibiotic in Korea as certain conditions are unmet and the antibiotic has not been introduced in the country. Considering the increasing demand for antibiotics for acute uncomplicated cystitis and the increasing antibiotic resistance of the causative bacteria, the introduction of nitrofurantoin is urgently needed.
(b) Pivmecillinam
Many guidelines recommend the use of pivmecillinam as a primary empirical antibiotic. It is currently not possible to use this antibiotic in Korea. A recent meta-analysis reported the dose and duration of use of pivmecillinam in the treatment of uncomplicated cystitis [66]. Currently, American guidelines recommend taking the antibiotic 400 mg at a time, twice daily, for 3–7 days. European guidelines recommend 400 mg at a time, three times daily. According to the meta-analysis, there are not enough data to determine the dose, frequency of use, and duration of use of pivmecillinam, and additional research is required. After the antibiotic is introduced in Korea, it should be used with caution.
C. The duration of antibiotic use for acute uncomplicated cystitis
The duration of use varies among antibiotics in the treatment of uncomplicated cystitis. Two Cochrane analyses have been performed to determine how many days of antibiotic use would produce the best treatment outcomes when an identical antibiotic is used. Although there was no difference in symptom improvement between 3 days and 5–10 days of antibiotic use in women with uncomplicated cystitis, the best therapeutic effects from the microbiological aspect were obtained at 5–10 days [67]. However, adverse events occurred significantly more frequently in the 5- to-10-day treatment group. Therefore, antibiotic treatment lasting 5–10 days may be considered only in cases where bacterial eradication is required. When the effects of antibiotics after one treatment session, short-term treatment lasting 3–6 days, and long-term treatment lasting 7–14 days were compared in older adults (aged over 60 years) with uncomplicated cystitis, there were no differences in therapeutic effects between the short-term and long-term treatments [68]. There was also no difference in the clinical and bacterial therapeutic effects of cefditoren pivoxil for uncomplicated cystitis between a 3-day and a 7-day treatment [69].
When the recurrence rate was compared between treatment lasting no more than 5 days and treatment lasting over 5 days regardless of the type of antibiotic used, the longer-term treatment did not reduce the recurrence rate [70].
Based on these results, short-term treatment lasting 3–5 days may be used to treat uncomplicated cystitis, and a longer treatment period may be considered in some cases. Three or more days of antibiotic treatment may be more effective in cases where bacterial treatment is required [71]. Patients who are suspected of having upper UTIs or complicated UTIs should be reassessed to determine the duration of antibiotic use.
② Is a urine culture test necessary for patients with acute uncomplicated cystitis?
<Recommendation>
ⓐ A urine culture test should be performed for the following patients: patients suspected of having pyelonephritis, patients who exhibit atypical symptoms, pregnant patients, male patients suspected of having UTIs, and patients whose symptoms did not improve within 2–4 weeks after treatment completion or whose symptoms recurred (level of evidence: low; recommendation grade: strong).
ⓑ In Korea, it is appropriate to perform a urine culture test owing to the increased antibiotic resistance of the causative bacteria of UTIs (level of evidence: very low; recommendation grade: strong).
<Summary of evidence>
A urine culture test is recommended to assess the possibility of an infection by causative bacteria or to assess antibiotic susceptibility in the following situations: 1) a patient suspected of having pyelonephritis, 2) a patient exhibiting atypical symptoms, 3) a pregnant patient, 4) a male patient suspected of having UTIs, and 4) a patient whose symptoms did not improve within 2–4 weeks after treatment completion or recurred.
Existing guidelines recommend immediately starting empirical treatment on patients with uncomplicated cystitis with typical symptoms without performing a urine culture test. This recommendation has low to very low levels of evidence based on a review of the related literature. According to studies on which this recommendation is based, a urinary culture test is associated with a contamination rate of 30% [72], and disinfection before urination does not affect the contamination rate [73]. In a cost-utility analysis performed in the United States in 1997, administration of empirical antibiotics for 7 days was more cost-effective than when a treatment decision was made after performing other tests [74]. It was thus suggested that a urine culture test may not be necessary based on its cost-effectiveness, which considers the antibiotic, medical, and examination costs related to treatment strategies [75].
However, it is difficult to apply the same cost-effectiveness concept in Korea where the standard treatment of acute uncomplicated cystitis has been changed to 3 days of empirical antibiotic treatment, and the fees for outpatient examinations, the dipstick test, and urine culture test are relatively inexpensive compared with those in the United States and Europe. According to recent data, approximately 24.6% of E. coli inducing community-acquired UTIs produce ESBL [42]. Therefore, systematic analysis is needed to investigate whether maintaining empirical antibiotic treatment without assessing antibiotic resistance of causative bacteria through a urine culture test would be appropriate in terms of treatment outcomes and prognoses.
An existing guideline recommends selecting and using empirical antibiotics when the antibiotic resistance of the causative bacteria of acute uncomplicated cystitis in the community is less than 20% [64]. With the increasing trend of antibiotic resistance in the country, performing empirical treatment alone without a urine culture test can lead to high treatment failure rates. Additional data on resistance trends based on culture test results are needed to make appropriate choices regarding empirical antibiotics. Therefore, it is necessary to perform a urine culture test even in the case of acute uncomplicated cystitis in Korea.
3. Acute uncomplicated pyelonephritis
1) Background and epidemiology
UTIs are among the most common bacterial infections. Approximately 40–50% of women are reported to experience UTIs at least once in their lifetime [76,77]. Uncomplicated UTIs are defined as symptoms of acute cystitis or pyelonephritis that occur in healthy premenopausal women without any structural or functional anomalies of the urinary tract [78].
Acute pyelonephritis or upper UTIs are kidney infections that manifest as pain during urination, fever, chills, flank pain, nausea, and vomiting. Their incidence has been reported at 35.7 cases per 10,000 persons in Korea [79], and 27.6 cases per 10,000 persons in the United States [80]. Although the worldwide prevalence and incidence of acute pyelonephritis is unknown, its incidence is reported to be the highest in the summer [81]. The number of hospital admissions owing to acute pyelonephritis is five times higher among women than among men [82]. Acute pyelonephritis mostly occurs when bacteria invade the kidney through the ureter or spread to the body through the bloodstream [78]. The most frequently isolated causative bacterium of acute pyelonephritis is E. coli (56–85%). Other causative bacteria include Enterococcus faecalis, K. pneumoniae, and Proteus mirabilis [80,83,84]. E. coli is the most common causative bacterium in Korea. Other bacteria such as K. pneumonia, P. mirabilis, Enterococcus spp., and Staphylococcus saprophyticus have also been isolated in Korea [40,85,86,87,88,89]. As shown in Table 6, the antibiotic susceptibility of E. coli isolated from patients with acute pyelonephritis to ciprofloxacin and TMP/SMX has gradually decreased. According to recent reports, the antibiotic susceptibility is 78.7% and 72.2%, respectively, for ciprofloxacin and TMP/SMX. This is lower than the antibiotic susceptibility reported in the United States of 82.9% and 75.8%, respectively [90,91].
Table 6. Antibiotic susceptibility of Escherichia coli isolated from Korean patients with acute pyelonephritis.
| Antibiotic susceptibility (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| AMK | AMP | SXT | GEN | CIP | CFZ | CFU | CTX | |
| Wie et al., 2002 [87] | 99.2 | NA | 63.3 | 81.8 | 92.5 | 41.7 | 99.2 | 99.2 |
| Hwang et al., 2003 [86] | NA | 31 | 42.6 | 83.6 | 88.5 | NA | NA | 100 |
| Wie et al., 2007 [85] | 98.7 | 38.3 | 62.1 | 81.3 | 86.3 | NA | 97.3 | 97.3 |
| Kim et al., 2008 [40] | 99.5 | 35.2 | 70.6 | 77.6 | 76.6 | 92.4 | NA | NA |
| Wie et al., 2014 [91] | 97.5 | 39.4 | 72.2 | 77.4 | 78.7a | 77.1 | 92.9 | 90.7 |
AMK, amikacin; AMP, ampicillin; SXT, trimethoprim/sulfamethoxazole; GEN, gentamicin; CIP, ciprofloxacin; CFZ, cefazolin; CFU, cefuroxime; CTX, cefotaxime.
aCiprofloxacin or levofloxacin.
2) Diagnosis
Acute pyelonephritis is characterized by the symptoms of upper UTIs and pyuria (a white blood cell count of 10 or more in a high-magnification analysis). Gram staining and a culture test should be performed if a patient is suspected to have acute pyelonephritis [78,88]. Although a blood culture test is not necessary, it is helpful [44,88]. A positive urine culture is defined as a bacterial count in properly collected clean midstream urine is greater than 105 colony forming units (CFU)/mL. However, this standard results in high specificity and low sensitivity. As a result, in 30–50% of cystitis cases caused by E. coli, S. saprophyticus, and Proteus spp., 102–104 CFU/mL are cultured, and therefore, the standard should be applied in clinical settings with caution [92]. In many laboratories, bacteria with less than 104 CFU/mL in a urine culture test are not quantified. Therefore, study reports of “no growth” in women exhibiting symptoms of UTIs should be interpreted carefully [78]. Approximately 80% of patients with acute pyelonephritis accompanied by bacteremia show over 105 CFU/mL, and 10–15% show 104–105 CFU/mL in their urine. Using bacterial growth of 104 CFU/mL in midstream urine as the diagnostic criteria for bacteriuria based on this observation, 90–95% sensitivity is obtained [92].
In general, acute pyelonephritis is diagnosed based on clinical symptoms and urine test results, and does not require medical imaging unless it is a complicated UTI. However, if a patient does not show any response to antibiotic administration even after 72 hours, medical imaging tests should be performed. Of the available medical imaging techniques, abdominal computed tomography (CT) is reported to be useful [93].
3) Guideline by key question
① What is an effective antibiotic treatment for acute uncomplicated pyelonephritis in adults?
<Recommendation>
ⓐ All patients with acute pyelonephritis should undergo a urinary culture test before empirical antibiotic administration (level of evidence: very low; recommendation grade: strong).
ⓑ The initial empirical antibiotics administered in the early period of treatment should be adjusted according to the antibiotic susceptibility test results of the causative bacteria (level of evidence: very low; recommendation grade: strong).
ⓒ For early empirical antibiotic administration for patients with acute pyelonephritis who do not require hospitalization, 1–2 g of intravenous ceftriaxone or 1 dose of amikacin should be administered, followed by oral fluoroquinolone until results are obtained from the culture test (level of evidence: very low; recommendation grade: strong).
ⓓ For early empirical antibiotic administration for patients with acute pyelonephritis who do not require hospitalization, 400 mg of intravenous ciprofloxacin may be administered, followed by oral ciprofloxacin (500 mg, twice daily) until results are obtained from the culture test (level of evidence: low; recommendation grade: weak).
ⓔ If the causative bacteria show susceptibility to antibiotics in the culture test, oral antimicrobial agents such as fluoroquinolone, TMP/SMX, and β-lactams may be used (level of evidence: high; recommendation grade: strong).
ⓕ If the causative bacteria show susceptibility in patients with acute pyelonephritis, oral antibiotics are administered for 7–14 days: ciprofloxacin (500 mg, twice daily for 7 days or sustained-release ciprofloxacin, 1000 mg, once daily for 7–14 days) (level of evidence: high; recommendation grade: strong), levofloxacin (500 mg, once daily for 7 days, or 750 mg, once daily for 5 days) (level of evidence: high; recommendation grade: strong), TMP/SMX (160/800 mg, twice daily for 14 days) (level of evidence: high; recommendation grade: strong), and oral β-lactams (10–14 days) (level of evidence: very low; recommendation grade: strong).
ⓖ For patients with acute pyelonephritis who require hospitalization, fluoroquinolone (level of evidence: low; recommendation grade: weak), aminoglycoside ± ampicillin (level of evidence: low; recommendation grade: weak), second-generation cephalosporin (level of evidence: low; recommendation grade: weak), broad-spectrum cephalosporin (level of evidence: high; recommendation grade: strong), β-lactam/β-lactamase inhibitor ± aminoglycoside (level of evidence: low; recommendation grade: weak), aminoglycoside ± β-lactam (level of evidence: low; recommendation grade: weak), and carbapenem (level of evidence: low; recommendation grade: weak) may be administered. Once the fever is alleviated, the antibiotic may be changed to an oral antibiotic agent chosen based on antibiotic susceptibility and resistance of the causative bacteria to the new antibiotic (level of evidence: low; recommendation grade: strong).
ⓗ For patients with acute pyelonephritis who should be admitted to an intensive care unit due to severe sepsis or septic shock, piperacillin/tazobactam or carbapenem are administered after considering the antibiotic resistance of the causative bacteria in the country (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
A. All patients with acute pyelonephritis should be subjected to a urinary culture test before empirical antibiotic administration.
A urinary culture test is performed to confirm the diagnosis of bacteremia and to obtain antibiotic susceptibility results. It should be performed for all patients suspected of having acute pyelonephritis [78,88,94]. Although patients with acute uncomplicated cystitis show a satisfactory response to treatment, and the infection rarely progresses to pyelonephritis, acute uncomplicated pyelonephritis can progress to severe disease and requires appropriate antibiotic treatment chosen based on the antibiotic susceptibility of the causative bacteria [94].
B. Early empirical antibiotic treatment should be carefully adjusted according to the antibiotic susceptibility test results of the causative bacteria.
If not treated properly, acute uncomplicated pyelonephritis can progress to severe diseases such as abscess formation, septic shock, and kidney damage including acute renal failure [78,95]. Therefore, it is necessary to treat the infection using antibiotics appropriately selected based on the antibiotic susceptibility of the causative bacteria and to prevent disease progression. Early empirical treatment should be appropriately regulated according to the bacterial sensitivity of the causative bacteria [94].
C. For early empirical antibiotic administration for patients with acute pyelonephritis who do not require hospitalization, 1–2 g of intravenous ceftriaxone or one day's dose of amikacin should be administered, followed by oral fluoroquinolone until results are obtained from the culture test.
The prevalence of antibiotic resistance to certain antibiotics that may require a treatment strategy to avoid early use of common empirical antibiotics owing to predicted resistance of the causative bacteria to the antibiotics, and instead to use more broad-spectrum antibiotics that are later replaced by selective antibiotics, in the empirical antibiotic treatment of acute pyelonephritis is relatively low compared with that in the treatment of uncomplicated cystitis. The threshold of the prevalence of antibiotic resistance to fluoroquinolones, which are currently the most effective recommended antibiotics, is 10%. If the prevalence of antibiotic resistance exceeds the threshold, early use of other empirical antibiotics is recommended. However, evidence to support the recommendation is limited, and the recommendation is made based on expert opinions [94]. The current status regarding antibiotic resistance in hospitals reflects the culture results obtained from inpatients, or patients with complicated UTIs or repeated infections, and may overestimate the antibiotic resistance of patients with uncomplicated UTIs [78]. In the antibiotic susceptibility results of E. coli isolated from Korean patients with acute pyelonephritis (Table 6), the subjects included patients with complicated UTIs, and this could have resulted in overestimation of the antibiotic resistance to fluoroquinolone in patients with uncomplicated pyelonephritis; nonetheless, the results indicate the increasing trend in antibiotic resistance to fluoroquinolone. When the patterns of antibiotic resistance of causative bacteria within a country or across regions are not well understood, appropriate empirical treatment may be continuous intravenous antibiotics and broad-spectrum antibiotics administered in the early period of treatment and later replaced by selective antibiotics based on antibiotic susceptibility results [94].
Treatments for acute uncomplicated pyelonephritis are mostly outpatient-based treatments [96,97]. In a recent study, only 7% of female patients with acute pyelonephritis required inpatient care [96]. The IDSA UTI guideline (2011) recommends fluoroquinolones only as oral antibiotics for outpatient-based empirical antibiotic treatment of acute uncomplicated pyelonephritis [94]. In regions where the prevalence of antibiotic resistance to fluoroquinolones exceeds 10%, administration of one day's dose of ceftriaxone or aminoglycoside is recommended as a method of empirical treatment [94].
Considering the antibiotic resistance of E. coli, which is the major causative bacteria of UTIs in Korea, to fluoroquinolones, 1 g of ceftriaxone or one day's dose of amikacin may be administered for empirical treatment, and oral fluoroquinolones may be administered (ciprofloxacin, 500 mg, twice daily, or sustained-release ciprofloxacin, 1000 mg, once daily, or 750 mg levofloxacin, once daily) until culture results are obtained (level of evidence: very low; recommendation grade: strong). Once the culture results are obtained, the initial empirical antibiotics should be adjusted according to the culture results (Table 7).
Table 7. Oral antibiotics and the duration of therapy for patients with acute uncomplicated pyelonephritis.
| Antibiotics | Daily dose | Duration of therapy, days | References |
|---|---|---|---|
| Ciprofloxacin | 500 mg twice daily | 7 | [99, 105] |
| Ciprofloxacin, sustained released | 1000 mg once daily | 7–14 | [102] |
| Levofloxacin | 750 mg once daily | 5 | [101] |
| Ceftibuten | 400 mg once daily | 10 | [103] |
| Cefpodoxime proxetil | 200 mg twice daily | 10 | [104] |
| TMP/SMX | 160/800 mg twice daily | 14 | [99] |
TMP, trimethoprim; SMX, sulfamethoxazole.
D. For early empirical antibiotic administration for patients with acute pyelonephritis who do not require hospitalization, 400 mg of intravenous ciprofloxacin may be administered, followed by oral ciprofloxacin (500 mg, twice daily) until results are obtained from the culture test.
There have been no RCTs on the use of fluoroquinolones and other alternative antibiotics in the early empirical antibiotic treatment of acute uncomplicated pyelonephritis when the prevalence of antibiotic resistance of the causative bacteria exceeds 10%. However, a single-institution prospective observation study has been conducted in Korea where the antibiotic resistance of E. coli, which induces UTIs in communities, to ciprofloxacin was approximately 20%. In the study, 400 mg of intravenous ciprofloxacin was administered early, followed by 500 mg of oral ciprofloxacin, twice daily, for 7–14 days, with doses subsequently readjusted based on culture results. Although there was no difference in the clinical cure rate between the ciprofloxacin-susceptible group (n = 216) and the ciprofloxacin-resistant group (n = 39) during the early follow-up (87.0% vs. 76.9%, P = 0.135) and later follow-up (98.6% vs. 94.0%, P = 0.177), the microbiological cure rate was significantly higher in the ciprofloxacin-susceptible group during the early follow-up (92.4% vs. 41.7%, P <0.001) [98]. Although the microbiological cure rate was lower in the ciprofloxacin resistance group during the early follow-up period, there was no difference in the clinical cure rate. This can be explained by the relatively low severity of acute uncomplicated pyelonephritis compared with infections affecting other sites and by the fact that antibiotics are appropriately administered even if the administration is delayed until culture results are obtained and that ciprofloxacin is maintained at high concentrations in urine. For appropriate administration of selective antibiotics according to susceptibility results of patients with acute uncomplicated pyelonephritis caused by ciprofloxacin-resistant bacteria, early empirical administration of intravenous ciprofloxacin followed by that of oral ciprofloxacin may be considered.
E. If the causative bacteria show susceptibility to antibiotics in the culture test, fluoroquinolone, TMP/SMX, and β-lactams may be used as oral antimicrobial agents.
Not many studies have compared the therapeutic effects of oral antibiotics against acute uncomplicated pyelonephritis. In an RCT involving 255 patients with acute uncomplicated pyelonephritis, the group receiving ciprofloxacin (500 mg, twice daily for 7 days) had a higher clinical cure rate (96% vs. 83%, P = 0.002) and microbiological cure rate (99% vs. 89%, P = 0.004) than the group receiving TMP/SMX (160/800 mg, twice daily for 14 days) [99]. Such results may be attributed to the fact that the causative bacteria had higher resistance to TMP/SMX. Therefore, when the causative bacteria show antibiotic susceptibility to TMP/SMX, TMP/SMX (160/800 mg, twice daily for 14 days) may be used as an oral antibiotic [100]. Fluoroquinolones, which can be administered once daily, are also effective (1000 mg of sustained-release ciprofloxacin or 750 mg of levofloxacin). There was no difference in the clinical cure rate between a group that was administered 750 mg of levofloxacin once daily for 5 days and a group that was administered 500 mg of ciprofloxacin twice daily for 10 days, with both groups consisting of patients with mild to moderate pyelonephritis (86.2% vs. 80.6%) [101]. In addition, administration of 1000 mg of sustained-release ciprofloxacin once daily was as effective as administration of 500 mg of ciprofloxacin twice daily [102]. Third-generation oral cephalosporins (ceftibuten and cefpodoxime proxetil) can also replace fluoroquinolones. Although they have resulted in a similar clinical cure rate compared with fluoroquinolones, the incidence of recurrent pyelonephritis was higher when third-generation cephalosporins were used [103,104].
F. If the causative bacteria show susceptibility to antibiotics in patients with acute pyelonephritis, oral antibiotics are administered for 7–14 days. Ciprofloxacin (500 mg, twice daily for 7 days or sustained-release ciprofloxacin, 1000 mg, once daily for 7–14 days), levofloxacin (500 mg, once daily for 7 days, or 750 mg, once daily for 5 days), TMP/SMX (160/800 mg, twice daily for 14 days), and oral β-lactams (10–14 days) are administered.
There is not much evidence regarding the duration of treatment for acute uncomplicated pyelonephritis using oral antibiotics. The treatment duration should be adjusted according to the disease severity and the immediacy of treatment response. In a recent RCT comparing a group receiving 500 mg of ciprofloxacin twice daily for 7 days and a group receiving the same drug at the same dose for 14 days, the short-term clinical cure rate was 97% and 96%, respectively, indicating that administration for 7 days was not inferior to administration for 14 days [105]. When bacterial susceptibility to ciprofloxacin is observed in a culture test, a 7-day treatment using ciprofloxacin may be effective. In another study, 7 days of treatment resulted in a lower short-term clinical cure rate than 14 days of treatment (86% vs. 98%) for male patients with acute pyelonephritis, whereas there was no difference between the two treatments for women [106]. When 1000 mg of sustained-released ciprofloxacin once daily was compared with 500 mg of ciprofloxacin twice daily for 7–14 days, there was no difference in the clinical and microbiological cure rates; therefore, administration of 1000 mg of sustained-release ciprofloxacin once daily for 7–14 days is also effective [102]. Antibiotic treatment in which 750 mg of levofloxacin is administered once daily for 5 days has also resulted in an excellent clinical cure rate [101].
TMP/SMX effectively treats acute pyelonephritis that shows antibiotic susceptibility in a culture test. Studies investigating the therapeutic effects of TMP/SMX against pyelonephritis have been based on 14 days of treatment [99]. Current research data on oral cephalosporins are limited, but they suggest that oral cephalosporins are inferior to fluoroquinolones in terms of therapeutic effects. The duration of treatment of pyelonephritis using oral cephalosporins is 10–14 days [94].
G. For patients with acute pyelonephritis who require hospitalization, fluoroquinolone, aminoglycoside ± ampicillin, second-generation cephalosporin, broad-spectrum cephalosporin, β-lactam/β-lactamase inhibitor ± aminoglycoside, aminoglycoside ± β-lactam, or carbapenem may be administered. Once the fever is alleviated, the antibiotic should be changed to an oral antibiotic chosen based on the antibiotic susceptibility and resistance of the causative bacteria to the new antibiotic.
It is unclear how the severity of uncomplicated pyelonephritis should be classified. However, patients can be divided into those who can take oral antibiotics and those who require intravenous antibiotics as well as into those who require outpatient treatment and those who require inpatient treatment (Table 8).
Table 8. Empirical intravenous antibiotics for inpatient treatment of patients with acute uncomplicated pyelonephritis.
| Antibiotics and dosing |
|---|
| Ciprofloxacin 400 mg iv twice daily |
| Levofloxacin 500–750 mg iv once daily |
| Cefuroxime 750 mg iv every 8 hours |
| Ceftriaxone 1–2 g iv once daily |
| Cefepime 1–2 g iv twice daily |
| Amikacin 15 mg/kg iv once daily ± ampicillin 1–2 g iv every 6 hours |
| Piperacillin-tazobactam 3.375 g iv every 6 hours |
| Meropenem 500–1000 mg iv every 8 hours |
| Imipenem-cilastatin 500 mg iv every 6–8 hours |
| Doripenem 500 mg every 8 hours |
| Ertapenem 1 g iv once daily |
Patients who consistently vomit, who experience dehydration, who are suspected of disease progression or sepsis, or who do not show any recovery during the early outpatient treatment require hospitalization [64]. There are not many studies on early empirical treatment using fluoroquinolones for inpatients when the antibiotic resistance of the causative bacteria of UTIs to fluoroquinolones exceeds 10%. Considering the results of a domestic study involving outpatients [98], fluoroquinolones may be used, but they should be replaced later with appropriate antibiotics based on antibiotic susceptibility test results. In a domestic retrospective study, early empirical administration of cefuroxime resulted in a significantly high early clinical cure rate in the cefuroxime-susceptible group compared with the cefuroxime-resistant group (90.8% vs. 68.2%, p = 0.001) [107]. However, there was no significant difference in the clinical cure rate (97.8% vs. 88.2%, p = 0.078) or the microbiological cure rate (93.4% vs. 90.8%) in a later follow-up [107]. In a retrospective study comparing cefuroxime and cefotaxime, the clinical effects of cefuroxime were on a par with those of cefotaxime [108]. Amikacin can be empirically used to treat pyelonephritis and complicated UTIs caused by antimicrobial-resistant gram-negative bacteria [109]. In a retrospective study, amikacin produced excellent therapeutic effects against UTIs caused by ESBL-producing bacteria [110].
As the antibiotic resistance of the causative bacteria of community-acquired UTIs increases, broad-spectrum antibiotics such as third-generation cephalosporins, piperacillin-tazobactam, or carbapenems may be used until susceptibility results are obtained. When treating patients with acute pyelonephritis accompanied by severe sepsis and septic shock requiring inpatient treatment in an intensive care unit, early empirical administration of carbapenems may be considered until susceptibility results are obtained in areas where the prevalence of ESBL-producing bacteria as the causative bacteria of UTIs is high [111].
② What are possible non-carbapenem-based antibiotic treatments for acute uncomplicated pyelonephritis caused by ESBL-producing bacteria?
<Recommendation>
ⓐ Fosfomycin, TMP/SMX, cefepime, ceftazidime-avibactam, ceftolozane-tazobactam, amoxicillin-clavulanate, piperacillin-tazobactam, and amikacin may be used in place of carbapenems against susceptible ESBL-producing bacteria (level of evidence: low; recommendation grade: weak).
<Summary of evidence>
There is not much evidence for non-carbapenem-based antibiotic treatment of acute uncomplicated pyelonephritis caused by ESBL-producing bacteria. According to a retrospective study, the therapeutic effects of oral fosfomycin (3 g per 48 or 72 hours) against UTIs caused by ESBL-producing bacteria were not inferior to those of ertapenem (1 g, once daily, intravenous injection) [112].
Although TMP/SMX is not recommended for empirical treatment of UTIs caused by ESBL-producing bacteria, it may be used in selective antibiotic treatments if ESBL-producing bacteria show susceptibility to the antibiotic [94,109].
AmpC β-lactamase-producing E. coli have shown susceptibility to cefepime, and therefore, the antibiotic may be a treatment option for UTIs including pyelonephritis caused by susceptible bacteria [113]. However, it should not be used against ESBL-producing bacteria, especially for patients with severe UTIs [109]. The U.S. Food and Drug Administration approved the use of the ceftazidime-avibactam therapy when there are limited or no treatment options for complicated UTIs including pyelonephritis [109].
The therapy may be performed empirically or selectively to treat acute uncomplicated pyelonephritis caused by ESBL-producing bacteria [114]. In a double-blind RCT comparing the effects of ceftolozane-tazobactam (1.5g every 8 hours for 7 days) with those of high-dose levofloxacin (750 mg, once daily for 7 days), ceftolozane-tazobactam was more effective than high-dose levofloxacin, and showed a higher cure rate associated with ESBL-producing bacteria in a post hoc analysis (58.3% vs 34.9%) [115]. Therefore, ceftolozane-tazobactam may be used in empirical and selective treatments for acute uncomplicated pyelonephritis caused by ESBL-producing bacteria.
There is a controversy regarding the use of β-lactam/β-lactamase inhibitors for treating infectious diseases caused by ESBL-producing bacteria. However, in a recent post hoc analysis, amoxicillin-clavulanate and piperacillin-tazobactam were not inferior to carbapenem in terms of their therapeutic effects against bacteremia caused by ESBL-producing E. coli, and these antibiotics were suggested to use in alternative treatment in place of carbapenems [116].
A domestic retrospective study also suggested piperacillin-tazobactam as an alternative for carbapenems in treating bacteremia caused by ESBL-producing E. coli and K. pneumoniae [117]. Therefore, amoxicillin-clavulanate may be used in alternative treatment for lower UTIs caused by susceptible ESBL-producing bacteria. Piperacillin-tazobactam may be used in alternative treatment for complicated UTIs including pyelonephritis caused by ESBL-producing bacteria.
Amikacin can be used to treat complicated UTIs including pyelonephritis caused by ESBL-producing bacteria. According to a retrospective study, the clinical and microbiological success rates of amikacin in treating lower UTIs caused by ESBL-producing bacteria (E. coli, in particular) were 97.2% and 94.1%, respectively [110]. In a domestic retrospective study, amikacin showed excellent therapeutic effects against UTIs unaccompanied by bacteremia [118]. In the treatment of uncomplicated pyelonephritis caused by ESBL-producing bacteria, the antibiotics mentioned above as replacements for carbapenem may be used under limited circumstances when the targeting bacteria show susceptibility to these antibiotics.
4. Complicated pyelonephritis related to urinary tract obstruction
1) Background and epidemiology
Urinary tract obstruction is important in the pathophysiology of infectious pyelonephritis. When normal urine excretion is obstructed, bacteria are introduced into the urinary tract. In the presence of foreign substances such as urinary stones, a biofilm forms on the surface of the urinary tract and bacteriuria continues. In addition, the extent of kidney damage is proportional to the period of urinary tract obstruction, and the recovery function of the kidney gradually deteriorates. The urine-concentrating ability can be completely recovered if the urinary tract obstruction lasts no more than a week or so. However, if the condition continues for 4 weeks, the ability may be permanently lost [119]. For UTIs resulting from urinary tract obstruction, prompt management of the urinary tract obstruction is needed in addition to antibiotic treatment for the infection itself, and an individualized approach should be made according to the causative disease [120].
The major diseases that induce urinary tract obstruction vary with age. The most common causes include prostatic hypertrophy, neurogenic bladder, and urinary stones. Urinary stones are the most common cause among young adults, and prostatic hypertrophy, prostate cancer, and abdominal mass are the most common among older adults [121]. For women, diseases that invade the organs in the pelvic cavity can cause urinary tract obstruction. Urinary tract obstruction can be classified as acute or chronic, partial or complete, unilateral or bilateral, and upper or lower urinary tract obstruction.
Owing to the diversity of the causes of UTIs related to urinary tract obstruction, there are no integrative data on the causative diseases published in or outside Korea. There has also been almost no research regarding the causative bacteria of the diseases that induce urinary tract obstruction. In domestic cases of hydronephrosis, E. coli, Pseudomonas, Enterobacter, Proteus, Enterococcus, and Citrobacter spp. were common causative bacteria of UTIs [112]. E. coli was the most causative bacteria for UTIs in patients with a neurogenic bladder, and others included E. faecalis, P. aeruginosa, P. mirabilis, K. pneumoniae, and Streptococcus agalactiae [123]. In patients with a neurogenic bladder or other neurologic anomalies, Enterococcus and Pseudomonas spp. are especially important causative bacteria compared with other UTIs. A UTI by urease-positive bacteria should occur in advance for infectious urinary stones (or struvite stones) or staghorn calculi to occur [124,125]. Proteus, Morganella, and Providencia spp. produce urease. Klebsiella, Pseudomonas, Serratia, and Staphylococcus spp. also produce urease using various mechanisms. Among patients with staghorn calculi, 82% are infected by urease-producing bacteria, and the most common causative bacteria in these patients are Proteus, Klebsiella, Pseudomonas, and Staphylococcus spp. [125].
2) Diagnosis
Following the treatment of infection, the causes of urinary tract obstruction should be identified and the condition treated using specialized, individualized diagnostic methods. During this process, consultations with urologists and radiologists are necessary [121]. Methods of early diagnosis of upper urinary tract obstruction include abdominal radiography (Kidney, Ureter, and Bladder X-ray study ± Intravenous pyelogram), ultrasonography, and CT. CT is the most useful for diagnosing emphysematous pyelonephritis and checking the scope of a lesion [126]. Lower urinary tract obstruction commonly presents as urinary retention. When urinary retention is suspected, the volume of the residual urine following urination is measured. Volumes exceeding 100 mL are suggestive of significant lower urinary tract obstruction. In the early period after urinary tract obstruction, a voiding diary, cytology, ultrasound examination, and CT are necessary. For male patients, a blood prostate-specific antigen (PSA) test and a transrectal ultrasound-guided prostate biopsy are additionally needed. If a patient has overflow incontinence, Parkinson's disease, diabetic neuropathy, cerebral infarction, spine injury, a neurological disorder, or recurrent UTIs, an obstructive UTI or neurogenic bladder should be considered as a possible cause of the urinary tract obstruction. When any anomalies are observed in the early neurological examinations and anal reflex tone and perianal sensation tests, a urodynamic study should be conducted or magnetic resonance imaging of the brain or spine should be performed when necessary.
Severe UTIs such as emphysematous pyelonephritis is more common among diabetic patients [127]. Emphysematous cystitis may be accompanied by hematuria or pneumaturia that can be grossly examined. Emphysematous pyelonephritis exhibits symptoms of severe sepsis and may be accompanied by a mass in the flank or crepitus upon touch. Emphysematous cystitis can be diagnosed when air shadows are observed on the bladder wall in a radiological examination. Emphysematous pyelonephritis can be diagnosed upon observation of air shadows around the kidney pelvis of the kidney parenchyma or around the kidney. Patients suspected of having emphysematous pyelonephritis require a CT examination for diagnosing the disease and understanding the extent of invasion. In a systematic literature review of 10 retrospective studies, abdominal radiographs showed 65–69% accuracy in diagnosing emphysematous pyelonephritis, whereas a significant inter-examiner variability was found for the accuracy of ultrasonography [128]. Although it was difficult to differentiate emphysema from stones or intestinal gas under ultrasound guidance, CT showed 100% accuracy in differentiating them [128].
3) Guideline by key question
① What are appropriate empirical antibiotics for initial administration in adult patients with complicated pyelonephritis related to urinary tract obstruction?
<Recommendation>
ⓐ Empirical antibiotics for patients with pyelonephritis related to urinary tract obstruction should be selected in accordance with the treatment protocol for uncomplicated pyelonephritis. However, if clinical symptoms are severe, antibiotic selection should be based on the treatment protocol for severe UTIs accompanied by sepsis (level of evidence: low; recommendation grade: strong).
ⓑ Fluoroquinolone, β-lactam/β-lactamase inhibitor, broad-spectrum cephalosporin, aminoglycoside, and carbapenem may be used as early empirical antibiotics (level of evidence: low; recommendation grade: strong).
ⓒ For pyelonephritis accompanied by sepsis or for recurrent pyelonephritis, piperacillin/tazobactam, broad-spectrum third-generation or fourth-generation cephalosporins, and carbapenem may be used. If the risk of infection by antibiotic-resistant bacteria is high, a combination therapy using broad-spectrum β-lactam and amikacin may be considered (level of evidence: low; recommendation grade: weak).
<Summary of evidence>
Patients with pyelonephritis related to urinary tract obstruction show different prognoses depending on whether the urinary tract obstruction is removed during antibiotic treatment, the severity of accompanying diseases, and the extent of damage to the urinary tract. However, the characteristics of patient groups have been too varied and the clinical standard for diagnosing pyelonephritis have been unclear in studies that have been conducted on UTIs related to urinary tract obstruction. These studies also did not consider urological prognoses. For this reason, it is difficult to propose treatment guidelines based on their results. No comparative clinical research has been conducted to determine which empirical antibiotics should be used for patients with pyelonephritis related to urinary tract obstruction. However, there is not enough evidence to make other antibiotic recommendations for such patients. The types of causative bacteria and their antibiotic susceptibility vary depending on the disease severity, region, causative diseases of urinary tract obstruction, and patient history of healthcare-associated infections or community-acquired infections. Therefore, antibiotic treatment should be selected after considering the severity of the infection, the causative diseases of urinary tract obstruction, the frequency of recurrence, history of healthcare-associated infections, and types of antibiotic resistance. In a recent multi-institutional study conducted in Japan, the most common causative bacteria of obstructive pyelonephritis associated with urinary stones was E. coli, followed by P. mirabilis and K. pneumoniae, and similar results were obtained from non-obstructive UTIs [129]. The mortality rate was 2.3%, and old age of 80 years or older, severe sepsis, reduced consciousness, and a single kidney were identified as risk factors of death [129]. The antibiotics for patients with pyelonephritis related to urinary tract obstruction may be empirically selected in accordance with the treatment protocol for general pyelonephritis. However, selection may need to be based on the treatment protocol for severe UTIs accompanied by sepsis [125,130]. It is advisable to use antibiotics in accordance with the treatment protocol for severe pyelonephritis and avoid the use of antibiotics that can induce nephrotoxicity. Fluoroquinolone, β-lactam/β-lactamase inhibitor, third-generation cephalosporin, aminoglycoside, and a carbapenem such as ertapenem may be used as early empirical antibiotics. However, E. coli, which induces UTIs in Korea, is highly resistant to ciprofloxacin, ampicillin/sulbactam, and gentamicin. Therefore, for UTIs accompanied by sepsis or recurrent UTIs, piperacillin/tazobactam, third- or fourth-generation cephalosporin, amikacin, and carbapenem are recommended as a priority. Some experts recommend antipseudomonal antibiotics in the early stage for severe infections suspected as sepsis or healthcare-associated infections. After beginning early empirical treatment using broad-spectrum antibiotics, the treatment should be readjusted according to obtained culture results. Before treatment, urine and blood culture tests should be performed. Although the duration of antibiotic use should be shortened as much as possible, the clinical reactions in each disease should be individually assessed since UTIs have various causes. If the causes of urinary tract obstruction are removed and there are no additional causes of infection, antibiotics are used for 7–14 days. If symptom improvement and treatment outcomes regarding urinary tract obstruction are unsatisfactory, the duration of antibiotic treatment may be extended to over 21 days.
② Is combination antibiotic therapy superior to monotherapy for adult patients with pyelonephritis related to urinary tract obstruction?
<Recommendation>
ⓐ If the identity of the causative bacteria and their antibiotic susceptibility are known in a patient with pyelonephritis related to urinary tract obstruction, monotherapy should be performed using commonly recommended antibiotics to which the causative bacteria are susceptible (level of evidence: low; recommendation grade: strong).
ⓑ For severe infections suspected as sepsis, recurrent infections, or healthcare-associated infections, early empirical treatment should be enforced, and combination therapy may be considered (level of evidence: low; recommendation grade: weak).
ⓒ In consideration of combination therapy, broad-spectrum β-lactams and aminoglycoside or fluoroquinolone may be used together (level of evidence: low; recommendation grade: weak).
<Summary of evidence>
There is not enough evidence to claim that combination antibiotic therapy is superior to appropriately performed monotherapy using antibiotics to which causative bacteria are highly susceptible. When the identity of the causative bacteria and their susceptibility are known, monotherapy using commonly recommended antibiotics is sufficient [130]. However, for severe infections suspected as sepsis, recurrent infections, or healthcare-associated infections, combination therapy may be considered. Data collected within Korea on ESBL-producing E. coli have shown that combined administration of piperacillin/tazobactam and amikacin produced similar results to those of carbapenem [131]. Although fluoroquinolone may be combined with piperacillin/tazobactam, antibacterial resistance to fluoroquinolone is significantly higher than that to amikacin [131].
③ Should urinary tract obstruction be treated in patients with pyelonephritis related to urinary tract obstruction? If yes, when is the appropriate timing for the intervention?
<Recommendation>
ⓐ Pyelonephritis related to urinary tract obstruction requires antibiotic treatment in addition to decompression of the urinary tract obstruction (level of evidence: high; recommendation grade: strong).
ⓑ A patient diagnosed with pyelonephritis related to urinary tract obstruction requiring drainage or decompression requires interventions as soon as possible (level of evidence: low, recommendation grade: strong).
ⓒ For hydronephrosis and UTIs accompanied by urinary stones, the percutaneous nephrostomy or urethral stent insertion should be performed as soon as possible (level of evidence: low; recommendation grade: strong).
ⓓ For UTIs accompanied by urinary tract obstruction caused by prostatic hypertrophy, a urinary catheter should be inserted as soon as possible (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
Pyelonephritis related to urinary tract obstruction requires antibiotic treatment and decompression. Acute urinary tract obstruction may urgently require decompression or even surgery. It is advisable to decompress using minimally invasive methods (such as catheterization, percutaneous nephrostomy, and ureteral stent insertion) initially and perform kidney resection as a last resort [130]. It has been reported that drainage and decompression should be performed promptly when they are needed and that the duration of the hospital stay significantly increased when drainage was delayed [129]. Although decompression and drainage are commonly performed in clinical settings, studies comparing and analyzing the differences between the effects and complications of the procedures are rare. For ureteral stones accompanied by hydronephrosis and UTIs, percutaneous nephrostomy is generally performed first. However, in a study comparing percutaneous nephrostomy and ureteral stent insertion, there were no differences in the treatment outcomes and complications between the two procedures [132].
The appropriate duration of catheter use in patients with acute urinary tract obstruction caused by prostatic hypertrophy has not been determined. Use of α-blockers (such as alfuzocin and tamsulosin) in the early catheterization period or at least 3 days before voluntary urination is attempted can increase the success of voluntary urination. If a catheter has been maintained over 14 days, suprapubic catheterization may be considered. Functional impairment caused by neurogenic bladder occurs as a result of pressure build-up within the bladder (overflow incontinence), or urinary retention, or both. Administration of a single anticholinergic agent in addition to infection treatment or combined administration of an anticholinergic agent and an α-blocker may be used.
④ For adult patients with pyelonephritis related to urinary tract obstruction, for how long should antibiotic treatment be performed following successful relief of urinary tract obstruction?
<Recommendation>
ⓐ If the causal factors of urinary tract obstruction are corrected, and there are no additional factors of infections for a patient with pyelonephritis related to urinary tract obstruction, antibiotics may be used for 7–14 days (level of evidence: low; recommendation grade: weak).
ⓑ If treatment outcome, symptom relief, and urinary tract obstruction relief are insufficient, the treatment can be extended to over 21 days in accordance with the treatment protocol for renal abscesses (level of evidence: low; recommendation: weak).
<Summary of evidence>
There are currently no studies that compare appropriate antibiotic treatment durations for pyelonephritis related to urinary tract obstruction. If the causal factors of urinary tract obstruction are corrected, and there are no additional factors of infections, antibiotics are administered for 7–14 days [124,130]. In case of insufficient treatment outcome, symptom relief, and urinary tract obstruction relief, the treatment may be extended to over 21 days. If stones are not fully removed, long-term antibiotic treatment is considered.
⑤ Which empirical antibiotics should be used for patients suspected of having emphysematous pyelonephritis?
<Recommendation>
ⓐ For patients suspected of having emphysematous pyelonephritis, empirical antibiotics may be selected in accordance with the treatment protocol for uncomplicated pyelonephritis. However, in the case of severe clinical symptoms, antibiotic selection should be selected based on the treatment protocol for severe UTIs accompanied by sepsis (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
The distribution of causal bacteria in diabetic patients with pyelonephritis or emphysematous pyelonephritis does not significantly differ from that of the causal bacteria of uncomplicated UTIs; therefore, E. coli is the most common causal bacterium [127,133]. However, the bacterial distribution differs between diabetic and non-diabetic patients, with K. pneumoniae being more common than E. coli in diabetic patients with pyelonephritis or emphysematous pyelonephritis. There is no clinical study comparing antibiotics to determine which empirical antibiotics should be used for patients with emphysematous pyelonephritis. It is recommended to use antibiotics in accordance with the treatment protocol for severe pyelonephritis and it is best to avoid nephrotoxic antibiotics. In a study on emphysematous pyelonephritis, E. coli and K. pneumoniae were the most common causal bacteria, as is the case among non-diabetic patients. Therefore, empirical antibiotics should be selected while considering the status of antibiotic resistance within each hospital.
⑥ Should percutaneous drainage be performed for patients suspected of having emphysematous pyelonephritis)? When should nephrectomy be considered?
<Recommendation>
ⓐ For patients suspected of having emphysematous pyelonephritis, antibiotics are administered if gas formation is localized in the kidney pelvis and there is no invasion in the kidney parenchyma. If there is invasion in the kidney parenchyma, percutaneous drainage or surgery should be performed in addition to antibiotic administration (level of evidence: low; recommendation grade: strong).
ⓑ If gas formation has widely spread to the kidney, and there is no improvement even after percutaneous drainage, nephrectomy should be considered (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
Antibiotics are administered only if gas formation is localized in the renal pelvis and there is no invasion in the kidney parenchyma [133]. If there is an invasion in the kidney parenchyma, percutaneous drainage or surgery should be performed in addition to antibiotic administration [133]. If gas formation has widely spread to the kidney, and there is no improvement even after percutaneous drainage, nephrectomy should be considered. In a study involving 48 patients with emphysematous pyelonephritis, the patients were divided into four groups based on their CT scans and then observed. Although 6% of patients died whose gas was localized in the kidney pelvis or parenchyma, and thus used antibiotics only, 21% and 50% of patients died whose gas formation had widely spread to the kidney or who had bilateral emphysematous pyelonephritis, respectively. Although 88% of the patients who underwent percutaneous drainage owing to symptom worsening survived, all those who were not operated died [134]. There is also a report that antibiotic administration alone improved symptoms in five patients with emphysematous pyelonephritis with gas formation localized in the renal pelvis [126]. A study on eight diabetic patients with emphysematous pyelonephritis who were treated with antibiotics and conservative therapy instead of surgery reported E. coli as the causative bacterium. Symptoms improved in all patients following antibiotic treatment, fluid therapy, and ureteral stent insertion [135]. In a study that analyzed 28 patients who were diagnosed with emphysematous pyelonephritis or emphysematous cystitis and underwent antibiotic treatment and percutaneous drainage, only one patient died, and the remaining patients were fully cured without any complications [136]. Of these 28 patients, 60% had diabetes, and the most common causal bacterium was E. coli [136].
5. Acute bacterial prostatitis
1) Background and epidemiology
Prostatitis refers to a group of various diseases from bacterial or non-bacterial prostatitis to chronic prostatitis pain syndrome. Prostatitis has a high prevalence. Approximately 10% of men suffer from symptoms of prostatitis, and 50% of men experience these symptoms at least once in their lifetime [137]. However, only 7% of these men have bacterial prostatitis with confirmed bacterial infection, whereas the rest have non-bacterial chronic prostatitis/chronic pelvic pain syndrome with an unconfirmed UTI [138]. In most cases, asymptomatic prostatitis is coincidentally diagnosed in patients with prostatic hypertrophy or prostatic cancer following a biopsy or surgery [139,140].
Prostatitis can be divided into four categories according to the National Institutes of Health (Table 9) [141].
Table 9. Prostatitis categories.
| Category | Description |
|---|---|
| I | Acute bacterial prostatitis |
| II | Chronic bacterial prostatitis |
| III | Chronic prostatitis/chronic pelvic pain syndrome |
| a. Inflammatory | |
| b. Non-inflammatory | |
| IV | Asymptomatic prostatitis |
This guideline discusses only acute bacterial prostatitis whose causes have been ascertained as bacterial and that requires antibiotic treatment.
In most cases, acute bacterial prostatitis is caused by E. coli, which also causes UTIs. Pseudomonas aeruginosa and Enterococci are common causes of healthcare-associated infections. The risk factors of acute bacterial prostatitis include urinary manipulation such as catheterization or anatomical anomalies such as benign prostatic hypertrophy.
2) Diagnosis
Acute bacterial prostatitis is a serious acute systemic disease. It can cause symptoms of UTIs such as painful urination, frequent urination, and urge incontinence; symptoms of prostatitis such as perineal pain, genital pain, painful urination, and rectal pain; and symptoms of bacteremia such as fever, chill, joint pain, and muscle pain [14,15,16].
Acute bacterial prostatitis is characterized by the sensation of heat around the prostate; a soft, swollen, and tense state of the prostate; and severe pressure pain during a digital rectal examination [141,142]. Complications of this infection include acute urinary tract obstruction, epididymitis, prostatic abscess, sepsis, and chronic bacterial prostatitis [143]. Progression to sepsis or chronic bacterial prostatitis can be prevented through appropriate treatment. Prostatic abscesses occur in 2–18% of patients with acute bacterial prostatitis and are especially common in cases of prostatitis that developed following urogenital manipulation [144,145].
Acute bacterial prostatitis is diagnosed upon the microbiological detection of pathogens in midstream urine. A blood culture test is performed to diagnose bacteremia. However, prostatic massage is contraindicated for patients with acute bacterial prostatitis as it can cause severe pain and induce bacteremia. Patients with acute bacterial prostatitis show elevated serum PSA levels, which can be relieved by antibiotic treatment. Transrectal ultrasonography or CT may be used to screen for prostatic abscesses.
3) Guideline by key question
① Is intravenous antibiotic treatment more effective than oral antibiotics for patients with acute bacterial prostatitis?
<Recommendation>
ⓐ Acute bacterial prostatitis is a serious acute disease requiring immediate inpatient management and empirical antibiotic administration (level of evidence: low; recommendation grade: strong).
ⓑ Immediately after adequate samples are collected for urine and blood culture tests, intravenous antibiotics should be administered. The patient should be sufficiently hydrated and rested, analgesics such as NSAIDs may be administered if necessary (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
There has been no meta-analysis or RCT on acute bacterial prostatitis. Antibiotics are the primary treatment choice for acute bacterial prostatitis [146,147,148,149]. Guidelines based upon domestic and foreign case reports, as well as expert opinions, recommend inpatient treatment and early empirical antibiotic treatment for acute prostatitis [145,150].
② Can fluoroquinolones be considered before third-generation cephalosporin in the selection of empirical antibiotics for treating acute bacterial prostatitis?
<Recommendation>
ⓐ For patients with acute bacterial prostatitis requiring hospitalization, third-generation cephalosporins, a broad-spectrum β-lactam/β-lactamase inhibitor, or carbapenem are recommended (level of evidence: low; recommendation grade: strong).
ⓑ Administration of empirical antibiotics should continue until susceptibility test results are obtained, and antibiotics are adjusted according to the test results (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
Early selection of empirical antibiotics is based on the ability to penetrate into the prostate tissue, local epidemiology of antibiotic resistance, and the identities of the suspected causative bacteria [151]. There has been no RCT on antibiotic selection and the duration of antibiotic use. Considering the resistance of the causative bacteria to fluoroquinolone in Korea, intravenous use of third-generation cephalosporins, a broad-spectrum β-lactam/β-lactamase inhibitor, and carbapenem is recommended [149]. When symptoms are relived following the administration of highly concentrated intravenous antibiotics, it is recommended to replace them with oral antibiotics to be administered for 2–4 weeks.
③ Is cephalosporin-aminoglycoside combination therapy more effective than monotherapy for patients with acute bacterial prostatitis?
<Recommendation>
ⓐ For severe infections suspected as sepsis or recurrent infections, combination antibiotic therapy may be considered as early empirical antibiotic treatment (level of evidence: low; recommendation grade: weak).
ⓑ A combination therapy using β-lactams and aminoglycosides may be considered (level of evidence: low; recommendation grade: weak).
<Summary of evidence>
There is not much evidence supporting the inclusion of aminoglycoside in early empirical antibiotics. According to retrospective studies conducted in and outside Korea, combination therapy was effective for patients who had previously undergone manipulation of the lower urinary tract [145,152]. In Korea, E. coli, which is the causative bacterium of UTIs, has higher resistance to gentamicin and tobramycin than to amikacin. Therefore, amikacin, a type of aminoglycoside, is recommended for use in combination therapy.
④ Can the administration of medications such as α-blockers in patients with acute bacterial prostatitis improve treatment efficiency and outcome?
<Recommendation>
ⓐ Use of α-blockers is recommended if residual urine is observed after urination or if the patient complains of urinary symptoms (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
Adjuvant treatment is necessary for relieving the symptoms of acute bacterial prostatitis. For pain and inflammation, NSAIDs are recommended [146]. For symptoms of lower UTIs, α-blockers are recommended. In Korea, α-blockers including tamsulosin, terazosin, alfuzosin, doxazosin, silodosin, and naftopidil have been used [153].
⑤ When urinary tract obstruction worsens or the residual urine volume increases in patients with acute bacterial prostatitis, should suprapubic catheterization be performed?
<Recommendation>
ⓐ The suprapubic catheter should be maintained in the presence of acute urinary tract obstruction (level of evidence: low; recommendation grade: strong).
ⓑ In patients showing no evidence of urinary tract obstruction, urethral catheterization can increase the risk of progression to chronic prostatitis (level of evidence: low; recommendation grade: weak).
<Summary of evidence>
Urinary tract obstruction frequently occurs in patients with acute bacterial prostatitis. If the volume of residual urine increases or if acute urinary tract obstruction is suspected, immediate catheterization should be considered. Suprapubic catheterization that does not pass through the prostate is recommended [154]. Intermittent catheterization and urethral catheterization may also be used to alleviate urinary tract obstruction at the expert's discretion. However, since urethral catheterization can increase the risk of progression to chronic prostatitis in patients without any evidence of urinary tract obstruction, ureteral catheterization should be performed with caution [155].
⑥ When prostatic abscesses occur in patients with acute bacterial prostatitis, can puncture and drainage procedures improve treatment outcomes?
<Recommendation>
ⓐ For prostatic abscesses that do not respond to antibiotic treatment, transrectal ultrasound-guided needle aspiration, transrectal ultrasound-guided catheter drainage, perineal ultrasound-guided drainage, or transurethral abscess resection may be performed (level of evidence: low; recommendation grade: strong).
<Summary of evidence>
If a patient shows no clinical improvement despite undergoing appropriate antibiotic treatment, a prostatic abscess should be suspected, and transrectal ultrasonography or CT should be performed [156]. If prostatic abscesses are detected, surgical puncture and drainage through the perineal region or the urethra are considered [157]. When there is no response to aspiration and drainage, surgical resection of prostatic abscess can be performed.
Acknowledgement
This research was supported by a fund (2017-E21002-00) by Research of Korea Centers for Disease Control and Prevention. This manuscript was translated from the original Korean version.
Footnotes
Conflicts of Interest: No conflicts of interest.
Supplementary materials
Guideline Korean version.
Supplementary material can be found with this article on-line https://icjournal.icjournal.org/src/sm/ic-50-67-s001.pdf.
References
- 1.Canadian Agency for Drugs and Technologies in Health (CADTH) CADTH databases search filters. [Accessed February 4, 2017]. Available at: https://www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#guide.
- 2.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM Infectious Diseases Society of America. American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 3.Grabe M, Bartoletti R, Bjerklund-Johansen TE, Cai T, Çek M, Köves B, Naber KG, Pickard RS, Tenke P, Wagenlehner F, Wullt B. Guidelines on urological infections. [Accessed 7 July 2017]. Available at: https://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf.
- 4.Dull RB, Friedman SK, Risoldi ZM, Rice EC, Starlin RC, Destache CJ. Antimicrobial treatment of asymptomatic bacteriuria in noncatheterized adults: a systematic review. Pharmacotherapy. 2014;34:941–960. doi: 10.1002/phar.1437. [DOI] [PubMed] [Google Scholar]
- 5.Nicolle LE. Asymptomatic bacteriuria. Curr Opin Infect Dis. 2014;27:90–96. doi: 10.1097/QCO.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 6.Nicolle LE. The paradigm shift to non-treatment of asymptomatic bacteriuria. Pathogens. 2016;5:E38. doi: 10.3390/pathogens5020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengtsson C, Bengtsson U, Björkelund C, Lincoln K, Sigurdsson JA. Bacteriuria in a population sample of women: 24-year follow-up study. Results from the prospective population-based study of women in Gothenburg, Sweden. Scand J Urol Nephrol. 1998;32:284–289. doi: 10.1080/003655998750015467. [DOI] [PubMed] [Google Scholar]
- 8.Evans DA, Williams DN, Laughlin LW, Miao L, Warren JW, Hennekens CH, Shimada J, Chapman WG, Rosner B, Taylor JO. Bacteriuria in a population-based cohort of women. J Infect Dis. 1978;138:768–773. doi: 10.1093/infdis/138.6.768. [DOI] [PubMed] [Google Scholar]
- 9.Jeon MH, Park WB, Kim SR, Chun HK, Han SH, Bang JH, Park ES, Jeong SY, Eom JS, Kim YK, Kim HB, Lee KY, Choi HJ, Kim HY, Kim KM, Sung JH, Uh Y, Chung HS, Kwon JW, Woo JH. Korean nosocomial infections surveillance system, intensive care unit module report: data summary from July 2010 through June 2011. Korean J Nosocomial Infect Control. 2012;17:28–39. [Google Scholar]
- 10.Shin JC, Yoo JH, Park JW, Park S, Ahn SJ, Park CI. Difference of organism and their antibiotics sensitivity from urine culture in symptomatic urinary tract infection of spinal cord injury patients. J Korean Acad Rehabil Med. 2008;32:38–44. [Google Scholar]
- 11.Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2015;(8):CD000490. doi: 10.1002/14651858.CD000490.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Kazemier BM, Koningstein FN, Schneeberger C, Ott A, Bossuyt PM, de Miranda E, Vogelvang TE, Verhoeven CJ, Langenveld J, Woiski M, Oudijk MA, van der Ven JE, Vlegels MT, Kuiper PN, Feiertag N, Pajkrt E, de Groot CJ, Mol BW, Geerlings SE. Maternal and neonatal consequences of treated and untreated asymptomatic bacteriuria in pregnancy: a prospective cohort study with an embedded randomised controlled trial. Lancet Infect Dis. 2015;15:1324–1333. doi: 10.1016/S1473-3099(15)00070-5. [DOI] [PubMed] [Google Scholar]
- 13.Angelescu K, Nussbaumer-Streit B, Sieben W, Scheibler F, Gartlehner G. Benefits and harms of screening for and treatment of asymptomatic bacteriuria in pregnancy: a systematic review. BMC Pregnancy Childbirth. 2016;16:336. doi: 10.1186/s12884-016-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, D'Elia C, Malossini G, Boddi V, Bartoletti R. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis. 2012;55:771–777. doi: 10.1093/cid/cis534. [DOI] [PubMed] [Google Scholar]
- 15.Cai T, Nesi G, Mazzoli S, Meacci F, Lanzafame P, Caciagli P, Mereu L, Tateo S, Malossini G, Selli C, Bartoletti R. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis. 2015;61:1655–1661. doi: 10.1093/cid/civ696. [DOI] [PubMed] [Google Scholar]
- 16.Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA. 1987;257:1067–1071. [PubMed] [Google Scholar]
- 17.Abrutyn E, Mossey J, Berlin JA, Boscia J, Levison M, Pitsakis P, Kaye D. Does asymptomatic bacteriuria predict mortality and does antimicrobial treatment reduce mortality in elderly ambulatory women? Ann Intern Med. 1994;120:827–833. doi: 10.7326/0003-4819-120-10-199405150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med. 1987;83:27–33. doi: 10.1016/0002-9343(87)90493-1. [DOI] [PubMed] [Google Scholar]
- 19.Ouslander JG, Schapira M, Schnelle JF, Uman G, Fingold S, Tuico E, Nigam JG. Does eradicating bacteriuria affect the severity of chronic urinary incontinence in nursing home residents? Ann Intern Med. 1995;122:749–754. doi: 10.7326/0003-4819-122-10-199505150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Harding GK, Zhanel GG, Nicolle LE, Cheang M Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med. 2002;347:1576–1583. doi: 10.1056/NEJMoa021042. [DOI] [PubMed] [Google Scholar]
- 21.Dalal S, Nicolle L, Marrs CF, Zhang L, Harding G, Foxman B. Long-term Escherichia coli asymptomatic bacteriuria among women with diabetes mellitus. Clin Infect Dis. 2009;49:491–497. doi: 10.1086/600883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol. 1987;138:336–340. doi: 10.1016/s0022-5347(17)43138-7. [DOI] [PubMed] [Google Scholar]
- 23.Maynard FM, Diokno AC. Urinary infection and complications during clean intermittent catheterization following spinal cord injury. J Urol. 1984;132:943–946. doi: 10.1016/s0022-5347(17)49959-9. [DOI] [PubMed] [Google Scholar]
- 24.Harding GK, Nicolle LE, Ronald AR, Preiksaitis JK, Forward KR, Low DE, Cheang M. How long should catheter-acquired urinary tract infection in women be treated? a randomized controlled study. Ann Intern Med. 1991;114:713–719. doi: 10.7326/0003-4819-114-9-713. [DOI] [PubMed] [Google Scholar]
- 25.Warren JW, Anthony WC, Hoopes JM, Muncie HL., Jr Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA. 1982;248:454–458. [PubMed] [Google Scholar]
- 26.Leone M, Perrin AS, Granier I, Visintini P, Blasco V, Antonini F, Albanèse J, Martin C. A randomized trial of catheter change and short course of antibiotics for asymptomatic bacteriuria in catheterized ICU patients. Intensive Care Med. 2007;33:726–729. doi: 10.1007/s00134-007-0534-1. [DOI] [PubMed] [Google Scholar]
- 27.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 28.Grabe M, Forsgren A, Björk T, Hellsten S. Controlled trial of a short and a prolonged course with ciprofloxacin in patients undergoing transurethral prostatic surgery. Eur J Clin Microbiol. 1987;6:11–17. doi: 10.1007/BF02097183. [DOI] [PubMed] [Google Scholar]
- 29.Olsen JH, Friis-Møller A, Jensen SK, Korner B, Hvidt V. Cefotaxime for prevention of infectious complications in bacteriuric men undergoing transurethral prostatic resection. A controlled comparison with methenamine. Scand J Urol Nephrol. 1983;17:299–301. doi: 10.3109/00365598309182135. [DOI] [PubMed] [Google Scholar]
- 30.Grabe M, Forsgren A, Hellsten S. The effect of a short antibiotic course in transurethral prostatic resection. Scand J Urol Nephrol. 1984;18:37–42. doi: 10.3109/00365598409182161. [DOI] [PubMed] [Google Scholar]
- 31.Allan WR, Kumar A. Prophylactic mezlocillin for transurethral prostatectomy. Br J Urol. 1985;57:46–49. doi: 10.1111/j.1464-410x.1985.tb08983.x. [DOI] [PubMed] [Google Scholar]
- 32.Rao PN, Dube DA, Weightman NC, Oppenheim BA, Morris J. Prediction of septicemia following endourological manipulation for stones in the upper urinary tract. J Urol. 1991;146:955–960. doi: 10.1016/s0022-5347(17)37974-0. [DOI] [PubMed] [Google Scholar]
- 33.Cafferkey MT, Falkiner FR, Gillespie WA, Murphy DM. Antibiotics for the prevention of septicaemia in urology. J Antimicrob Chemother. 1982;9:471–477. doi: 10.1093/jac/9.6.471. [DOI] [PubMed] [Google Scholar]
- 34.Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35:1–12. v. doi: 10.1016/j.ucl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Health Insurance Review & Assessment service (HIRA) In-depth analysis of drug consumption. Wonju: HIRA; 2015. [Google Scholar]
- 36.Mazzulli T. Resistance trends in urinary tract pathogens and impact on management. J Urol. 2002;168:1720–1722. doi: 10.1097/01.ju.0000028385.10311.c9. [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ, Lee DS, Choe HS, Shim BS, Kim CS, Kim ME, Cho YH. Antimicrobial resistance in community-acquired urinary tract infections: results from the Korean Antimicrobial Resistance Monitoring System. J Infect Chemother. 2011;17:440–446. doi: 10.1007/s10156-010-0178-x. [DOI] [PubMed] [Google Scholar]
- 38.Kim B, Kim J, Wie SH, Park SH, Cho YK, Lim SK, Shin SY, Yum JS, Lee JS, Kweon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. Is it acceptable to select antibiotics for the treatment of community-acquired acute cystitis based on the antibiotics susceptibility results for uropathogens from community-acquired acute pyelonephritis in Korea? Infect Chemother. 2012;44:269–274. [Google Scholar]
- 39.Lee SJ, Lee SD, Cho IR, Sim BS, Lee JG, Kim CS, Kim ME, Cho YH, Woo YN. Antimicrobial susceptibility of uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2002. Int J Antimicrob Agents. 2004;24(Suppl 1):S61–S64. doi: 10.1016/j.ijantimicag.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Kim ME, Ha US, Cho YH. Prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2006. Int J Antimicrob Agents. 2008;31(Suppl 1):S15–S18. doi: 10.1016/j.ijantimicag.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 41.Kim HY, Lee SJ, Lee DS, Yoo JM, Choe HS. Microbiological characteristics of unresolved acute uncomplicated cystitis. Microb Drug Resist. 2016;22:387–391. doi: 10.1089/mdr.2015.0241. [DOI] [PubMed] [Google Scholar]
- 42.Kim WB, Cho KH, Lee SW, Yang HJ, Yun JH, Lee KW, Kim JM, Kim YH, Jeon YS, Kim ME. Recent antimicrobial susceptibilities for uropathogenic Escherichia coli in patients with community acquired urinary tract infections: a multicenter study. Urogenit Tract Infect. 2017;12:28–34. [Google Scholar]
- 43.Kim KY, Kim CS, Lim DH. The ciprofloxacin resistance pattern of Escherichia coli isolated from female patients with community-acquired urinary tract infection in the Jeonnam and Gwangju region for the recent 2-years. Korean J Urol. 2008;49:540–548. [Google Scholar]
- 44.The Korean Society of Infectious Diseases, The Korean Society for Chemotherapy, Korean Association of Urogenital Tract Infection and Inflammation, The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of urinary tractiInfections: asymptomatic bacteriuria, uncomplicated & complicated urinary tract infections, bacterial prostatitis. Infect Chemother. 2011;43:1–25. [Google Scholar]
- 45.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287:2701–2710. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 46.Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am. 2014;28:75–89. doi: 10.1016/j.idc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simerville JA, Maxted WC, Pahira JJ. Urinalysis: a comprehensive review. Am Fam Physician. 2005;71:1153–1162. [PubMed] [Google Scholar]
- 48.Rubin RH, Shapiro ED, Andriole VT, Davis RJ, Stamm WE. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992;15(Suppl 1):S216–S227. doi: 10.1093/clind/15.supplement_1.s216. [DOI] [PubMed] [Google Scholar]
- 49.Zalmanovici Trestioreanu A, Green H, Paul M, Yaphe J, Leibovici L. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2010;(10):CD007182. doi: 10.1002/14651858.CD007182.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Rafalsky V, Andreeva I, Rjabkova E. Quinolones for uncomplicated acute cystitis in women. Cochrane Database Syst Rev. 2006;(3):CD003597. doi: 10.1002/14651858.CD003597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.U.S. Food & Drug. Fluoroquinolone antimicrobial drugs information. [Accessed 20 March 2018]. Available at: https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm.
- 52.Falagas ME, Vouloumanou EK, Togias AG, Karadima M, Kapaskelis AM, Rafailidis PI, Athanasiou S. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2010;65:1862–1877. doi: 10.1093/jac/dkq237. [DOI] [PubMed] [Google Scholar]
- 53.Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L, Marchese A. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34:407–413. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Zhanel GG, Walkty AJ, Karlowsky JA. Fosfomycin: a first-line oral therapy for acute uncomplicated cystitis. Can J Infect Dis Med Microbiol. 2016;2016:2082693. doi: 10.1155/2016/2082693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seo MR, Kim SJ, Kim Y, Kim J, Choi TY, Kang JO, Wie SH, Ki M, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. Susceptibility of Escherichia coli from community-acquired urinary tract infection to fosfomycin, nitrofurantoin, and temocillin in Korea. J Korean Med Sci. 2014;29:1178–1181. doi: 10.3346/jkms.2014.29.8.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA. 2012;307:583–589. doi: 10.1001/jama.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kavatha D, Giamarellou H, Alexiou Z, Vlachogiannis N, Pentea S, Gozadinos T, Poulakou G, Hatzipapas A, Koratzanis G. Cefpodoxime-proxetil versus trimethoprim-sulfamethoxazole for short-term therapy of uncomplicated acute cystitis in women. Antimicrob Agents Chemother. 2003;47:897–900. doi: 10.1128/AAC.47.3.897-900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raz R, Rottensterich E, Leshem Y, Tabenkin H. Double-blind study comparing 3-day regimens of cefixime and ofloxacin in treatment of uncomplicated urinary tract infections in women. Antimicrob Agents Chemother. 1994;38:1176–1177. doi: 10.1128/aac.38.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galkin VV, Malev IV, Dovgan' EV, Kozlov SN, Rafal'skiĭ VV. Efficacy and safety of cefixim and ciprofloxacin in acute cystitis (a multicenter randomized trial) Urologiia. 2011;(1):13–16. [PubMed] [Google Scholar]
- 60.Lee SJ, Ha US, Kim HW, Cho YH. Efficacy of cefcapene pivoxil for empirical therapy of acute uncomplicated cystitis. Infect Chemother. 2008;40:162–166. [Google Scholar]
- 61.Sohn DW, Ha US, Seo HJ, Lee JY, Kim SW, Cho YH. In-vitro susceptibility study of oral antibiotics to Escherichia coli, isolated from acute uncomplicated cystitis in female outpatients. Infect Chemother. 2006;38:140–145. [Google Scholar]
- 62.Roh KH, Yum JH, Yong D, Choi SH, Rhee JK, Yoo M, Lee K, Chong Y. In vitro activities of cefatrizine-clavulanic acid against gram-negative bacilli isolated from community-acquired urinary track infection. Korean J Lab Med. 2005;25:411–415. [Google Scholar]
- 63.Lee SY, Lee JH, Kim JH, Hur JK, Kim SM, Ma SH, Kang JH. Susceptibility tests of oral antibiotics including cefixime against Escherichia coli, isolated from pediatric patients with community-acquired urinary tract infections. Korean J Pediatr. 2006;49:777–783. [Google Scholar]
- 64.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29:745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 65.Kim JH, Sun HY, Kim TH, Shim SR, Doo SW, Yang WJ, Lee EJ, Song YS. Prevalence of antibiotic susceptibility and resistance of Escherichia coli in acute uncomplicated cystitis in Korea: systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4663. doi: 10.1097/MD.0000000000004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinart M, Kranz J, Jensen K, Proctor T, Naber K, Kunath F, Wagenlehner F, Schmidt S. Optimal dosage and duration of pivmecillinam treatment for uncomplicated lower urinary tract infections: a systematic review and meta-analysis. Int J Infect Dis. 2017;58:96–109. doi: 10.1016/j.ijid.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Milo G, Katchman EA, Paul M, Christiaens T, Baerheim A, Leibovici L. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2005;(2):CD004682. doi: 10.1002/14651858.CD004682.pub2. [DOI] [PubMed] [Google Scholar]
- 68.Lutters M, Vogt-Ferrier NB. Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women. Cochrane Database Syst Rev. 2008;(3):CD001535. doi: 10.1002/14651858.CD001535.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Sadahira T, Wada K, Araki M, Ishii A, Takamoto A, Kobayashi Y, Watanabe M, Watanabe T, Nasu Y, Kumon H Okayama Urological Research Group (OURG) Efficacy and safety of 3 day versus 7 day cefditoren pivoxil regimens for acute uncomplicated cystitis: multicentre, randomized, open-label trial. J Antimicrob Chemother. 2017;72:529–534. doi: 10.1093/jac/dkw424. [DOI] [PubMed] [Google Scholar]
- 70.Grigoryan L, Zoorob R, Wang H, Horsfield M, Gupta K, Trautner BW. Less workup, longer treatment, but no clinical benefit observed in women with diabetes and acute cystitis. Diabetes Res Clin Pract. 2017;129:197–202. doi: 10.1016/j.diabres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Katchman EA, Milo G, Paul M, Christiaens T, Baerheim A, Leibovici L. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196–1207. doi: 10.1016/j.amjmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Lifshitz E, Kramer L. Outpatient urine culture: does collection technique matter? Arch Intern Med. 2000;160:2537–2540. doi: 10.1001/archinte.160.16.2537. [DOI] [PubMed] [Google Scholar]
- 73.Bradbury SM. Collection of urine specimens in general practice: to clean or not to clean? J R Coll Gen Pract. 1988;38:363–365. [PMC free article] [PubMed] [Google Scholar]
- 74.Barry HC, Ebell MH, Hickner J. Evaluation of suspected urinary tract infection in ambulatory women: a cost-utility analysis of office-based strategies. J Fam Pract. 1997;44:49–60. [PubMed] [Google Scholar]
- 75.Carlson KJ, Mulley AG. Management of acute dysuria. A decision-analysis model of alternative strategies. Ann Intern Med. 1985;102:244–249. doi: 10.7326/0003-4819-102-2-244. [DOI] [PubMed] [Google Scholar]
- 76.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 77.Kunin CM. Urinary tract infections in females. Clin Infect Dis. 1994;18:1–10. doi: 10.1093/clinids/18.1.1. [DOI] [PubMed] [Google Scholar]
- 78.Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 79.Ki M, Park T, Choi B, Foxman B. The epidemiology of acute pyelonephritis in South Korea, 1997-1999. Am J Epidemiol. 2004;160:985–993. doi: 10.1093/aje/kwh308. [DOI] [PubMed] [Google Scholar]
- 80.Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med. 2005;142:20–27. doi: 10.7326/0003-4819-142-1-200501040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Czaja CA, Hooton TM. Update on acute uncomplicated urinary tract infection in women. Postgrad Med. 2006;119:39–45. doi: 10.3810/pgm.2006.06.1639. [DOI] [PubMed] [Google Scholar]
- 82.Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician. 2005;71:933–942. [PubMed] [Google Scholar]
- 83.Efstathiou SP, Pefanis AV, Tsioulos DI, Zacharos ID, Tsiakou AG, Mitromaras AG, Mastorantonakis SE, Kanavaki SN, Mountokalakis TD. Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch Intern Med. 2003;163:1206–1212. doi: 10.1001/archinte.163.10.1206. [DOI] [PubMed] [Google Scholar]
- 84.Farrell DJ, Morrissey I, De Rubeis D, Robbins M, Felmingham D. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect. 2003;46:94–100. doi: 10.1053/jinf.2002.1091. [DOI] [PubMed] [Google Scholar]
- 85.Wie SH, Chang UI, Kim HW, Kim YS, Kim SY, Hur J, Kim SI, Kim YR, Kang MW. Clinical features and antimicrobial resistance among clinical isolates of women with community-acquired acute pyelonephritis in 2001-2006. Infect Chemother. 2007;39:9–16. [Google Scholar]
- 86.Hwang BY, Lee JG, Park DW, Lee YJ, Kim SB, Eom JS, Sohn JW, Cheong HJ, Kim WJ, Kim MJ, Park SC. Antimicrobial susceptibility of causative microorganisms in adults with acute pyelonephritis at on Univeristy-affiliated hospital in southwestern Seoul. Infect Chemother. 2003;35:277–282. [Google Scholar]
- 87.Wie SH, Choi SM, Lee DG, Kim SY, Kim SI, Yoo JH, Shin WS, Kang MW. Antibiotic sensitivity of the causative organisms and use of antibiotics in women with community-acquired acute pyelonephritis. Korean J Infect Dis. 2002;34:353–359. [Google Scholar]
- 88.Lee HN, Yoon H. Management of antibiotic-resistant acute pyelonephritis. Urogenit Tract Infect. 2017;12:95–102. [Google Scholar]
- 89.Kim B, Kim J, Seo MR, Wie SH, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Ki M, Pai H. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection. 2013;41:603–612. doi: 10.1007/s15010-013-0441-z. [DOI] [PubMed] [Google Scholar]
- 90.Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother. 2012;56:2181–2183. doi: 10.1128/AAC.06060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wie SH, Ki M, Kim J, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. Clinical characteristics predicting early clinical failure after 72 h of antibiotic treatment in women with community-onset acute pyelonephritis: a prospective multicentre study. Clin Microbiol Infect. 2014;20:O721–O729. doi: 10.1111/1469-0691.12500. [DOI] [PubMed] [Google Scholar]
- 92.Wie SH. The Korean Society for Infectious Diseases, editors. Infectious Diseases. 2nd ed. Seoul: Koonja Publishing Inc.; 2014. Urinary tract infections; pp. 225–237. [Google Scholar]
- 93.American College of Radiology. ACR Appropriateness Criteria®. [Accessed 20 March 2018]. Available at: https://acsearch.acr.org/docs/69489/Narrative/
- 94.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE Infectious Diseases Society of America. European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 95.Neumann I, Moore P. Pyelonephritis (acute) in non-pregnant women. BMJ Clin Evid. 2014;2014:pii:0807. [PMC free article] [PubMed] [Google Scholar]
- 96.Czaja CA, Scholes D, Hooton TM, Stamm WE. Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis. 2007;45:273–280. doi: 10.1086/519268. [DOI] [PubMed] [Google Scholar]
- 97.van der Starre WE, van Dissel JT, van Nieuwkoop C. Treatment duration of febrile urinary tract infections. Curr Infect Dis Rep. 2011;13:571–578. doi: 10.1007/s11908-011-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeon JH, Kim K, Han WD, Song SH, Park KU, Rhee JE, Song KH, Park WB, Kim ES, Park SW, Kim NJ, Oh MD, Kim HB. Empirical use of ciprofloxacin for acute uncomplicated pyelonephritis caused by Escherichia coli in communities where the prevalence of fluoroquinolone resistance is high. Antimicrob Agents Chemother. 2012;56:3043–3046. doi: 10.1128/AAC.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Talan DA, Stamm WE, Hooton TM, Moran GJ, Burke T, Iravani A, Reuning-Scherer J, Church DA. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA. 2000;283:1583–1590. doi: 10.1001/jama.283.12.1583. [DOI] [PubMed] [Google Scholar]
- 100.Stamm WE, McKevitt M, Counts GW. Acute renal infection in women: treatment with trimethoprim-sulfamethoxazole or ampicillin for two or six weeks. A randomized trial. Ann Intern Med. 1987;106:341–345. doi: 10.7326/0003-4819-106-3-341. [DOI] [PubMed] [Google Scholar]
- 101.Klausner HA, Brown P, Peterson J, Kaul S, Khashab M, Fisher AC, Kahn JB. A trial of levofloxacin 750 mg once daily for 5 days vs. ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr Med Res Opin. 2007;23:2637–2645. doi: 10.1185/030079907x233340. [DOI] [PubMed] [Google Scholar]
- 102.Talan DA, Klimberg IW, Nicolle LE, Song J, Kowalsky SF, Church DA. Once daily, extended release ciprofloxacin for complicated urinary tract infections and acute uncomplicated pyelonephritis. J Urol. 2004;171:734–739. doi: 10.1097/01.ju.0000106191.11936.64. [DOI] [PubMed] [Google Scholar]
- 103.Cronberg S, Banke S, Bergman B, Boman H, Eilard T, Elbel E, Hugo-Persson M, Johansson E, Kuylenstierna N, Lanbeck P, Lindblom A, Paulsen O, Schönbeck C, Walder M, Wieslander P. Fewer bacterial relapses after oral treatment with norfloxacin than with ceftibuten in acute pyelonephritis initially treated with intravenous cefuroxime. Scand J Infect Dis. 2001;33:339–343. doi: 10.1080/003655401750173922. [DOI] [PubMed] [Google Scholar]
- 104.Wagenlehner FM, Weidner W, Naber KG. An update on uncomplicated urinary tract infections in women. Curr Opin Urol. 2009;19:368–374. doi: 10.1097/MOU.0b013e32832ae18c. [DOI] [PubMed] [Google Scholar]
- 105.Sandberg T, Skoog G, Hermansson AB, Kahlmeter G, Kuylenstierna N, Lannergård A, Otto G, Settergren B, Ekman GS. Ciprofloxacin for 7 days vs. 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484–490. doi: 10.1016/S0140-6736(12)60608-4. [DOI] [PubMed] [Google Scholar]
- 106.van Nieuwkoop C, van der Starre WE, Stalenhoef JE, van Aartrijk AM, van der Reijden TJ, Vollaard AM, Delfos NM, van 't Wout JW, Blom JW, Spelt IC, Leyten EM, Koster T, Ablij HC, van der Beek MT, Knol MJ, van Dissel JT. Treatment duration of febrile urinary tract infection: a pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med. 2017;15:70. doi: 10.1186/s12916-017-0835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang UI, Kim HW, Wie SH. Use of cefuroxime for women with community-onset acute pyelonephritis caused by cefuroxime-susceptible or -resistant Escherichia coli . Korean J Intern Med. 2016;31:145–155. doi: 10.3904/kjim.2016.31.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang UI, Kim HW, Wie SH. Comparison of second- and third-generation cephalosporin as initial therapy for women with community-onset uncomplicated acute pyelonephritis. Yonsei Med J. 2015;56:1266–1273. doi: 10.3349/ymj.2015.56.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129:242–258. doi: 10.1080/00325481.2017.1246055. [DOI] [PubMed] [Google Scholar]
- 110.Ipekci T, Seyman D, Berk H, Celik O. Clinical and bacteriological efficacy of amikacin in the treatment of lower urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae . J Infect Chemother. 2014;20:762–767. doi: 10.1016/j.jiac.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 111.Pietrucha-Dilanchian P, Hooton TM. Diagnosis, treatment, and prevention of urinary tract infection. Microbiol Spectr. 2016;4:UTI-0021–UTI-2015. doi: 10.1128/microbiolspec.UTI-0021-2015. [DOI] [PubMed] [Google Scholar]
- 112.Veve MP, Wagner JL, Kenney RM, Grunwald JL, Davis SL. Comparison of fosfomycin to ertapenem for outpatient or step-down therapy of extended-spectrum beta-lactamase urinary tract infections. Int J Antimicrob Agents. 2016;48:56–60. doi: 10.1016/j.ijantimicag.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 113.Tamma PD, Girdwood SC, Gopaul R, Tekle T, Roberts AA, Harris AD, Cosgrove SE, Carroll KC. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781–788. doi: 10.1093/cid/cit395. [DOI] [PubMed] [Google Scholar]
- 114.Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG. Yates K, Gasink LB. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63:754–762. doi: 10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI) Lancet. 2015;385:1949–1956. doi: 10.1016/S0140-6736(14)62220-0. [DOI] [PubMed] [Google Scholar]
- 116.Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á Extended-Spectrum Beta-Lactamases-Red Española de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group. β-Lactam/ β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 117.Kang CI, Park SY, Chung DR, Peck KR, Song JH. Piperacillin-tazobactam as an initial empirical therapy of bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae . J Infect. 2012;64:533–534. doi: 10.1016/j.jinf.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Cho SY, Choi SM, Park SH, Lee DG, Choi JH, Yoo JH. Amikacin therapy for urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Korean J Intern Med. 2016;31:156–161. doi: 10.3904/kjim.2016.31.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klahr S. Obstructive nephropathy. Intern Med. 2000;39:355–361. doi: 10.2169/internalmedicine.39.355. [DOI] [PubMed] [Google Scholar]
- 120.Reyner K, Heffner AC, Karvetski CH. Urinary obstruction is an important complicating factor in patients with septic shock due to urinary infection. Am J Emerg Med. 2016;34:694–696. doi: 10.1016/j.ajem.2015.12.068. [DOI] [PubMed] [Google Scholar]
- 121.Heyns CF. Urinary tract infection associated with conditions causing urinary tract obstruction and stasis, excluding urolithiasis and neuropathic bladder. World J Urol. 2012;30:77–83. doi: 10.1007/s00345-011-0725-9. [DOI] [PubMed] [Google Scholar]
- 122.Kim SC, Wang CS. A clinical observation on 131 cases of hydronephrosis. Korean J Urol. 1978;19:89–97. [Google Scholar]
- 123.Romero Cullerés G, Sugrañes JC, Planells Romeo I, Giménez Pérez M. Characteristics of urinary tract infections in different patient subpopulations and depending on the bladder emptying system. Actas Urol Esp. 2010;34:251–257. [PubMed] [Google Scholar]
- 124.Marien T, Miller NL. Treatment of the Infected Stone. Urol Clin North Am. 2015;42:459–472. doi: 10.1016/j.ucl.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 125.Brown PD. Management of urinary tract infections associated with nephrolithiasis. Curr Infect Dis Rep. 2010;12:450–454. doi: 10.1007/s11908-010-0141-0. [DOI] [PubMed] [Google Scholar]
- 126.Roy C, Pfleger DD, Tuchmann CM, Lang HH, Saussine CC, Jacqmin D. Emphysematous pyelitis: findings in five patients. Radiology. 2001;218:647–650. doi: 10.1148/radiology.218.3.r01fe14647. [DOI] [PubMed] [Google Scholar]
- 127.Olvera-Posada D, Armengod-Fischer G, Vázquez-Lavista LG, Maldonado-Ávila M, Rosas-Nava E, Manzanilla-García H, Castillejos-Molina RA, Méndez-Probst CE, Sotomayor M, Feria-Bernal G, Rodríguez-Covarrubias F. Emphysematous pyelonephritis: multicenter clinical and therapeutic experience in Mexico. Urology. 2014;83:1280–1284. doi: 10.1016/j.urology.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 128.Somani BK, Nabi G, Thorpe P, Hussey J, Cook J, N'Dow J ABACUS Research Group. Is percutaneous drainage the new gold standard in the management of emphysematous pyelonephritis? Evidence from a systematic review. J Urol. 2008;179:1844–1849. doi: 10.1016/j.juro.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 129.Hamasuna R, Takahashi S, Nagae H, Kubo T, Yamamoto S, Arakawa S, Matsumoto T. Obstructive pyelonephritis as a result of urolithiasis in Japan: diagnosis, treatment and prognosis. Int J Urol. 2015;22:294–300. doi: 10.1111/iju.12666. [DOI] [PubMed] [Google Scholar]
- 130.Wagenlehner FM, Lichtenstern C, Rolfes C, Mayer K, Uhle F, Weidner W, Weigand MA. Diagnosis and management for urosepsis. Int J Urol. 2013;20:963–970. doi: 10.1111/iju.12200. [DOI] [PubMed] [Google Scholar]
- 131.Cha MK, Kang CI, Kim SH, Cho SY, Ha YE, Wi YM, Chung DR, Peck KR, Song JH Korean Network for Study on Infectious Diseases (KONSID) In vitro activities of 21 antimicrobial agents alone and in combination with aminoglycosides or fluoroquinolones against extended-spectrum-β-lactamase-producing Escherichia coli isolates causing bacteremia. Antimicrob Agents Chemother. 2015;59:5834–5837. doi: 10.1128/AAC.01121-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramsey S, Robertson A, Ablett MJ, Meddings RN, Hollins GW, Little B. Evidence-based drainage of infected hydronephrosis secondary to ureteric calculi. J Endourol. 2010;24:185–189. doi: 10.1089/end.2009.0361. [DOI] [PubMed] [Google Scholar]
- 133.Aboumarzouk OM, Hughes O, Narahari K, Coulthard R, Kynaston H, Chlosta P, Somani B. Emphysematous pyelonephritis: Time for a management plan with an evidence-based approach. Arab J Urol. 2014;12:106–115. doi: 10.1016/j.aju.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;160:797–805. doi: 10.1001/archinte.160.6.797. [DOI] [PubMed] [Google Scholar]
- 135.Kolla PK, Madhav D, Reddy S, Pentyala S, Kumar P, Pathapati RM. Clinical profile and outcome of conservatively managed emphysematous pyelonephritis. ISRN Urol. 2012;2012:931982. doi: 10.5402/2012/931982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bjurlin MA, Hurley SD, Kim DY, Cohn MR, Jordan MD, Kim R, Divakaruni N, Hollowell CM. Clinical outcomes of nonoperative management in emphysematous urinary tract infections. Urology. 2012;79:1281–1285. doi: 10.1016/j.urology.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 137.Nickel JC, Alexander RB, Anderson R, Berger R, Comiter CV, Datta NS, Fowler JE, Krieger JN, Landis JR, Litwin MS, McNaughton-Collins M, O'Leary MP, Pontari MA, Schaeffer AJ, Shoskes DA, White P, Kusek J, Nyberg L Chronic Prostatitis Collaborative Research Network Study Groups. Category III chronic prostatitis/chronic pelvic pain syndrome: insights from the National Institutes of Health Chronic Prostatitis Collaborative Research Network studies. Curr Urol Rep. 2008;9:320–327. doi: 10.1007/s11934-008-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weidner W, Schiefer HG, Krauss H, Jantos C, Friedrich HJ, Altmannsberger M. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection. 1991;19(Suppl 3):S119–S125. doi: 10.1007/BF01643680. [DOI] [PubMed] [Google Scholar]
- 139.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 140.Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–S11. doi: 10.1097/01.ju.0000142058.99614.ff. discussion S11-2. [DOI] [PubMed] [Google Scholar]
- 141.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 142.Leigh DA. Prostatitis--an increasing clinical problem for diagnosis and management. J Antimicrob Chemother. 1993;32(Suppl A):1–9. doi: 10.1093/jac/32.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 143.Ludwig M. Diagnosis and therapy of acute prostatitis, epididymitis and orchitis. Andrologia. 2008;40:76–80. doi: 10.1111/j.1439-0272.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- 144.Kravchick S, Cytron S, Agulansky L, Ben-Dor D. Acute prostatitis in middle-aged men: a prospective study. BJU Int. 2004;93:93–96. doi: 10.1111/j.1464-410x.2004.04642.x. [DOI] [PubMed] [Google Scholar]
- 145.Ha US, Kim ME, Kim CS, Shim BS, Han CH, Lee SD, Cho YH. Acute bacterial prostatitis in Korea: clinical outcome, including symptoms, management, microbiology and course of disease. Int J Antimicrob Agents. 2008;31(Suppl 1):S96–S101. doi: 10.1016/j.ijantimicag.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 146.Brede CM, Shoskes DA. The etiology and management of acute prostatitis. Nat Rev Urol. 2011;8:207–212. doi: 10.1038/nrurol.2011.22. [DOI] [PubMed] [Google Scholar]
- 147.Sharp VJ, Takacs EB, Powell CR. Prostatitis: diagnosis and treatment. Am Fam Physician. 2010;82:397–406. [PubMed] [Google Scholar]
- 148.Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, Karsenty G, Kessler TM, Schneider M, 't Hoen L, Blok B. Summary of European Association of Urology (EAU) guidelines on Neuro-Urology. Eur Urol. 2016;69:324–333. doi: 10.1016/j.eururo.2015.07.071. [DOI] [PubMed] [Google Scholar]
- 149.Lipsky BA, Byren I, Hoey CT. Treatment of bacterial prostatitis. Clin Infect Dis. 2010;50:1641–1652. doi: 10.1086/652861. [DOI] [PubMed] [Google Scholar]
- 150.Etienne M, Chavanet P, Sibert L, Michel F, Levesque H, Lorcerie B, Doucet J, Pfitzenmeyer P, Caron F. Acute bacterial prostatitis: heterogeneity in diagnostic criteria and management. Retrospective multicentric analysis of 371 patients diagnosed with acute prostatitis. BMC Infect Dis. 2008;8:12. doi: 10.1186/1471-2334-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Charalabopoulos K, Karachalios G, Baltogiannis D, Charalabopoulos A, Giannakopoulos X, Sofikitis N. Penetration of antimicrobial agents into the prostate. Chemotherapy. 2003;49:269–279. doi: 10.1159/000074526. [DOI] [PubMed] [Google Scholar]
- 152.Millán-Rodríguez F, Palou J, Bujons-Tur A, Musquera-Felip M, Sevilla-Cecilia C, Serrallach-Orejas M, Baez-Angles C, Villavicencio-Mavrich H. Acute bacterial prostatitis: two different sub-categories according to a previous manipulation of the lower urinary tract. World J Urol. 2006;24:45–50. doi: 10.1007/s00345-005-0040-4. [DOI] [PubMed] [Google Scholar]
- 153.Nickel JC. The use of alpha1-adrenoceptor antagonists in lower urinary tract symptoms: beyond benign prostatic hyperplasia. Urology. 2003;62(Suppl 1):34–41. doi: 10.1016/s0090-4295(03)00472-2. [DOI] [PubMed] [Google Scholar]
- 154.Wagenlehner FM, Pilatz A, Bschleipfer T, Diemer T, Linn T, Meinhardt A, Schagdarsurengin U, Dansranjavin T, Schuppe HC, Weidner W. Bacterial prostatitis. World J Urol. 2013;31:711–716. doi: 10.1007/s00345-013-1055-x. [DOI] [PubMed] [Google Scholar]
- 155.Yoon BI, Kim S, Han DS, Ha US, Lee SJ, Kim HW, Han CH, Cho YH. Acute bacterial prostatitis: how to prevent and manage chronic infection? J Infect Chemother. 2012;18:444–450. doi: 10.1007/s10156-011-0350-y. [DOI] [PubMed] [Google Scholar]
- 156.Meares EM., Jr Prostatic abscess. J Urol. 1986;136:1281–1282. doi: 10.1016/s0022-5347(17)45313-4. [DOI] [PubMed] [Google Scholar]
- 157.Meares EM., Jr Acute and chronic prostatitis: diagnosis and treatment. Infect Dis Clin North Am. 1987;1:855–873. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.