Abstract
Neuropeptide Y (NPY) plays an important role in the regulation of energy homeostasis in the level of central and sympathetic nervous systems (SNSs). Genetic silencing of peripheral Y2-receptors have anti-obesity effects, but it is not known whether pharmacological blocking of peripheral Y2-receptors would similarly benefit energy homeostasis. The effects of a peripherally administered Y2-receptor antagonist were studied in healthy and energy-rich conditions with or without excess NPY. Genetically obese mice overexpressing NPY in brain noradrenergic nerves and SNS (OE-NPYDβH) represented the situation of elevated NPY levels, while wildtype (WT) mice represented the normal NPY levels. Specific Y2-receptor antagonist, BIIE0246, was administered (1.3 mg/kg/day, i.p.) for 2 or 4.5 weeks to OE-NPYDβH and WT mice feeding on chow or Western diet. Treatment with Y2-receptor antagonist increased body weight gain in both genotypes on chow diet and caused metabolic disturbances (e.g., hyperinsulinemia and hypercholesterolemia), especially in WT mice. During energy surplus (i.e., on Western diet), blocking of Y2-receptors induced obesity in WT mice, whereas OE-NPYDβH mice showed reduced fat mass gain, hepatic glycogen and serum cholesterol levels relative to body adiposity. Thus, it can be concluded that with normal NPY levels, peripheral Y2-receptor antagonist has no potential for treating obesity, but oppositely may even induce metabolic disorders. However, when energy-rich diet is combined with elevated NPY levels, e.g., stress combined with an unhealthy diet, Y2-receptor antagonism has beneficial effects on metabolic status.
Keywords: neuropeptide Y, Y2-receptor, BIIE0246, obesity, the metabolic syndrome
Introduction
Neuropeptide Y (NPY), a 36-amino-acid neurotransmitter, plays a well-known role in the regulation of energy homeostasis aiming at energy storage during negative energy balance (Billington et al., 1991; Zarjevski et al., 1993; Sainsbury et al., 1997). In the central nervous system (CNS), NPY nerves densely innervate the hypothalamus, especially the arcuate nucleus (Arc) and the paraventricular nucleus (PVN), which are responsible for regulation of feeding (Chronwall et al., 1985). Additionally, NPY is co-expressed with noradrenaline in central noradrenergic neurons and peripheral sympathetic nervous system (SNS) (Ekblad et al., 1984), where NPY promotes weight gain by inhibiting lipolysis, and stimulating adipogenesis and angiogenesis in the adipose tissue, and is indispensable for stress-induced obesity (Zukowska-Grojec et al., 1998; Bradley et al., 2005; Kuo et al., 2007; Zhang et al., 2014). NPY acts via six G-protein coupled Y-receptors (Y1–Y5 and y6), which bind also the other members of the NPY family, i.e., peptide YY (PYY) and pancreatic polypeptide (PP) (Silva et al., 2002). Most of them (Y1, Y2, Y5, and y6) have been implicated in feeding and in the control of energy homeostasis (Ekblad et al., 1984; Bowers et al., 2004; Yulyaningsih et al., 2014).
Presynaptic Y2-auto-receptors regulate the release of NPY in the hypothalamus and SNS. Gut-derived Y2-receptor agonist, PYY3-36, reduces food intake by inhibiting NPY release via presynaptic Y2-receptors in the Arc (Batterham et al., 2002), which is abolished by Arc-specific administration of Y2-receptor antagonist (Abbott et al., 2005). Knock-out of hypothalamic Y2-receptors in mice leads to hyperphagia and predisposes to obesity (Shi et al., 2010). Similarly, several human single nucleotide polymorphisms (SNPs) in Y2-receptor (rs1047214, rs12649641, rs6857715, and rs17376826) are associated with obesity (Lavebratt et al., 2006; Torekov et al., 2006; Siddiq et al., 2007; Hunt et al., 2011). In contrast, extra-hypothalamic, postsynaptic Y2-receptors mediate the obesogenic effects of NPY. Knock-out of germline Y2-receptors has been shown to reduce body weight and adiposity both on regular chow and on high-fat diet (Soloveva et al., 1997; Sainsbury et al., 2006), and also to improve glucose and cholesterol metabolism of genetically obese and type 2 diabetic ob/ob mice (Naveilhan et al., 2002; Sainsbury et al., 2002). Mice with an adult-onset knock-down of peripheral Y2-receptors are resistant to diet-induced obesity (DIO) and have improved glucose clearance (Shi et al., 2011). Treatment with a peripheral Y2-receptor antagonist has been shown to improve the metabolic status of diabetic rats by decreasing serum cholesterol and triglyceride levels (Liu et al., 2013). Furthermore, inhibition of adipose tissue Y2-receptors has been shown to prevent NPY-induced growth of fat mass in vivo and pre-adipocyte (3T3-L1) differentiation in vitro (Kuo et al., 2007; Rosmaninho-Salgado et al., 2012).
Based on these findings, the obesogenic role of peripheral Y2-receptors seems quite convincing and antagonism of peripheral Y2-receptors a plausible anti-obesity drug strategy. To this end, we aimed to test the anti-obesity effects of chronic treatment with a specific Y2-receptor antagonist with a potential clinical application to be obesity and metabolic disorders due to NPY excess induced by chronic stress or genetic factors (gain-of-function polymorphisms) (Karvonen et al., 1998; Ding et al., 2005; Masoudi-Kazemabad et al., 2013). In order to study this, transgenic mice overexpressing NPY in noradrenergic neurons (OE-NPYDβH) and wildtype (WT) control mice were subjected to chow or DIO with Western type diet and treated with peripheral Y2-receptor antagonist, BIIE0246, which is highly selective for its receptors (Doods et al., 1999) and unable to penetrate the blood-brain barrier (Brothers et al., 2010). OE-NPYDβH mice present a genetic obesity model with the metabolic syndrome-like phenotype and with increased peripheral NPY (Ruohonen et al., 2008; Vahatalo et al., 2015; Ailanen et al., 2017). We hypothesized that Y2-receptor antagonism would improve the metabolic status especially in OE-NPYDβH mice and in DIO. As expected, it decreased fat mass gain in an energy-rich environment in OE-NPYDβH mice, but surprisingly impaired the metabolic status in WT mice on both diets.
Materials and Methods
Animals
Homozygous transgenic male OE-NPYDβH and WT mice on a C57Bl/6N background were used in the experiments. The transgene construction, delivery to noradrenergic neurons with dopamine-β-hydroxylase (DβH) gene promoter (Ruohonen et al., 2008), and the metabolic phenotyping of homozygous OE-NPYDβH mice has been previously published (Vahatalo et al., 2015; Ailanen et al., 2017). The mice were housed in an animal room maintained at 21 ± 3°C with a 12-h light/12-h dark cycle (lights on at 6 a.m.). To study the effect of Y2-receptor antagonism in healthy conditions, standard rodent chow (9 kcal% fat, 22 kcal% protein, 69 kcal% carbohydrates, SDS, Essex, United Kingdom) was fed ad libitum to OE-NPYDβH (NPY) and WT mice. To study the effect in DIO, Western diet (41 kcal% fat, 17 kcal% protein, 43 kcal% carbohydrates, D12079B, Research Diets, New Brunswick, NJ, United States) was fed for 8 weeks prior to the drug administration, and the groups were named DIO-NPY and DIO-WT. Tap water was freely available. Animals (1-3/group) were excluded from the study if they did not gain weight on Western diet (before habituation body weight gain < 3 g and fat mass gain < 2 g). Animal care was in accordance with the guidelines of the International Council of Laboratory Animal Science (ICLAS), and all the experimental procedures were approved by the Finnish national animal care and use committee.
Experimental Procedures
Drug treatment was studied at the age of 20 weeks. Prior to treatments the mice were habituated for 2 weeks to the handling stress with daily saline injections (i.p.). Half of the chow-fed mice (n = 10–13/group) were treated for 4.5 weeks, and half were sacrificed and tissues collected already after 2-week treatment (n = 7–14/group). DIO mice (n = 7–12/group) were treated for 2 weeks. (The study protocol is presented in Supplementary Figure 1). Mice received 1.3 mg/kg of Y2-receptor antagonist (BIIE0246, Tocris Bioscience, Bristol, United Kingdom) or vehicle (DMSO, Tween® 80) (Fisher Scientific, Fair Lawn, NJ, United States and 0.9% NaCl, 1:1:18, respectively) with daily intraperitoneal (i.p.) injections. BIIE0246 with a dose of 2 mg/kg has previously been used in a study with acute administration (Forbes et al., 2012), which supports rationality of the dose (1.3 mg/kg) being used with repeated dosing in the present study. The half-life of BIIE0246 in mouse is less than 3 h, but markedly longer than the half-life of the other Y2-receptor antagonists, thus making it the most suitable Y2-receptor antagonist for chronic administration (Brothers et al., 2010).
Mice were weighed twice a week and food intake per cage was measured once a week. Food intake per cage (n = 3–6 cages/group) was divided by the number of animals in each cage and presented as an average daily energy intake per cage. Body composition was measured from conscious mice with EchoMRI-700 (Echo Medical Systems LLC, Houston, TX, United States) at the initiation of the Western diet, prior to the habituation and the drug treatment periods, after 2-week drug treatment, and before euthanasia. Each animal was scanned twice and the average values for body fat and lean tissue mass were calculated. Mice were divided into treatment groups based on their body weights, and at the initiation of the drug treatments the body compositions were similar between treatment groups within the same genotype (Supplementary Figure 2). As previously shown, NPY mice had increased body weight and fat mass, and decreased lean mass compared to WT on chow, whereas DIO-NPY and DIO-WT mice had similar body weights and adiposity at the beginning of drug treatment (Ruohonen et al., 2012). Energy intake was similar between groups during the habituation period (Supplementary Figure 2).
At termination, mice were fasted for 3 h and blood glucose was measured from awake animals with the Precision Xtra Glucose Monitoring Device (Abbott Diabetes Care, Abbott Park, CA, United States). Mice were then anesthetized with ketamine (75 mg/kg i.p. Ketaminol, Intervet Oy, Espoo, Finland) and medetomidine (1 mg/kg i.p. Cepetor, ScanVet Oy, Vantaa, Finland). Serum was obtained by cardiac puncture, after which the animal was euthanized by cervical dislocation. Subcutaneous, epididymal, retroperitoneal and mesenteric white adipose tissue (WAT) pads, interscapular brown adipose tissue (BAT) and liver were collected and weighed. Medial basal hypothalamus was isolated with a mouse brain block using a 3-mm section caudal to the optic nerve chiasma. The 3-mm brainstem section extended 2-mm caudal from the hypothalamic section excluding the cerebellum and cerebral cortex.
Blood Parameters
Serum insulin levels were quantitated with an ultrasensitive mouse ELISA kit (Mercodia AB, Uppsala, Sweden), and serum cholesterol with the Cholesterol Fluorometric Assay kit (Cayman Chemical Company, Ann Arbor, MI, United States) according to the manufacturer’s instructions.
Liver Histology and Adiposity
In order to detect liver histology, samples were fixed with formalin and embedded in paraffin. Liver morphology and glycogen content were analyzed with standard light microscope from paraffin sections (5 μm) on microscopic slides stained with haematoxylin and eosin (H&E) or Periodic acid-Schiff (PAS), respectively. In order to analyze the hepatic lipids, frozen liver samples were homogenized in PBS with 0.1% NP-40, and centrifuged (2 min at 16 000 rpm). Triglycerides and cholesterol were determined from the supernatant with TR0100 Serum triglyceride determination kit (Sigma-Aldrich, St. Louis, MO, United States) and Cholesterol (Total) CHOD-PAP Kit (Biolabo, Maizy, France), respectively.
Real-Time qPCR
Total RNA was isolated from tissue samples stored in RNA Stabilization Reagent (RNAlater, Qiagen, Hilden, Germany). Retroperitoneal WAT and BAT were snap frozen in liquid nitrogen without RNAlater. RNAs from BAT and WAT samples were extracted with the Trizol Reagent (Invitrogen, Carlsbad, CA, United States), from liver with RNeasy Mini Kit (Qiagen), from the brain sections with RNeasy Lipid Tissue Mini Kit (Qiagen) and from adrenal gland with Arcturus® PicoPure® RNA Isolation Kit (Applied Biosystems, Foster City, CA, United States). RNA was converted to cDNA with High Capacity RNA-to-cDNA Kit (Applied Biosystems) and SYBR Green (KAPA SYBR® FAST ABI Prism®, Kapa Biosystems, Woburn, MA, United States) technique with separate primers (available upon request) was used for quantification. Target genes were quantified with 7300 Real-Time PCR System (Applied Biosystems) relative to the housekeeping gene β-actin (Actb) (NM_007393.5) or ribosomal protein S29 (Rps29) (NM_009093.2), and formula 2-ΔΔCT was used for calculating the gene expression. Expression levels were presented relatively to the expression levels of vehicle-treated WT mice.
The mRNA expression was analyzed from the samples collected after 2 weeks of treatment both in chow and DIO groups in order to detect the changes in energy metabolism and sympathetic tone induced by drug treatment. Npy (NM_023456) and Y2-receptor (Y2r) (NM_008731.3) mRNA expression were quantitated in adrenal gland, Npy, tyrosine hydroxylase (Th) (NM_009377.1), pro-opiomelanocortin (Pomc) (NM_001278582.1) and Y2r expression in the hypothalamus, and Npy and Th expression in the brainstem. In addition, carboxylesterase 3 (Ces3) (BC019198.2), fatty acid binding protein 4 (Fabp4) (NM_024406.3), hormone sensitive lipase (Lipe), (NM_010719.5) lipoprotein lipase (Lpl) (NM_008509.2) and matrix metallopeptidase (Mmp3) (NM_010809.2) mRNA expressions were analyzed in retroperitoneal WAT, uncoupling protein 1 (Ucp1) (NM_009463.3) mRNA expression in BAT, and glycogen metabolism related genes glycogen synthase (Gys2) (NM_145572.2) and phosphorylase (Pygl) (NM_133198.2) mRNA expressions in the livers of DIO groups.
Statistical Analyses
Statistical analyses were carried out using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, United States). Data are presented as means ± SEM and the results were considered statistically significant at P < 0.05. The comparisons between the genotypes and the treatments were analyzed with two-way ANOVA, except for food intake, which was analyzed over time with repeated measures ANOVA. Bonferroni post hoc test was performed in case the interaction between treatment and genotype was significant to analyze the treatment effect within each genotype. Correlations were analyzed with the Pearson correlation test, and linear regression analysis was used to test the differences between regression slopes and intercepts.
Results
Body Composition
First, the effect of BIIE0246 on body composition was studied. On chow diet, genetically obese NPY mice showed increased gain in body weight and adiposity (Figures 1A–C). Treatment with BIIE0246 promoted body weight gain in both genotypes after 4.5 weeks (Figure 1A), and already at 2 weeks (Supplementary Figure 3). BIIE0246 had no significant effect on fat mass gain (Figure 1B). However, there was a different effect of BIIE0246 on lean mass gain between the genotypes (treatment × genotype interaction P = 0.05) suggesting that weight gain in NPY (but not in WT) mice is due to lean mass gain (Figure 1C).
FIGURE 1.
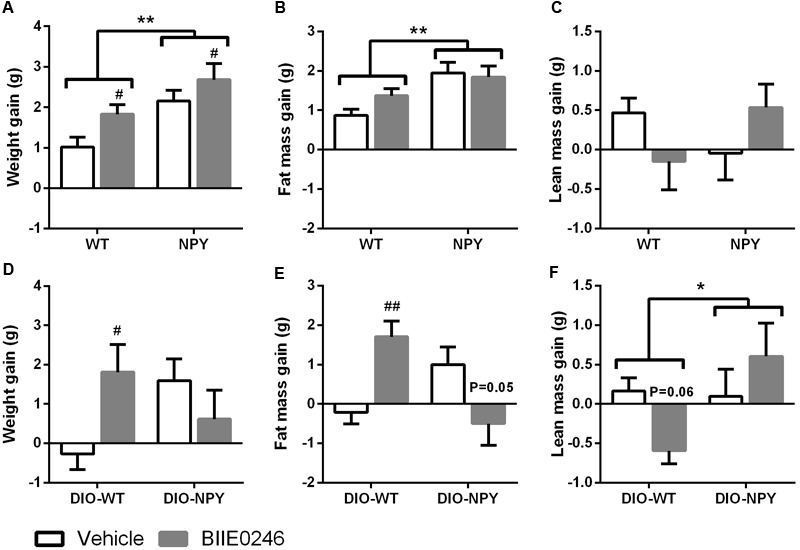
(A,D) Body weight, (B,E) fat mass and (C,F) lean mass gain in OE-NPYDβH and WT mice on chow (n = 11–12/group) (A–C) or Western diet (n = 7–11/group) (D–F) treated with Y2-receptor antagonist (BIIE0246) or vehicle for 4.5 or 2 weeks, respectively. Values are expressed as means ± SEM. ∗P < 0.05 and ∗∗P < 0.01 comparing the different genotypes with two-way ANOVA, and #P < 0.05, ##P < 0.01, P = 0.05 and P = 0.06 comparing BIIE0246 treatment and vehicle treatment with two-way ANOVA (A), or with Bonferroni post hoc test following a significant interaction between treatment and genotype in two-way ANOVA (D–F). White bars, vehicle treated mice; gray bars, BIIE0246 treated mice; WT, wildtype mice on chow diet; NPY, OE-NPYDβH mice on chow diet; DIO-WT, wildtype mice on Western diet; DIO-NPY, OE-NPYDβH mice on Western diet.
In DIO, BIIE0246 had different effects on body weight and composition depending on the genotype (treatment × genotype interaction in body weight P < 0.05, in fat mass P < 0.001 and in lean mass P < 0.05). In DIO-WT group, post hoc analysis revealed increased body weight and fat mass gain, and a tendency to decreased lean mass gain. In DIO-NPY, BIIE0246 inhibited fat mass gain (P = 0.05), and did not change body weight or lean mass gain compared to vehicle (Figures 1D–F).
Food Intake
Treatment with BIIE0246 did not change the energy intake compared to vehicle treatment on chow diet or in DIO (Figure 2).
FIGURE 2.
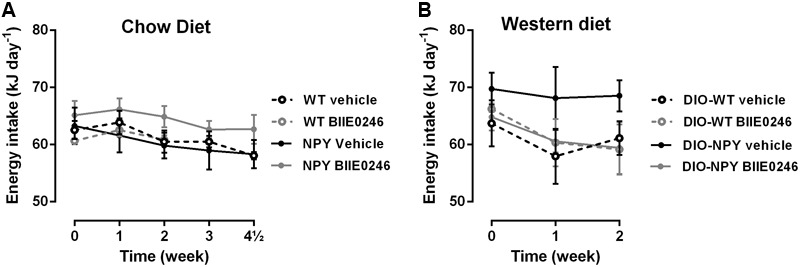
(A,B) Energy intake of chow and western diet-fed OE-NPYDβH and WT mice (n = 3–6 cages/group) treated with Y2-receptor antagonist (BIIE0246) or vehicle for 4.5 or 2 weeks, respectively. Values are expressed as means ± SEM and present an average value per mouse per cage. Statistics is analyzed with repeated measures two-way ANOVA. WT, wildtype mice on chow diet; NPY, OE-NPYDβH mice on chow diet; DIO-WT, wildtype mice on Western diet; DIO-NPY, OE-NPYDβH mice on Western diet.
Blood Glucose and Lipids
In order to elucidate the effects of BIIE0246 on glucose and lipid metabolism, relevant markers in serum and liver were measured in the 4.5-week chow cohort with more pronounced weight effect than the 2-week cohort, and in the DIO cohort. BIIE0246 or NPY genotype had no effect on glucose levels on either diet (Table 1). However, serum insulin levels and HOMA-IR index were increased in NPY and DIO-NPY compared to their control groups suggesting insulin resistance. BIIE0246 increased insulin and HOMA-IR in chow fed groups but not in DIO.
Table 1.
Serum glucose and cholesterol parametres.
| Chow diet |
||||
|---|---|---|---|---|
| WT (n = 11–13) |
NPY (n = 11–12) |
|||
| Vehicle | BIIE0246 | Vehicle | BIIE0246 | |
| Glucose (mM) | 8.4 ± 0.6 | 8.0 ± 0.5 | 8.6 ± 0.4P=0.05 | 8.9 ± 0.3P=0.05 |
| Insulin (μg l-1) | 0.28 ± 0.03 | 0.44 ± 0.04# | 0.47 ± 0.04∗∗∗ | 0.51 ± 0.06∗∗∗# |
| HOMA-IR | 2.5 ± 0.3 | 3.6 ± 0.3# | 4.3 ± 0.4∗∗∗ | 5.1 ± 0.5∗∗∗# |
| Cholesterol (mM) | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.1∗∗ | 2.7 ± 0.1∗∗ |
|
Western diet |
||||
|
DIO-WT (n = 7–12) |
DIO-NPY (n = 6–7) |
|||
| Vehicle | BIIE0246 | Vehicle | BIIE0246 | |
| Glucose (mM) | 9.3 ± 0.4 | 9.3 ± 0.6 | 9.4 ± 0.6 | 9.5 ± 0.6 |
| Insulin (μg l-1) | 0.75 ± 0.14 | 0.76 ± 0.10 | 1.03 ± 0.25P=0.06 | 1.51 ± 0.55 P=0.06 |
| HOMA-IR | 7.6 ± 1.4 | 6.7 ± 0.9 | 9.8 ± 1.8∗ | 14.8 ± 4.8∗ |
| Cholesterol (mM) | 6.1 ± 0.4 | 6.4 ± 0.4 | 6.5 ± 0.5 | 5.8 ± 0.8 |
The data is presented from a chow diet-fed cohort after 4.5 weeks of treatment and from a Western diet-fed cohort after 2 weeks of treatment with BIIE0246 or vehicle. Values are expressed as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, P = 0.05 and P = 0.06 between the genotypes with two-way ANOVA. #P < 0.05 comparing BIIE0246 treatment and vehicle treatment with two-way ANOVA. WT, wildtype mice on chow diet; NPY, OE-NPYDβH mice on chow diet; DIO-WT, wildtype mice on Western diet; DIO-NPY, OE-NPYDβH mice on Western diet.
Excess NPY increased serum cholesterol levels on healthy diet (Table 1). Interestingly, increased cholesterol levels were detected also in WT mice treated with BIIE0246 for 2 weeks (Supplementary Figure 3), but not in the 4.5-week cohort (Table 1). In DIO, BIIE0246 did not have significant effect on absolute cholesterol levels (Table 1). However, in DIO-NPY mice in both treatment groups, cholesterol levels correlated positively with body fat mass (DIO-NPY vehicle R2 = 0.90, P < 0.01; DIO-NPY BIIE0246 R2 = 0.96, P < 0.001), but not in any other group, and the slope of the regression curve of cholesterol and fat mass was significantly decreased in BIIE0246-treated DIO-NPY group when compared with vehicle-treated group (Figure 3). This suggests that serum cholesterol in DIO-NPY mice is highly dependent on body fat mass, and with similar body adiposity, serum cholesterol levels are lower after treatment with BIIE0246.
FIGURE 3.
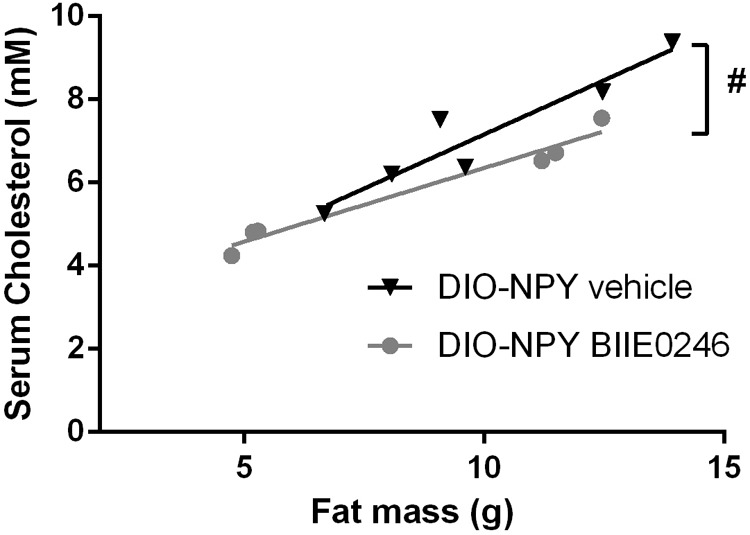
Positive correlation between serum cholesterol values and body fat mass and the difference between the correlation slopes in OE-NPYDβH and WT mice on Western diet treated with Y2-receptor antagonist (BIIE0246) and vehicle for 2 weeks (n = 6–7/group). Values are expressed as means ± SEM. #P < 0.05 comparing correlation curves between BIIE0246 and vehicle treatment with linear regression analysis. DIO-NPY, OE-NPYDβH mice on Western diet.
Liver Weight and Morphology
Similarly to previous findings, NPY mice displayed ballooning degeneration and hepatic accumulation of triglycerides and cholesterol compared to WT mice on chow diet (Figures 4A–C). BIIE0246 had no influence on liver morphology (Figure 4A). However, it induced different responses between the genotypes in hepatic triglyceride and cholesterol levels (interaction P < 0.05 and P = 0.05, respectively) with a tendency to increased contents in WT mice and no change in NPY mice compared to vehicle treated mice (Figures 4B,C).
FIGURE 4.
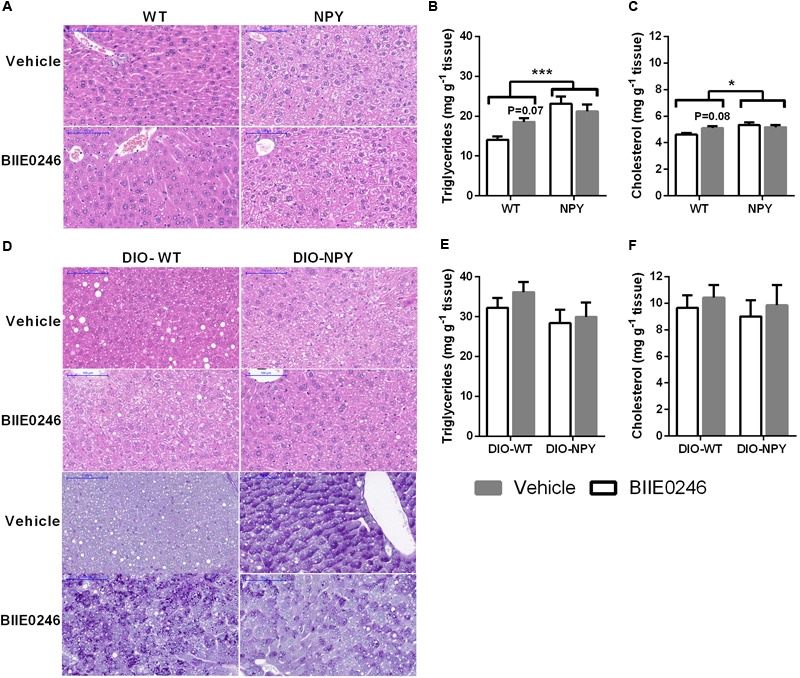
(A) Representative H&E stainings of liver slides with × 20 magnification (scale bar 100 μm), and (B) triglycerides and (C) cholesterol in the livers of mice on chow diet treated with Y2-receptor antagonist (BIIE0246) or vehicle for 4.5 weeks (n = 8–12/group). (D) Representative H&E (upper panel) and PAS stainings (lower panel) of liver slides with × 20 magnification (scale bar 100 μm), and (E) triglycerides and (F) cholesterol in the livers of mice on Western diet treated with Y2-receptor antagonist (BIIE0246) or vehicle for 2 weeks (n = 7–11/group). Values are expressed as means ± SEM. ∗P < 0.05 and ∗∗∗P < 0.001 comparing the different genotypes with two-way ANOVA. P-values present comparison of BIIE0246 with vehicle treatment within WT mice with Bonferroni post hoc test following a significant or near-significant interaction between treatment and genotype in two-way ANOVA. White bars, vehicle treated mice; gray bars, BIIE0246 treated mice; WT, wildtype mice on chow diet; NPY, OE-NPYDβH mice on chow diet; DIO-WT, wildtype mice on Western diet; DIO-NPY, OE-NPYDβH mice on Western diet.
Also in DIO, ballooning degeneration was detected in vehicle-treated DIO-NPY mice compared with DIO-WT mice. Similarly, treatment with BIIE0246 induced ballooning degeneration in DIO-WT mice compared to vehicle-treated mice. Oppositely, in BIIE0246-treated DIO-NPY mice ballooning degeneration was less pronounced when compared with their vehicle controls (Figure 4D, upper panel). PAS staining revealed that the ballooning degeneration pointed to hepatic glycogen accumulation in vehicle-treated DIO-NPY and BIIE0246-treated DIO-WT mice, whereas in BIIE0246-treated DIO-NPY mice glycogen content was decreased (Figure 4D, lower panel). However, mRNA expressions of genes related to glycogen metabolism were not changed (data not shown). The hepatic contents of triglycerides and cholesterol were similar between the genotypes and the treatment groups (Figures 4E,F).
Sympathetic and Central Noradrenergic Nervous System
To elucidate the mechanisms of the metabolic effects of BIIE0246 mRNA levels of key genes in the sympathetic and central noradrenergic nervous systems were analyzed. The 2-week chow cohort was used in order to detect the primary effects of the drug rather than compensatory changes induced by weight change. First, the effect of BIIE0246 on peripheral NPY in SNS in adrenal glands was elucidated. As it did not have significant effect on the expression of Npy or Y2r in adrenal glands (data not shown), we next studied the central NPY and noradrenergic systems in the hypothalamus and the brainstem, the brain regions lacking an effective blood-brain barrier. First, the expression of Th and Npy were measured to study whether BIIE0246 directly or by regulating Npy expression could influence the production of noradrenaline. In DIO (but not on chow diet), BIIE0246 increased Npy expression in both genotypes (Figures 5A–D). Instead on chow diet (but not in DIO), BIIE0246 decreased Th expression in the hypothalamus of NPY mice and in the brainstem of both genotypes (Figures 5E–H), suggesting that on healthy diet, BIIE0246 directly decreases central sympathetic tone. On the other hand, fitting with the changes in Npy expression, blocking of Y2-receptors with BIIE0246 in DIO (but not on chow diet) tended (P = 0.06) to decrease hypothalamic Pomc expression, especially in DIO-WT mice (Figures 5I,J), suggesting that with excess calories, BIIE0246 directly potentiates the expression of Npy, which in turn inhibits Pomc expression. Second, the effect of Y2-receptor antagonism on hypothalamic autoinhibitory Y2-receptor was studied. BIIE0246 significantly increased Y2r expression in NPY and DIO-NPY mice, whereas it had no effect in WT and DIO-WT mice [treatment × genotype interaction P < 0.05 (chow) and P < 0.01 (DIO)] (Figures 5K,L).
FIGURE 5.
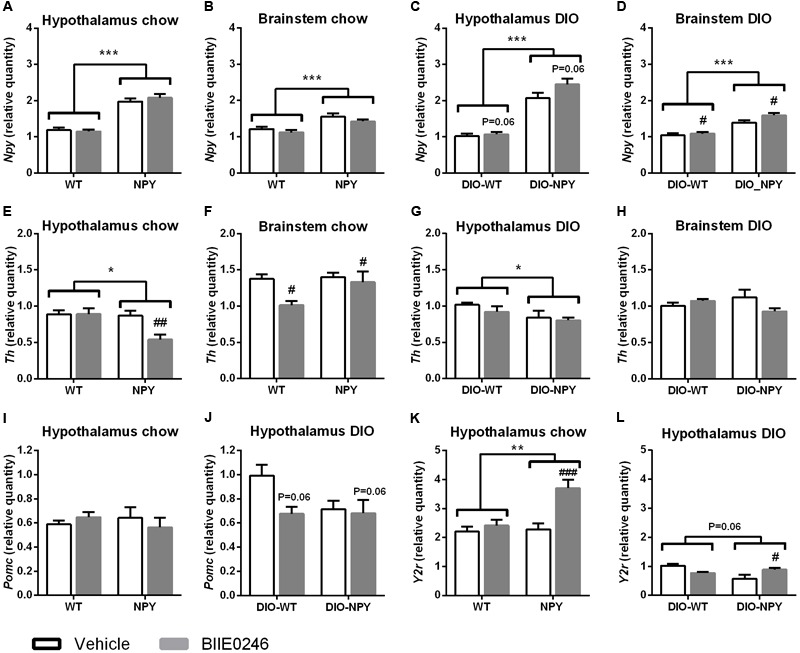
(A–D) mRNA expression of Npy, (E–H) Th (I,J) Pomc and (K,L) Y2r in the hypothalamus and the brainstem of chow- (A,B,E,F,I,K) or Western-diet-fed (C,D,G,H,J,L) OE-NPYDβH and WT mice (n = 6–12/group) after 2-week Y2-receptor antagonist (BIIE0246) or vehicle treatment. Values are expressed as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 and P = 0.06 comparing the different genotypes with two-way ANOVA, and #P < 0.05, ##P < 0.01, ###P < 0.001 and P = 0.06 comparing BIIE0246 treatment and vehicle treatment with two-way ANOVA (C,D,F,J), or with Bonferroni post hoc test following a significant interaction between treatment and genotype in two-way ANOVA (E,K,L). Npy, Neuropeptide Y; Pomc, pro-opiomelanocortin; Th, Tyrosine hydroxylase; Y2r, Y2-receptor; white bars, vehicle treated mice; gray bars, BIIE0246 treated mice; WT, wildtype mice on chow diet; NPY, OE-NPYDβH mice on chow diet; DIO-WT, wildtype mice on Western diet; DIO-NPY, OE-NPYDβH mice on Western diet.
WAT and BAT on Western Diet
Last, to elucidate whether the inhibition of fat accumulation in DIO-NPY mice could be explained by direct effects of BIIE0246 on adipose tissue, mRNA expression of selected genes involved in adipogenesis and angiogenesis in retroperitoneal WAT (WAT/r) and thermogenesis in BAT were studied. Weights of WAT depots did not differ between the treatments (data not shown). In WAT/r the lipases Lipe and Lpl tended or were significantly increased by BIIE0246, respectively, without difference between the genotypes (Figures 6A,B). The adipogenic Fabp4 was decreased in DIO-NPY compared to DIO-WT mice without difference between the treatments. Another adiopogenic marker, Ces3, and angiogenesis marker Mmp3 did not differ between the treatments or the genotypes (Figures 6C–E). BAT weight or the expression of the thermogenesis marker Ucp1 in BAT were not changed by BIIE0246 (data not shown).
FIGURE 6.
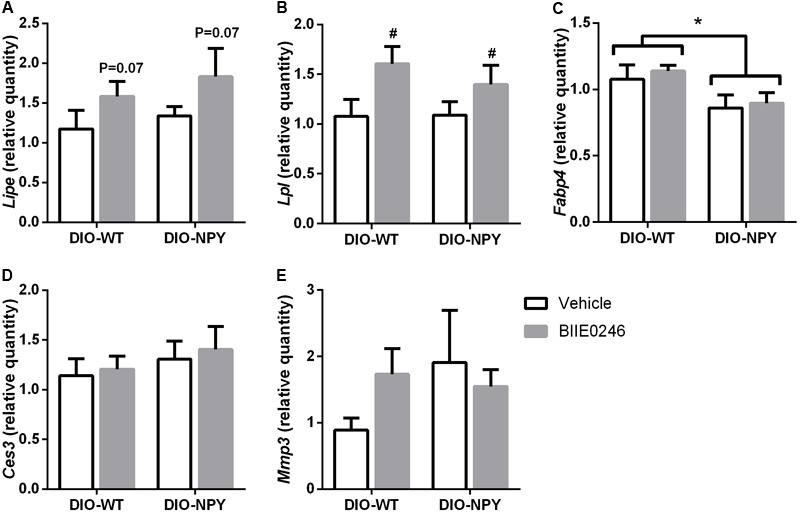
(A) mRNA expression of Lipe, (B) Lpl, (C) Fabp4, (D) Ces3, and (E) Mmp3 in retroperitoneal WAT of Western-diet-fed OE-NPYDβH and WT mice (n = 7–11/group) after 2-week Y2-receptor antagonist (BIIE0246) or vehicle treatment. Values are expressed as means ± SEM. ∗P < 0.05 comparing the different genotypes with two-way ANOVA. #P < 0.05 and P = 0.07 treatment effect with two-way ANOVA with non-significant interaction genotype × treatment. Lipe, hormone sensitive lipase; Lpl, lipoprotein lipase; Fabp4, fatty acid binding protein 4; Ces3, carboxylesterase 3; Mmp3, matrix metallopeptidase; white bars, vehicle treated mice; gray bars, BIIE0246 treated mice; DIO-WT, wildtype mice on Western diet; DIO-NPY, OE-NPYDβH mice on Western diet.
Discussion
Genetic down-regulation of peripheral Y2-receptors has previously been shown to inhibit NPY’s ability to reduce energy expenditure and to increase WAT mass (Kuo et al., 2007; Shi et al., 2011). In this study, we elucidated whether pharmacological blockage of peripheral Y2-receptors with BIIE0246 would have beneficial effects on energy metabolism in a situation of excess energy and/or NPY, usually associated with obesity (Shi et al., 2013). This study confirms the finding that blocking of Y2-receptors has beneficial effects on fat deposition and the metabolic status, but only when energy-rich environment is combined with excess NPY. Instead with normal NPY and excess calories, an Y2-receptor antagonist induces obesity. On a healthy diet, a peripherally administered BIIE0246 increases body weight gain and causes metabolic disturbances, especially when NPY levels are normal.
Y2-receptor antagonism-induced reduction in fat mass gain in the situation of elevated NPY levels and energy surplus, i.e., in DIO-NPY mice, fits with the findings by Kuo et al. (2007). They showed that chronic stress-induced NPY release from SNS in combination with energy-rich diet promoted fat mass gain via direct effects on adipose tissue, and that this was prevented with adipose tissue-targeted treatment with BIIE0246. NPY was suggested to induce fat mass gain directly by stimulating the proliferation and differentiation of adipocytes, and indirectly by promoting angiogenesis (Kuo et al., 2007). Although in our study BIIE0246 did not induce significant differences in the gene expressions of adipogenic and angiogenic genes in the retroperitoneal WAT, it does not exclude the fact that this is the most probable mechanism-of-action in inhibition of fat mass gain in presence of excess NPY (i.e., in DIO-NPY mice). Instead, BIIE0246 increased the expression of enzymes responsible for both triglyceride uptake and breakdown suggesting that the accelerated lipid turn-over may influence the outcome in both genotypes. The catabolic effect of BIIE0246 in DIO-NPY mice was also reflected in decreased hepatic glycogen accumulation and serum cholesterol relative to body weight suggesting a role for Y2-receptors in regulation of these traits in a state of excess NPY and energy surplus. This fits with the improved serum cholesterol in Y2-receptor antagonist treated diabetic rats (Liu et al., 2013), which have also been shown to have elevated NPY levels (Morris and Pavia, 2004; Ruipan et al., 2014).
In contrast, without excess peripheral NPY (i.e., in DIO-WT mice) treatment with BIIE0246 does not lead to beneficial effects but rather increases fat and glycogen accumulation suggesting different mechanisms depending on the level of NPY. This difference between genotypes supports that Y2-receptor plays a key role in mediating the obesogenic effects of NPY in WAT in situations of NPY excess due to stress (Kuo et al., 2007) or genetic overexpression as presented here. The induction of metabolic disturbances is even more prominent when the mice are fed with a healthy diet. In chow-fed WT mice, BIIE0246 increased weight gain, and caused hypercholesterolemia, hyperinsulinemia, and hepatic triglyceride and cholesterol accumulation, which actually resemble the metabolic phenotype of genetically obese NPY mice (Vahatalo et al., 2015; Ailanen et al., 2017). These findings in WT and DIO-WT mice are opposite to the results of peripheral Y2-receptor knock-down that induced resistance to DIO and had no effects on chow (Shi et al., 2011). The discrepancy in the results could be influenced by the method of receptor inhibition, i.e., by the stress induced by the drug administration and the fluctuation in drug concentration over the day due to once daily dosing combined with the relatively short half-life of BIIE0246. However, even more likely it could result from the effects of neural Y2-autoreceptor inhibition that was avoided by the knock-down approach.
Therefore, we hypothesized that antagonism of presynaptic Y2-autoreceptors increased NPY release in WT mice. As no differences in the expression of Npy or Y2r were detected in the adrenal glands, the central effects of Y2-receptor antagonism were considered. As the hypothalamus and brainstem are some of the rare brain regions that lack an effective blood-brain barrier (Wang et al., 2008; Rodriguez et al., 2010), peripherally administered BIIE0246 could have reached Y2-receptors in these key areas regulating energy balance. NPY neuron-specific Y2-receptor deletion in the hypothalamus has been shown to increase Npy and decrease Pomc expression in the Arc (Shi et al., 2010), and Npy overexpression in the Arc to increase food intake and to inhibit SNS activity via decreased expression of hypothalamic Th, the rate-limiting enzyme of catecholamine synthesis (Shi et al., 2013). Brainstem Npy overexpression in turn downregulates brainstem Th, but does not increase food intake (Vahatalo et al., 2015). In the current study, a small increase in hypothalamic and brainstem Npy, and a tendency to decreased Pomc were detected, but only in the DIO group. This could have contributed to the obesogenic effect of BIIE0246 in mice with normal NPY levels, although it did not affect food intake or Th expression. Instead, in chow-fed mice, no changes in Npy expression or food intake were detected, but brainstem Th was downregulated and thus lower SNS activity could play a role in the metabolic disturbances induced by BIIE0246 similar to genetic Npy overexpression in NPY mice (Vahatalo et al., 2015). Interestingly, BIIE0246 changed Y2r expression differently between the genotypes. Hypothalamic Y2r expression was increased in NPY mice treated with BIIE0246 on both diets. This could have contributed to somewhat milder adverse metabolic effect in NPY mice compared to WT mice, and to the beneficial metabolic effect in DIO-NPY mice compared to DIO-WT. Thus, in addition to the blockage of peripheral Y2-receptors, it is likely that hypothalamic and brainstem Y2-receptor antagonism contributed to the metabolic outcomes in the current study. Central effects are also supported by the resemblance of the BIIE0246-induced phenotype in WT mice with the obese phenotype of adult-onset hypothalamic Y2-receptor knock-out mice (Shi et al., 2013).
Taken together, the peripheral Y2-receptors are clearly participating in the regulation of energy metabolism, but whether their pharmacological blockage is beneficial in the treatment of obesity and metabolic disorders, remains even more controversial than previous studies have suggested. Although we cannot exclude the fact that the effect of the incomplete antagonism of Y2-receptors due to the fluctuation of the drug concentration may influence the results of this study, it seems that on a regular chow diet, independent of normal or excess NPY, Y2-receptors accessible to a peripherally administered Y2-receptor antagonist seem to be beneficial to energy metabolism, and their blockage leads to weight gain and metabolic disorders possibly via centrally decreased sympathetic activity. In an energy-rich environment, pharmacological Y2-receptor blockage is metabolically beneficial supporting the key role of Y2-receptor in expanding adipose tissue. However, this is true only when NPY levels are increased. In humans, elevated NPY levels are associated with increased risk for metabolic disorders in individuals with gain-of-function polymorphism in the NPY gene (Kallio et al., 2001; Ding et al., 2005; Yeung et al., 2011). Thus, since the trend in medical care is moving toward more personalized pharmacotherapy, the blockade of peripheral Y2-receptors could be targeted for the treatment of the obesity accompanied with elevated noradrenergic NPY levels (e.g., during stress or genetic NPY overexpression) in energy rich environment. In this sense, OE-NPYDβH mice could be a potential tool for the pharmacological studies of Y2-receptor specific compounds.
Author Contributions
LV and LA conceived and performed the study, analyzed the data, and wrote the manuscript with equal contributions. HS-M, SM, and WO took part in performing the study. SR and ES conceived the study and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Sanna Bastman, Elina Kahra, and Raija Kaartosalmi for skillful technical assistance.
Footnotes
Funding. This study was financially supported by Academy of Finland (130882 to ES and 252441 to SR), the Finnish Funding Agency for Technology and Innovation (143/31/2010), and Turku University Hospital Research Fund (L3826).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00319/full#supplementary-material
References
- Abbott C. R., Small C. J., Kennedy A. R., Neary N. M., Sajedi A., Ghatei M. A., et al. (2005). Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3-36) on food intake. Brain Res. 1043 139–144. 10.1016/j.brainres.2005.02.065 [DOI] [PubMed] [Google Scholar]
- Ailanen L., Ruohonen S. T., Vahatalo L. H., Tuomainen K., Eerola K., Salomaki-Myftari H., et al. (2017). The metabolic syndrome in mice overexpressing neuropeptide Y in noradrenergic neurons. J. Endocrinol. 234 57–72. 10.1530/JOE-16-0223 [DOI] [PubMed] [Google Scholar]
- Batterham R. L., Cowley M. A., Small C. J., Herzog H., Cohen M. A., Dakin C. L., et al. (2002). Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418 650–654. 10.1038/nature00887 [DOI] [PubMed] [Google Scholar]
- Billington C. J., Briggs J. E., Grace M., Levine A. S. (1991). Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am. J. Physiol. 260 R321–R327. 10.1152/ajpregu.1991.260.2.R321 [DOI] [PubMed] [Google Scholar]
- Bowers R. R., Festuccia W. T., Song C. K., Shi H., Migliorini R. H., Bartness T. J. (2004). Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286 R1167–R1175. 10.1152/ajpregu.00558.2003 [DOI] [PubMed] [Google Scholar]
- Bradley R. L., Mansfield J. P., Maratos-Flier E. (2005). Neuropeptides, including neuropeptide Y and melanocortins, mediate lipolysis in murine adipocytes. Obes. Res. 13 653–661. 10.1038/oby.2005.73 [DOI] [PubMed] [Google Scholar]
- Brothers S. P., Saldanha S. A., Spicer T. P., Cameron M., Mercer B. A., Chase P., et al. (2010). Selective and brain penetrant neuropeptide y y2 receptor antagonists discovered by whole-cell high-throughput screening. Mol. Pharmacol. 77 46–57. 10.1124/mol.109.058677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall B. M., DiMaggio D. A., Massari V. J., Pickel V. M., Ruggiero D. A., O’Donohue T. L. (1985). The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience 15 1159–1181. 10.1016/0306-4522(85)90260-X [DOI] [PubMed] [Google Scholar]
- Ding B., Kull B., Liu Z., Mottagui-Tabar S., Thonberg H., Gu H. F., et al. (2005). Human neuropeptide Y signal peptide gain-of-function polymorphism is associated with increased body mass index: possible mode of function. Regul. Pept. 127 45–53. 10.1016/j.regpep.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Doods H., Gaida W., Wieland H. A., Dollinger H., Schnorrenberg G., Esser F., et al. (1999). BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur. J. Pharmacol. 384 R3–R5. 10.1016/S0014-2999(99)00650-0 [DOI] [PubMed] [Google Scholar]
- Ekblad E., Edvinsson L., Wahlestedt C., Uddman R., Hakanson R., Sundler F. (1984). Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul. Pept. 8 225–235. 10.1016/0167-0115(84)90064-8 [DOI] [PubMed] [Google Scholar]
- Forbes S., Herzog H., Cox H. M. (2012). A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br. J. Pharmacol. 166 2307–2316. 10.1111/j.1476-5381.2012.01939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. C., Hasstedt S. J., Xin Y., Dalley B. K., Milash B. A., Yakobson E., et al. (2011). Polymorphisms in the NPY2R gene show significant associations with BMI that are additive to FTO, MC4R, and NPFFR2 gene effects. Obesity 19 2241–2247. 10.1038/oby.2011.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio J., Pesonen U., Kaipio K., Karvonen M. K., Jaakkola U., Heinonen O. J., et al. (2001). Altered intracellular processing and release of neuropeptide Y due to leucine 7 to proline 7 polymorphism in the signal peptide of preproneuropeptide Y in humans. FASEB J. 15 1242–1244. 10.1096/fj.00-0436fje [DOI] [PubMed] [Google Scholar]
- Karvonen M. K., Pesonen U., Koulu M., Niskanen L., Laakso M., Rissanen A., et al. (1998). Association of a leucine(7)-to-proline(7) polymorphism in the signal peptide of neuropeptide Y with high serum cholesterol and LDL cholesterol levels. Nat. Med. 4 1434–1437. 10.1038/4027 [DOI] [PubMed] [Google Scholar]
- Kuo L. E., Kitlinska J. B., Tilan J. U., Li L., Baker S. B., Johnson M. D., et al. (2007). Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 13 803–811. 10.1038/nm1611 [DOI] [PubMed] [Google Scholar]
- Lavebratt C., Alpman A., Persson B., Arner P., Hoffstedt J. (2006). Common neuropeptide Y2 receptor gene variant is protective against obesity among Swedish men. Int. J. Obes. (Lond.) 30 453–459. 10.1038/sj.ijo.0803188 [DOI] [PubMed] [Google Scholar]
- Liu J., Wang N., Xie F., Sun L. H., Chen Q. X., Ye J. H., et al. (2013). Blockage of peripheral NPY Y1 and Y2 receptors modulates barorefex sensitivity of diabetic rats. Cell. Physiol. Biochem. 31 421–431. 10.1159/000343379 [DOI] [PubMed] [Google Scholar]
- Masoudi-Kazemabad A., Jamialahmadi K., Moohebati M., Mojarrad M., Manshadi R. D., Akhlaghi S., et al. (2013). Neuropeptide Y Leu7Pro polymorphism associated with the metabolic syndrome and its features in patients with coronary artery disease. Angiology 64 40–45. 10.1177/0003319711435149 [DOI] [PubMed] [Google Scholar]
- Morris M. J., Pavia J. M. (2004). Increased endogenous noradrenaline and neuropeptide Y release from the hypothalamus of streptozotocin diabetic rats. Brain Res. 1006 100–106. 10.1016/j.brainres.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Naveilhan P., Svensson L., Nystrom S., Ekstrand A. J., Ernfors P. (2002). Attenuation of hypercholesterolemia and hyperglycemia in ob/ob mice by NPY Y2 receptor ablation. Peptides 23 1087–1091. 10.1016/S0196-9781(02)00042-6 [DOI] [PubMed] [Google Scholar]
- Rodriguez E. M., Blazquez J. L., Guerra M. (2010). The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31 757–776. 10.1016/j.peptides.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Rosmaninho-Salgado J., Cortez V., Estrada M., Santana M. M., Goncalves A., Marques A. P., et al. (2012). Intracellular mechanisms coupled to NPY Y2 and Y5 receptor activation and lipid accumulation in murine adipocytes. Neuropeptides 46 359–366. 10.1016/j.npep.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Ruipan Z., Xiangzhi M., Li L., Ying Z., Mingliang Q., Peng J., et al. (2014). Differential expression and localization of neuropeptide Y peptide in pancreatic islet of diabetic and high fat fed rats. Peptides 54 33–38. 10.1016/j.peptides.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Ruohonen S. T., Pesonen U., Moritz N., Kaipio K., Roytta M., Koulu M., et al. (2008). Transgenic mice overexpressing neuropeptide Y in noradrenergic neurons: a novel model of increased adiposity and impaired glucose tolerance. Diabetes Metab. Res. Rev. 57 1517–1525. 10.2337/db07-0722 [DOI] [PubMed] [Google Scholar]
- Ruohonen S. T., Vahatalo L. H., Savontaus E. (2012). Diet-induced obesity in mice overexpressing neuropeptide y in noradrenergic neurons. Int. J. Pept. 2012:452524. 10.1155/2012/452524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A., Bergen H. T., Boey D., Bamming D., Cooney G. J., Lin S., et al. (2006). Y2Y4 receptor double knockout protects against obesity due to a high-fat diet or Y1 receptor deficiency in mice. Diabetes Metab. Res. Rev. 55 19–26. 10.2337/diabetes.55.01.06.db05-0472 [DOI] [PubMed] [Google Scholar]
- Sainsbury A., Rohner-Jeanrenaud F., Cusin I., Zakrzewska K. E., Halban P. A., Gaillard R. C., et al. (1997). Chronic central neuropeptide Y infusion in normal rats: status of the hypothalamo-pituitary-adrenal axis, and vagal mediation of hyperinsulinaemia. Diabetologia 40 1269–1277. 10.1007/s001250050820 [DOI] [PubMed] [Google Scholar]
- Sainsbury A., Schwarzer C., Couzens M., Herzog H. (2002). Y2 receptor deletion attenuates the type 2 diabetic syndrome of ob/ob mice. Diabetes Metab. Res. Rev. 51 3420–3427. 10.2337/diabetes.51.12.3420 [DOI] [PubMed] [Google Scholar]
- Shi Y. C., Lau J., Lin Z., Zhang H., Zhai L., Sperk G., et al. (2013). Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell. Metab. 17 236–248. 10.1016/j.cmet.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Shi Y. C., Lin S., Castillo L., Aljanova A., Enriquez R. F., Nguyen A. D., et al. (2011). Peripheral-specific y2 receptor knockdown protects mice from high-fat diet-induced obesity. Obesity 19 2137–2148. 10.1038/oby.2011.99 [DOI] [PubMed] [Google Scholar]
- Shi Y. C., Lin S., Wong I. P., Baldock P. A., Aljanova A., Enriquez R. F., et al. (2010). NPY neuron-specific Y2 receptors regulate adipose tissue and trabecular bone but not cortical bone homeostasis in mice. PLoS One 5:e11361. 10.1371/journal.pone.0011361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A., Gueorguiev M., Samson C., Hercberg S., Heude B., Levy-Marchal C., et al. (2007). Single nucleotide polymorphisms in the neuropeptide Y2 receptor (NPY2R) gene and association with severe obesity in French white subjects. Diabetologia 50 574–584. 10.1007/s00125-006-0555-2 [DOI] [PubMed] [Google Scholar]
- Silva A. P., Cavadas C., Grouzmann E. (2002). Neuropeptide Y and its receptors as potential therapeutic drug targets. Clin. Chim. Acta 326 3–25. 10.1016/S0009-8981(02)00301-7 [DOI] [PubMed] [Google Scholar]
- Soloveva V., Graves R. A., Rasenick M. M., Spiegelman B. M., Ross S. R. (1997). Transgenic mice overexpressing the beta 1-adrenergic receptor in adipose tissue are resistant to obesity. Mol. Endocrinol. 11 27–38. [DOI] [PubMed] [Google Scholar]
- Torekov S. S., Larsen L. H., Andersen G., Albrechtsen A., Glumer C., Borch-Johnsen K., et al. (2006). Variants in the 5′ region of the neuropeptide Y receptor Y2 gene (NPY2R) are associated with obesity in 5,971 white subjects. Diabetologia 49 2653–2658. 10.1007/s00125-006-0425-y [DOI] [PubMed] [Google Scholar]
- Vahatalo L. H., Ruohonen S. T., Makela S., Kovalainen M., Huotari A., Makela K. A., et al. (2015). Neuropeptide Y in the noradrenergic neurones induces obesity and inhibits sympathetic tone in mice. Acta Physiol. 213 902–919. 10.1111/apha.12436 [DOI] [PubMed] [Google Scholar]
- Wang Q. P., Guan J. L., Pan W., Kastin A. J., Shioda S. (2008). A diffusion barrier between the area postrema and nucleus tractus solitarius. Neurochem. Res. 33 2035–2043. 10.1007/s11064-008-9676-y [DOI] [PubMed] [Google Scholar]
- Yeung E. H., Zhang C., Chen J., Bowers K., Hu F. B., Kang G., et al. (2011). Polymorphisms in the neuropeptide Y gene and the risk of obesity: findings from two prospective cohorts. J. Clin. Endocrinol. Metab. 96 E2055–E2062. 10.1210/jc.2011-0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulyaningsih E., Loh K., Lin S., Lau J., Zhang L., Shi Y., et al. (2014). Pancreatic polypeptide controls energy homeostasis via Npy6r signaling in the suprachiasmatic nucleus in mice. Cell. Metab. 19 58–72. 10.1016/j.cmet.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Zarjevski N., Cusin I., Vettor R., Rohner-Jeanrenaud F., Jeanrenaud B. (1993). Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology 133 1753–1758. 10.1210/endo.133.4.8404618 [DOI] [PubMed] [Google Scholar]
- Zhang L., Lee I. C., Enriquez R. F., Lau J., Vahatalo L. H., Baldock P. A., et al. (2014). Stress- and diet-induced fat gain is controlled by NPY in catecholaminergic neurons. Mol. Metab. 3 581–591. 10.1016/j.molmet.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukowska-Grojec Z., Karwatowska-Prokopczuk E., Fisher T. A., Ji H. (1998). Mechanisms of vascular growth-promoting effects of neuropeptide Y: role of its inducible receptors. Regul. Pept. 75–76, 231–238. 10.1016/S0167-0115(98)00073-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


